Significance
Self-administered insulin is the most important therapeutic to provide control over blood glucose levels for patients with type-1 diabetes. However, standard insulin therapy introduces a number of complications and subsequent issues with control of blood glucose levels. Here, we prepared a derivative of insulin with a molecular switch to provide glucose-mediated activation of the insulin molecule, toward the generation of more autonomous therapy with improved blood glucose control. This modified insulin, when administered in a diabetic mouse model, restores blood glucose levels following a glucose challenge (i.e., a simulated meal) faster than both standard insulin and a clinically used long-lasting insulin derivative.
Keywords: diabetes, protein modification, molecular engineering, glucose sensing, smart therapy
Abstract
Since its discovery and isolation, exogenous insulin has dramatically changed the outlook for patients with diabetes. However, even when patients strictly follow an insulin regimen, serious complications can result as patients experience both hyperglycemic and hypoglycemic states. Several chemically or genetically modified insulins have been developed that tune the pharmacokinetics of insulin activity for personalized therapy. Here, we demonstrate a strategy for the chemical modification of insulin intended to promote both long-lasting and glucose-responsive activity through the incorporation of an aliphatic domain to facilitate hydrophobic interactions, as well as a phenylboronic acid for glucose sensing. These synthetic insulin derivatives enable rapid reversal of blood glucose in a diabetic mouse model following glucose challenge, with some derivatives responding to repeated glucose challenges over a 13-h period. The best-performing insulin derivative provides glucose control that is superior to native insulin, with responsiveness to glucose challenge improved over a clinically used long-acting insulin derivative. Moreover, continuous glucose monitoring reveals responsiveness matching that of a healthy pancreas. This synthetic approach to insulin modification could afford both long-term and glucose-mediated insulin activity, thereby reducing the number of administrations and improving the fidelity of glycemic control for insulin therapy. The described work is to our knowledge the first demonstration of a glucose-binding modified insulin molecule with glucose-responsive activity verified in vivo.
Insulin is a key component in the treatment of both type I and type II diabetes, diseases that are steadily increasing in prevalence (1–3). For insulin-dependent patients, best therapeutic outcomes are observed when a regimented insulin administration schedule is followed (4). Some patients respond well to insulin therapy, and a cohort exists as part of the Joslin Medalist program that has survived for 50 or more years with total insulin dependence (5). However, most insulin-dependent patients suffer from complications that arise from poor adherence to therapy or from inadequate glycemic control (6). Acute hypoglycemic episodes can result in coma or death (7). Furthermore, chronic instability in glucose levels can lead to cardiovascular disease and nonhealing wounds and is an independent predictor of total, cardiovascular, and cancer mortality (8). There remains a need to address the kinetics of insulin therapy to more closely match the natural dynamics in insulin signaling and blood glucose levels and to improve the autonomy of insulin therapy.
Extensive efforts have been taken to develop and commercialize insulin variants with tunable pharmacokinetics (9, 10). These efforts have led to variants with rapid activity and those with prolonged activity; combinations of various types may be useful to improve patient-specific glycemic control. Native unmodified insulin has an onset time of 30–60 min, a peak window of action from 2 to 3 h, and a duration of action up to 8 h (4). In comparison, fast-acting variants have been developed, for example, by switching the position of the B29 lysine residue and the B28 proline residue (Insulin lispro) to inhibit dimer formation and improve uptake (11). Insulin lispro reduces onset time to just 5–15 min, with peak action at 30–60 min and duration of action up to 5 h (4). Long-acting variants have also been developed, for example, by covalently attaching a saturated 14-carbon alkyl segment to the ε-amine of B29 lysine (Insulin detemir), enabling the insulin to bind to and be sequestered by circulating serum albumin. As a result, Insulin detemir has an onset of action of 1–2 h, with a peak action at 3–9 h and duration lasting up to 24 h (4). As a component in diabetes management, long-acting insulin is particularly useful as a daily injection to provide basal insulin and limit spikes in blood glucose levels throughout the day (12).
