Significance
Interleukin-1 family members are highly inflammatory but IL-37 member broadly suppresses inflammation and specific immunity. Initially, the mechanism of this suppression was shown to be via translocation to the nucleus following cleavage of the precursor by intracellular caspase-1. We now show that recombinant forms of IL-37 limit inflammation by extracellular binding to surface receptors but require the IL-1 family decoy receptor IL-1R8. Unexpectedly, picomolar concentrations of the IL-37 precursor optimally suppress IL-1β, IL-6, and TNFα production from human blood M1 macrophages, suggesting a unique function for a coreceptor function of IL-1R8. Assessment of IL-37 as well as IL-1R8 levels may provide previously unidentified insights into how the host limits inflammation.
Keywords: endotoxemia, immunity, inflammasome
Abstract
Similar to IL-1α and IL-33, IL-1 family member IL-37b translocates to the nucleus and is associated with suppression of innate and adaptive immunity. Here we demonstrate an extracellular function of the IL-37 precursor and a processed form. Recombinant IL-37 precursor reduced LPS-induced IL-6 by 50% (P < 0.001) in highly inflammatory human blood-derived M1 differentiated macrophages derived from selective subjects but not M2 macrophages. In contrast, a neutralizing monoclonal anti–IL-37 increased LPS-induced IL-6, TNFα and IL-1β (P < 0.01). The suppression by IL-37 was consistently observed at low picomolar but not nanomolar concentrations. Whereas LPS induced a 12-fold increase in TNFα mRNA, IL-37 pretreatment decreased the expression to only 3-fold over background (P < 0.01). Mechanistically, LPS-induced p38 and pERK were reduced by IL-37. Recombinant IL-37 bound to the immobilized ligand binding α-chain of the IL-18 receptor as well as to the decoy receptor IL-1R8. In M1 macrophages, LPS increased the surface expression of IL-1R8. Compared with human blood monocytes, resting M1 cells express more surface IL-1R8 as well as total IL-1R8; there was a 16-fold increase in IL-1R8 mRNA levels when pretreated with IL-37. IL-37 reduced LPS-induced TNFα and IL-6 by 50–55% in mouse bone marrow-derived dendritic cells, but not in dendritic cells derived from IL-1R8–deficient mice. In mice subjected to systemic LPS-induced inflammation, pretreatment with IL-37 reduced circulating and organ cytokine levels. Thus, in addition to a nuclear function, IL-37 acts as an extracellular cytokine by binding to the IL-18 receptor but using the IL-1R8 for its anti-inflammatory properties.
IL-37, previously known as IL-1 family member 7, broadly reduces innate inflammation as well as acquired immune responses (1). In human peripheral blood mononuclear cells (PBMCs), a knockdown of endogenous IL-37 results in increased production of LPS- as well as IL-1β–induced cytokines (2). Mice transgenic for full-length human IL-37 (IL-37tg) are protected against LPS-induced systemic inflammation (2), chemical colitis (3), metabolic syndrome (4), and acute myocardial infarction (5). IL-37tg mice also have suppressed immune responses following challenge by specific antigen (6). We believe that full-length IL-37 expressed in the transgenic mice is processed extracellularly.
In mouse macrophages stably transfected with human IL-37, ∼20% of IL-37 translocates to the nucleus (7), which is associated with decreased cytokine production (2, 7). However, in the presence of a caspase-1 inhibitor, there is no translocation to the nucleus and no reduction in LPS-induced cytokines (7). Mutation of aspartic acid at the caspase-1 cleavage position 20 to alanine also results in failure to translocate to the nucleus and loss of the suppression of cytokine production (8). Thus, as with IL-1α and IL-33, IL-37 is the third member of the IL-1 family that translocates to the nucleus and affects cellular responses. Nevertheless, it remains unclear whether the reduction in cytokines in vitro or in vivo is due solely to nuclear translocation of IL-37.