Here, we synthesized and developed a class of insulin derivatives that is designed to afford long-lasting and glucose-mediated activity toward the generation of glucose-responsive chemically modified insulin. To prepare these insulin derivatives, small molecules containing both an aliphatic moiety and a phenylboronic acid (PBA) moiety were synthesized for covalent conjugation to insulin. The use of an aliphatic domain was inspired by the design of long-acting Insulin detemir to afford binding to serum albumin, or other hydrophobic components in serum, for prolonged circulation half-life (13, 14). Meanwhile, incorporation of PBA provides a glucose-sensing element within the conjugate, as PBAs are known to bind reversibly to cis-1,2 or cis-1,3 diols such as glucose, which stabilizes a negative charge on the boronic acid (15). The inclusion of PBA groups within polymers has been previously used for glucose sensing and insulin delivery through modulation of electrostatics upon glucose binding to PBA (16), and PBA has also been evaluated as an element in optical glucose sensors (17). Here, we demonstrate that derivatizing insulin with aliphatic PBA conjugates affords long-lasting and glucose-responsive activity, providing a potentially improved therapeutic strategy for diabetes management.
Results
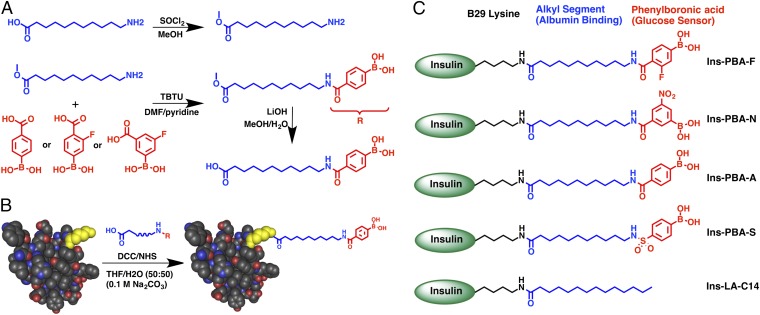
The desired conjugates, containing both an aliphatic domain and a PBA, were synthesized according to methods outlined in Fig. 1A and Scheme S1. The PBA moieties were coupled to the aliphatic domain by direct amidation of either commercially available PBAs or phenyl bromides followed by a Suzuki coupling reaction. This approach afforded aliphatic groups terminally substituted with four different PBA moieties. The design of these conjugates included fluoro-, nitro-, and sulfo-containing PBAs, intended to provide electron-withdrawing character to lower the pKa of the phenylboronic acid and facilitate the glucose-binding species at physiologic pH (18). Using dicyclohexylcarbodiimide (DCC)/N-hydroxysuccinimide (NHS) coupling under basic conditions, conjugates were attached to insulin preferentially at the ε-amine of the B29 lysine residue (Fig. 1B). Similar methods have been previously demonstrated to afford selectivity in functionalizing this primary amine instead of either the A1 or B1 N-terminal amines of insulin (19, 20). These methods resulted in four PBA-containing aliphatic insulin derivatives (Fig. 1C), with amido- (Ins-PBA-A), fluoro- (Ins-PBA-F), nitro- (Ins-PBA-N), or sulfo- (Ins-PBA-S) modified PBA groups. In addition, control long-acting insulin (Ins-LA-C14) was prepared through myristic acid conjugation, producing modified insulin with the same structure as the commercially used long-acting Insulin detemir (13, 14). All modified insulin derivatives were purified using preparative scale HPLC to afford the purified product specifically modified at the B29 lysine (Fig. S1).
Fig. 1.
Schematic illustrating the strategy for insulin modification. (A) Generalized scheme to prepare small molecules used in the preparation of Ins-PBA-F, Ins-PBA-N, and Ins-PBA-A. (B) Final carboxylic acid-containing small molecule is conjugated to the native human insulin protein through the ε-amine of the lysine at the B29 position (yellow). (C) Structures of the four PBA-modified long-acting insulin derivatives used in this work along with the positive control, Ins-LA-C14, commercially known as insulin detemir. Detailed schematic and methods, including those for synthesis of Ins-PBA-S, can be found in SI Methods.
To determine whether covalent modification had an effect on secondary structure of insulin, we evaluated all of the modified derivatives using near-UV circular dichroism (CD) (Fig. S2). All insulin derivatives were observed to have CD spectra consistent primarily with α-helical secondary structure. Modified derivatives had a subtle blue-shift in their CD signature, consistent with previous findings for CD spectra of acylated insulin (21), but overall the α-helical character of native insulin was maintained. To ensure there were no deleterious effects of modification on insulin function, a cell-based insulin receptor activation assay was performed (Fig. S3). Modification of insulin with aliphatic PBA conjugates or myristic acid was found to have no significant effect on insulin activity relative to native unmodified insulin.
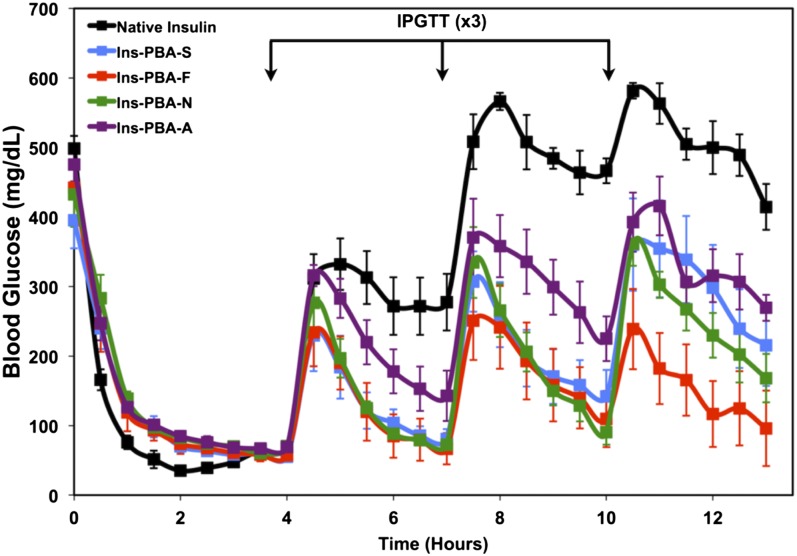
A mouse model of insulin-deficient diabetes, prepared using streptozotocin (STZ) to induce pancreatic β-cell death (22), was used to evaluate the function of the modified insulin derivatives developed here. All PBA-modified insulins reduced blood glucose levels to a normoglycemic level, <200 mg/dL for a mouse, following a single s.c. administration of 5 IU/kg insulin or mass equivalent of modified insulins (Fig. 2). Four hours following administration, an i.p. glucose tolerance test (IPGTT) was administered. Following a spike in blood glucose, all four PBA-modified insulin derivatives restored blood glucose to a normogylcemic level, whereas native insulin did not have an effect on reducing blood glucose. Of note, Ins-PBA-S, Ins-PBA-F, and Ins-PBA-N derivatives completely reversed blood glucose to prechallenge levels. After a second IPGTT, performed 7 h following initial insulin administration, Ins-PBA-S, Ins-PBA-F, and Ins-PBA-N were again able to restore normoglycemic levels. Ins-PBA-A was not able to restore normoglycemia following the second challenge. A third IPGTT performed at 10 h following insulin administration revealed that Ins-PBA-F and Ins-PBA-N were still able to restore normoglycemia. Ins-PBA-F was especially potent in reversing blood glucose levels to prechallenge levels, and thus, this insulin was identified as a promising candidate for further evaluation.
Fig. 2.
Initial screen of PBA-modified insulin derivatives in an STZ-induced diabetic mouse model. Insulin derivatives or native insulin were injected at a dose of 5 IU/kg (173.5 μg/kg) at the beginning of the experiment (0 h), with a series of IPGTTs performed at 4, 7, and 10 h following insulin administration. Blood glucose from peripheral tail vein puncture was monitored throughout the study to follow insulin activity.