Support for an extracellular function for IL-37 comes from early studies reported over 10 y ago that demonstrated binding of IL-37 to the α-chain of IL-18 receptor (IL-18Rα). We therefore hypothesized that extracellular IL-37 can function through the IL-18Rα surface receptor to mediate its anti-inflammatory effects but that a negative or decoy receptor would be required. The candidate decoy receptor would likely be IL-1R8 [formerly, single IgG IL-1–related receptor (SIGIRR)] because, similar to IL-18BP, IL-1R8 has only a single Ig domain and is known for providing a brake on inflammation (9). In the present study, we have characterized the function of full-length recombinant IL-37b in inhibiting inflammation in vitro and in vivo and the role of IL-1R8.
Results
Recombinant IL-37 Suppresses LPS-Induced IL-1β, IL-6, and TNFα in M1 Differentiated Human Blood Macrophages.
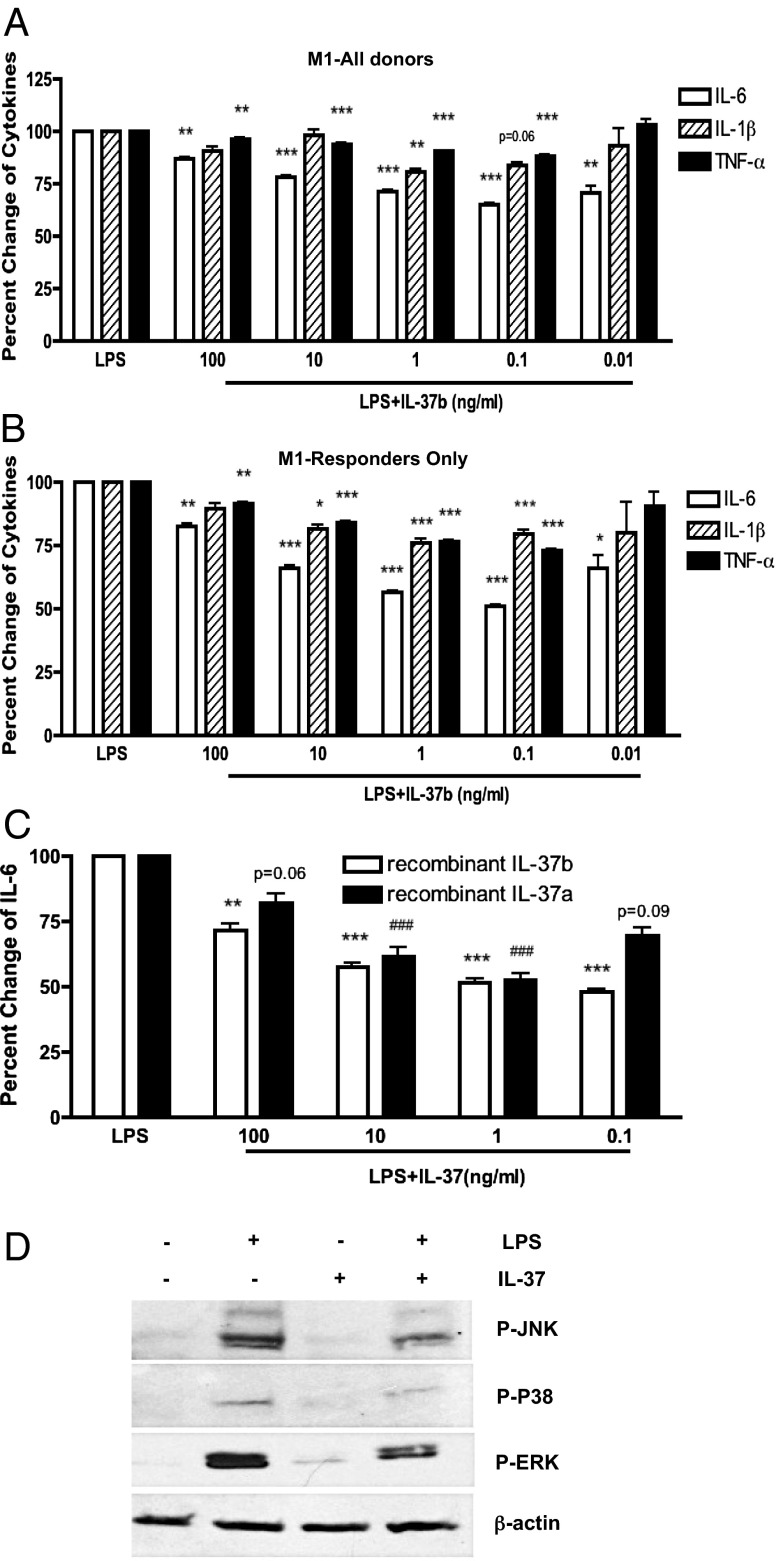
As shown in Fig. S1A, the precursor form of IL-37b was expressed in Escherichia coli and purified to homogeneity. The initial studies examined whether pretreatment with recombinant IL-37b reduces LPS-induced cytokines in PBMCs, an observation based on the reduction in plasma cytokines observed in mice transgenic for IL-37 following LPS challenge (2). Only a modest but consistent reduction (10–15%) was observed (Fig. S1B). We next differentiated adherent cells in the PBMC preparations into M1 and M2 macrophages and dendritic cells (DCs) and, after 5 d, pretreated the cells for 2 h with IL-37 and then challenged the cultures with LPS. As shown in Fig. 1A, LPS-induced IL-6 production decreased by 35% in M1 macrophages but not in M2 differentiated cells (Fig. S1C) nor in dendritic cells (Fig. S1D). Donor variation was observed, but in a subgroup of 35 responsive donors IL-6 decreased to 51% (Fig. 1B). As shown, maximal reduction in IL-1β, IL-6, and TNFα took place at picomolar concentrations of the IL-37 precursor. We also tested the recombinant IL-37a isoform (Lys27-Asp192) in the same cultures; we observed that the recombinant IL-37a isoform functions in a pattern similar to that of the IL-37b precursor (Fig. 1C). The recombinant IL-37b precursor and the recombinant IL-37a isoform share exons 4–6.