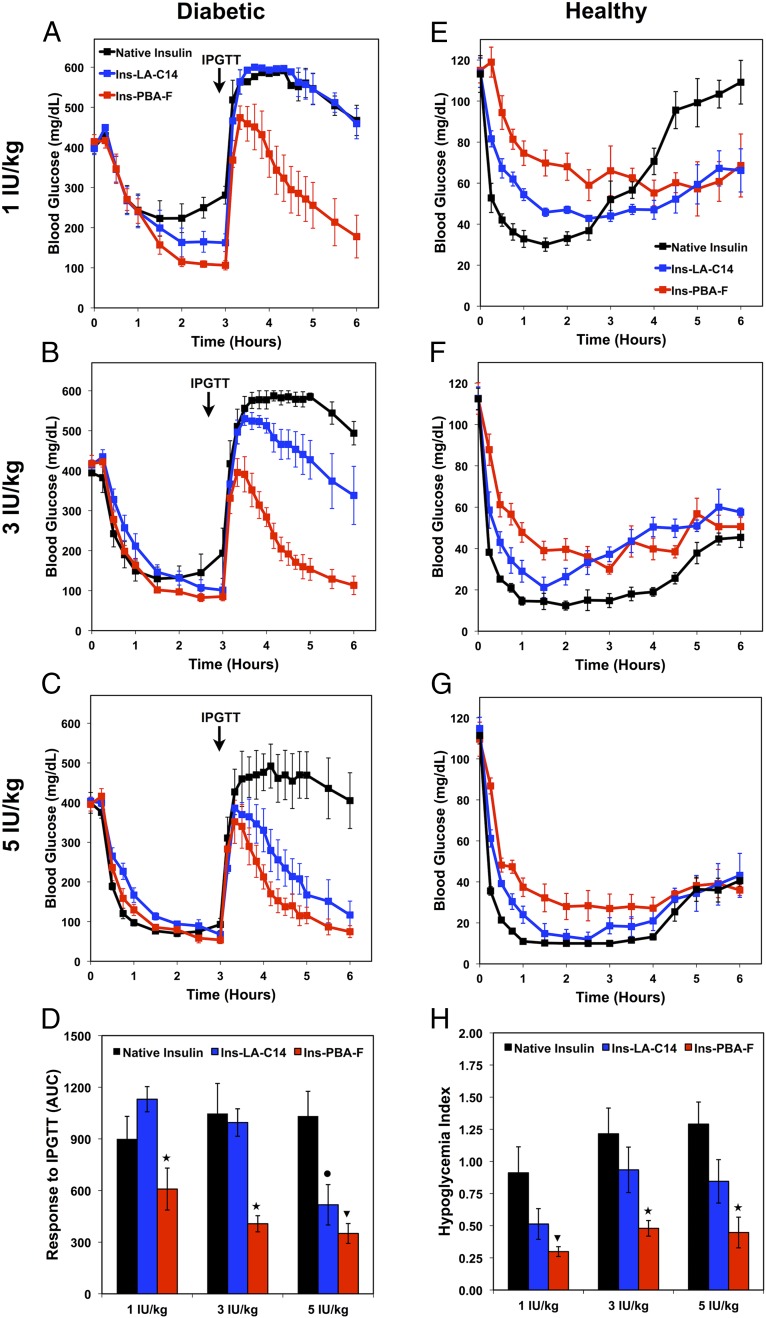
To establish the glucose dependence of Ins-PBA-F, we performed dosing studies in both STZ-induced diabetic mice and healthy mice (Fig. 3). In these studies, our best performing insulin (Ins-PBA-F) was compared with the active ingredient of a clinically used long-acting insulin formulation (Ins-LA-C14, Insulin detemir) in insulin-deficient mice to investigate whether the observed effect was not simply a result of long-lasting activity. Doses equivalent to 1, 3, and 5 IU/kg of native insulin were chosen as a therapeutically relevant dose range. In diabetic mice, following insulin administration, blood glucose levels for all mice dropped. At 3 h following dosing with insulin, an IPGTT was performed. To measure insulin responsiveness, the area under the curve was calculated for each insulin between 3 and 6 h (Fig. 3D). As shown, at both 1 and 3 IU/kg, Ins-PBA-F was significantly (P < 0.05) more responsive to glucose challenge than either Ins-LA-C14 or native insulin. At 5 IU/kg, both Ins-PBA-F and Ins-LA-C14 were significantly (P < 0.05) more responsive than native insulin, but there were no significant difference between the responsiveness of these two groups. Dosing studies were also performed in a healthy normoglycemic mouse. In these studies, the extent to which insulin elicited hypoglycemia was quantified using a hypoglycemia index, a measure of the drop from initial blood glucose reading to the nadir (i.e., lowest observed) value divided by the time over which this drop occurred (Fig. 3H). A similar metric is used clinically in the evaluation of diabetic patients (23). For healthy mice dosed at 1 IU/kg, the hypoglycemia index for Ins-PBA-F was significantly (P < 0.05) less than that for native insulin, whereas the hypoglycemia index for Ins-LA-C14 was not less that for native insulin. At 3 and 5 IU/kg, Ins-PBA-F resulted in a significant (P < 0.05) reduction in the hypoglycemic index compared with both Ins-LA-C14 and native insulin. Taken together, the results in diabetic and healthy mice indicate higher activity of Ins-PBA-F in response to glucose challenge, with a reduced hypoglycemic index when administered in a normoglycemic state.
Fig. 3.
Dose escalation studies in diabetic (left column) and healthy (right column) mice to evaluate the potency of Ins-PBA-F compared with Ins-LA-C14 and native insulin. Insulins were dosed corresponding to insulin-equivalent doses of 1 IU/kg (34.7 μg/kg, first row), 3 IU/kg (104.1 μg/kg, second row), and 5 IU/kg (173.5 μg/kg, third row) at time 0, and blood glucose was monitored for 6 h. (A–C) Dosing studies in diabetic mice, with insulin administered s.c. at time 0 and an IPGTT performed 3 h following insulin administration. (D) The responsiveness of each insulin was calculated based on the area under the curve from 3 to 6 h, with the baseline set at the 3-h blood glucose reading. (E–G) Dosing studies in healthy mice, with insulin administered s.c. at time 0. (H) Quantification of hypoglycemia index, which was determined from the difference between the initial and nadir blood glucose readings divided by the time at which nadir (i.e., lowest observed value) was reached. For D and H, ANOVA with Bonferroni multiple comparisons post hoc test was performed. ★P < 0.05 for Ins-PBA-F compared with both Ins-LA-C14 and native insulin; ▾P < 0.05 for Ins-PBA-F compared with native insulin; •P < 0.05 for Ins-LA-C14 compared with native insulin.
To further evaluate the kinetics and responsiveness of PBA-modified insulin analogs in response to IPGTT, continuous glucose monitoring was used to provide 5-min resolution for blood glucose measurements (Fig. S4A). Healthy age-matched mice were used as a positive control as a comparison for the responsiveness of a fully functioning pancreas. Following administration, both insulin derivatives reversed blood glucose to normoglycemic levels, but the slope of this decrease was significantly faster for Ins-PBA-F than for Ins-LA-C14 (Fig. S4C). An IPGTT was performed 3 h following insulin administration, which prompted a comparable rise in blood glucose levels in all groups. However, when mice were treated with Ins-PBA-F, a rapid reversal was observed following challenge that was similar in slope to the response seen for a healthy mouse with no insulin deficiency (Fig. S4D). In the case of the long-acting variant, Ins-LA-C14, the response following glucose challenge was much slower than both the Ins-PBA-F and healthy control. Although the slopes between different insulins varied considerably, the slopes for each individual treatment after initial administration and following IPGTT were similar. To quantify responsiveness of Ins-PBA-F and Ins-LA-C14 compared with the healthy control, area under the curve was calculated from the beginning of IPGTT at 3 h until the 6-h end point of the study. The responsiveness of Ins-PBA-F was comparable to that for a healthy animal, whereas the Ins-LA-C14 had a much larger area and did not return to baseline over the timeframe integrated (Fig. S4B).
It has been reported that PBAs bind more strongly to fructose than they do to glucose (24). To examine diol-mediated activation of Ins-PBA-F, an IP challenge was performed using fructose instead of glucose. For treatment with Ins-PBA-F, glucose challenge resulted in a spike and reversal of blood glucose levels (Fig. S5A), as previously demonstrated in other experiments. However, when fructose was instead used for challenge, blood glucose levels were only slightly elevated and quickly returned to baseline for the duration of the study. When glucose was used to challenge following Ins-LA-C14 treatment, the behavior was similar to that seen in previous studies. In contrast, treatment with Ins-LA-C14 demonstrated a gradual rise in blood glucose levels following fructose challenge (Fig. S5B), likely due to the conversion of administered fructose into glucose (25).
We hypothesized that the glucose-responsive activity demonstrated by these aliphatic PBA conjugates functioned through glucose-mediate binding to serum proteins, such as serum albumin, as the mechanism for insulin activation. We compared the binding kinetics of Ins-PBA-F, Ins-LA-C14, and native insulin to human serum albumin (HSA) using biolayer interferometry (Fig. S6). The mechanism underlying the long-lasting activity of Ins-LA-C14 (i.e., Insulin detemir) has been reported to be binding to serum albumin (26). The calculated binding constant, fitting to a 1:1 binding model, for Ins-PBA-F to HSA was comparable to that for Ins-LA-C14 (2.81 vs. 2.18 nM, respectively) in the absence of glucose. In the presence of glucose, the binding constant was not significantly changed (3.65 nM for Ins-PBA-F and 2.56 nM for Ins-LA-C14). These data indicate that binding of PBA-modified insulin to HSA is insensitive to the presence of physiologically relevant glucose concentrations in this specific assay and though this mechanism may still hold, an alternate glucose-responsive mechanism could also be responsible for the differential activity observed in vivo.
Discussion
Previous work toward the preparation of self-regulated insulin therapy has explored, for example, the use of glycosylated insulins in combination with glucose-binding lectins such as Con A (27–31). This technology has generally comprised a device, capsule, or pouch containing lectin-bound glycosylated insulin to be released as circulating glucose levels increase. Here, we developed a strategy for the covalent modification of insulin intended to couple an aliphatic domain for long-lasting activity with PBA as a glucose-sensing element to prepare a soluble, circulating, glucose-sensing modified insulin molecule. Although PBAs have been fused to insulin previously as a method for preparing glucose-responsive self-assemblies of insulin (19, 20), our report here illustrates, to our knowledge, the first demonstration of glucose-responsive activity for a PBA-modified insulin in an animal model of diabetes. Through screening of different PBA-containing aliphatic conjugates, Ins-PBA-F was identified as the most promising modification for long-lasting, glucose-responsive behavior. To analyze glucose-responsive activity, a dosing study in both diabetic and healthy mice was performed. In diabetic mice, Ins-PBA-F exhibited significantly higher potency and responsiveness compared with native insulin or a control of clinically used long-lasting modified insulin (Ins-LA-C14) across a range of doses. Conversely, in healthy mice with lower basal blood glucose levels, the hypoglycemia index for Ins-PBA-F was significantly reduced. Enhanced potency and responsiveness in a hyperglycemic state coupled with reduced activity in a euglycemic/hypoglycemic state suggests that Ins-PBA-F has glucose-mediated potency. Through continuous glucose monitoring, it was also established that the kinetics of Ins-PBA-F in responding to a glucose challenge were comparable to those for insulin produced from a healthy pancreas. Glucose-mediated binding to serum albumin could not be confirmed as the mechanism of action for the glucose-dependent activity of Ins-PBA-F observed here. Though glucose-responsive binding to hydrophobic domains in proteins such as serum albumin may still be the underlying mechanism, another possible mechanism is that PBA-modified insulin binds reversibly to immobilized diols, such as those on glycoproteins, glycosylated proteins, proteoglycans, or glycosaminoglycans. We note that the diabetic state increases the extent of protein glycosylation (32), increasing the number of potential binding sites available by this mechanism.
Insulin therapy could be improved by greater autonomy, increased fidelity in glycemic control, and reduced hypoglycemia (33). Even with perfect use, traditional insulin therapy fails to match the kinetics of normal insulin signaling. Even small improvements in glycemic control over time have the potential to reduce the frequency of serious complications, including blindness, cardiovascular disease, stroke, nonhealing wounds, and cancer (8). Glucose-responsive insulin, with bioactivity that is regulated by glucose levels in the body, could improve glycemic control. The safety and toxicity for covalent modification of insulin with aliphatic PBA conjugates remains to be established. Insulin therapy necessitates repeated administrations over a lifetime, and risk for both allergic reaction and antibody formation must be minimized before clinical use. Of note, the clinical incidence of adverse reaction to insulin is less than 1% (34), and B29-modified variants such as detemir and degludec have generally demonstrated excellent safety profiles with limited evidence of antibody formation or allergic reaction (34–37).
In summary, to our knowledge, this is the first demonstration of glucose-responsive behavior in vivo with a modified insulin derivative. Covalent modification of insulin with conjugates containing an aliphatic domain and a PBA afforded long-lasting insulin with glucose-mediated activity. The lead candidate demonstrated enhanced responsiveness to glucose challenge in diabetic mice but a reduction in hypoglycemic index in healthy mice. It is possible that these modified insulins could interface with insulin pumps, infusion devices, or controlled release materials to further improve performance.
Methods
Detailed experimental methods can be found in SI Methods. Briefly, small molecule conjugates were synthesized starting from 12-aminododecanoic acid and were coupled to insulin using DCC/NHS chemistry under basic conditions. Ins-LA-C14 was prepared using commercially available myristic acid. Insulin derivatives were purified by HPLC to afford the species specifically modified at the B29 lysine. Site-specific modification of insulin was confirmed using tandem MS/MS electrospray ionization (ESI) mass spectrometry on DTT/trypsin digests with Mascot proteomic analysis. Circular dichroism was used to verify secondary structure of insulin derivatives, whereas a cell-based protein kinase B (AKT) phosphorylation assay was used to confirm insulin bioactivity. An STZ-induced animal model was used to evaluate insulin derivatives in vivo. Mice were fasted overnight and then injected with insulin s.c. at a range of doses. In cases where glucose challenge was administered, this was done through i.p. injection of a glucose solution. Studies in healthy mice to assess hypoglycemia were dosed identically but were not subjected to a glucose challenge. Continuous glucose monitoring was performed using clinically used s.c. monitoring devices. All animal studies were conducted through protocols approved by the MIT animal care and use committee, following all institutional, state, and federal guidelines for the use of research animals. Binding to human serum albumin was established using biolayer interferometry, with binding constants obtained through fitting the complete data to a 1:1 binding model.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Leona M. and Harry B. Helmsley Charitable trust (Award 2014PG-T1D002) along with a generous gift from the Tayebati Family Foundation. M.J.W. acknowledges support from the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases) through Ruth L. Kirschstein National Research Service Award F32DK101335. B.C.T. and D.H.-C.C. acknowledge support from Juvenile Diabetes Research Foundation Postdoctoral Fellowships 3-2011-310 and 3-2013-56, respectively.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424684112/-/DCSupplemental.
References
- 1.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G. EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: A multicentre prospective registration study. Lancet. 2009;373(9680):2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: Scientific review. JAMA. 2003;289(17):2254–2264. doi: 10.1001/jama.289.17.2254. [DOI] [PubMed] [Google Scholar]
- 5.Vinik A. The question is, my dear watson, why did the dog not bark?: The joslin 50-year medalist study. Diabetes Care. 2011;34(4):1060–1063. doi: 10.2337/dc11-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29(5):682–689. doi: 10.1111/j.1464-5491.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCoy RG, et al. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35(9):1897–1901. doi: 10.2337/dc11-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muggeo M, et al. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: The Verona Diabetes Study. Diabetes Care. 2000;23(1):45–50. doi: 10.2337/diacare.23.1.45. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch IB. Insulin analogues. N Engl J Med. 2005;352(2):174–183. doi: 10.1056/NEJMra040832. [DOI] [PubMed] [Google Scholar]
- 10.Owens DR. New horizons—Alternative routes for insulin therapy. Nat Rev Drug Discov. 2002;1(7):529–540. doi: 10.1038/nrd836. [DOI] [PubMed] [Google Scholar]
- 11.Holleman F, Hoekstra JB. Insulin lispro. N Engl J Med. 1997;337(3):176–183. doi: 10.1056/NEJM199707173370307. [DOI] [PubMed] [Google Scholar]
- 12.Rosenstock J, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24(4):631–636. doi: 10.2337/diacare.24.4.631. [DOI] [PubMed] [Google Scholar]
- 13.Kurtzhals P, et al. Albumin binding and time action of acylated insulins in various species. J Pharm Sci. 1996;85(3):304–308. doi: 10.1021/js950412j. [DOI] [PubMed] [Google Scholar]
- 14.Kurtzhals P, et al. Albumin binding of insulins acylated with fatty acids: Characterization of the ligand-protein interaction and correlation between binding affinity and timing of the insulin effect in vivo. Biochem J. 1995;312(Pt 3):725–731. doi: 10.1042/bj3120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James TD, Sandanayake KRAS, Shinkai S. Saccharide sensing with molecular receptors based on boronic acid. Angew Chem Int Ed Engl. 1996;35(17):1910–1922. [Google Scholar]
- 16.Kitano S, Koyama Y, Kataoka K, Okano T, Sakurai Y. A novel drug delivery system utilizing a glucose responsive polymer complex between poly(vinyl alcohol) and poly(N-vinyl-2-pyrrolidone) with a phenylboronic acid moiety. J Control Release. 1992;19(1-3):162–170. [Google Scholar]
- 17.Mu B, et al. A structure-function relationship for the optical modulation of phenyl boronic acid-grafted, polyethylene glycol-wrapped single-walled carbon nanotubes. J Am Chem Soc. 2012;134(42):17620–17627. doi: 10.1021/ja307085h. [DOI] [PubMed] [Google Scholar]
- 18.Yan J, Springsteen G, Deeter S, Wang BH. The relationship among pK(a),pH, and binding constants in the interactions between boronic acids and diols - it is not as simple as it appears. Tetrahedron. 2004;60(49):11205–11209. [Google Scholar]
- 19.Hoeg-Jensen T, Havelund S, Nielsen PK, Markussen J. Reversible insulin self-assembly under carbohydrate control. J Am Chem Soc. 2005;127(17):6158–6159. doi: 10.1021/ja051038k. [DOI] [PubMed] [Google Scholar]
- 20.Hoeg-Jensen T, et al. Insulins with built-in glucose sensors for glucose responsive insulin release. J Peptide Sci. 2005;11(6):339–346. doi: 10.1002/psc.624. [DOI] [PubMed] [Google Scholar]
- 21.Ettinger MJ, Timasheff SN. Optical activity of insulin. I. On the nature of the circular dichroism bands. Biochemistry. 1971;10(5):824–831. doi: 10.1021/bi00781a015. [DOI] [PubMed] [Google Scholar]
- 22.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: New model of diabetes mellitus. Science. 1976;193(4251):415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 23.Lev-Ran A, Anderson RW. The diagnosis of postprandial hypoglycemia. Diabetes. 1981;30(12):996–999. doi: 10.2337/diab.30.12.996. [DOI] [PubMed] [Google Scholar]
- 24.Springsteen G, Wang BH. A detailed examination of boronic acid-diol complexation. Tetrahedron. 2002;58(24):5291–5300. [Google Scholar]
- 25.Sun SZ, Empie MW. Fructose metabolism in humans - what isotopic tracer studies tell us. Nutr Metab (Lond) 2012;9(1):89. doi: 10.1186/1743-7075-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Havelund S, et al. The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm Res. 2004;21(8):1498–1504. doi: 10.1023/b:pham.0000036926.54824.37. [DOI] [PubMed] [Google Scholar]
- 27.Brownlee M, Cerami A. A glucose-controlled insulin-delivery system: Semisynthetic insulin bound to lectin. Science. 1979;206(4423):1190–1191. doi: 10.1126/science.505005. [DOI] [PubMed] [Google Scholar]
- 28.Liu F, Song SC, Mix D, Baudys M, Kim SW. Glucose-induced release of glycosylpoly(ethylene glycol) insulin bound to a soluble conjugate of concanavalin A. Bioconjug Chem. 1997;8(5):664–672. doi: 10.1021/bc970128e. [DOI] [PubMed] [Google Scholar]
- 29.Baudys M, et al. Glycosylated insulins. J Control Release. 1995;36(1-2):151–157. [Google Scholar]
- 30.Seminoff LA, Olsen GB, Kim SW. A self-regulating insulin delivery system. 1. Characterization of a synthetic glycosylated insulin derivative. Int J Pharm. 1989;54(3):241–249. [Google Scholar]
- 31.Seminoff LA, et al. A self-regulating insulin delivery system. 2. In vivo characteristics of a synthetic glycosylated insulin. Int J Pharm. 1989;54(3):251–257. [Google Scholar]
- 32.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 33.Shalitin S, Phillip M. Hypoglycemia in type 1 diabetes: A still unresolved problem in the era of insulin analogs and pump therapy. Diabetes Care. 2008;31(Suppl 2):S121–S124. doi: 10.2337/dc08-s228. [DOI] [PubMed] [Google Scholar]
- 34.Darmon P, Castera V, Koeppel MC, Petitjean C, Dutour A. Type III allergy to insulin detemir. Diabetes Care. 2005;28(12):2980. doi: 10.2337/diacare.28.12.2980. [DOI] [PubMed] [Google Scholar]
- 35.Heller S, et al. BEGIN Basal-Bolus Type 1 Trial Investigators Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): A phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1489–1497. doi: 10.1016/S0140-6736(12)60204-9. [DOI] [PubMed] [Google Scholar]
- 36.Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(7):CD006383. doi: 10.1002/14651858.CD006383.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenstock J, et al. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia. 2008;51(3):408–416. doi: 10.1007/s00125-007-0911-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.