Fig. 1.
Recombinant IL-37 inhibits LPS-induced proinflammatory cytokine production and MAPK activation in granulocyte-macrophage colony-stimulating factor (GM-CSF)–differentiated human M1 macrophages. (A) Mean ± SEM percentage change of LPS-induced cytokine production from GM-CSF–differentiated human M1 macrophages incubated with decreasing concentrations of recombinant IL-37b precursor (n = 51). (B) Mean ± SEM percentage change of LPS-induced cytokine production in M1 macrophages from consistent responders (derived from data shown in A) (n = 35, except for 0.01 ng/mL where n = 6). ***P < 0.001, **P < 0.01, and *P < 0.05, compared with LPS alone. (C) Mean ± SEM percentage change IL-6 from M1 macrophages incubated with recombinant IL-37a, compared with IL-37b (n = 6). ***P < 0.001 and **P < 0.01, compared with LPS alone. (D) IL-37b inhibits LPS-induced MAPK activation in human M1 macrophages. The cells were stimulated with or without 10 ng/mL LPS for 30 min. Cells of lane 3 and lane 4 of the blots are pretreated with 0.1 ng/mL IL-37 for 2 h. Representative of three independent experiments.
Recombinant IL-37 Inhibits p38 MAPK Activation in Response to LPS in M1 Macrophages.
To examine changes in intracellular kinases that may be modulated by recombinant IL-37, we treated M1 differentiated macrophages with recombinant IL-37b precursor followed by LPS and assessed the lysates in a phospho-kinase array. As shown in Fig. S2, exposure to LPS increased the p38 MAPK phosphorylation to ninefold over nonstimulated cells. However, in IL-37–pretreated cells, the increase was markedly reduced to twofold. We then further confirmed the findings using individual immunoblotting for specific p-P38, p-ERK, and p-JNK kinases (Fig. 1D). Consistently, LPS-induced p38, ERK, and JNK phosphorylations were reduced by recombinant IL-37b in the M1 cells.
Effects of Recombinant IL-37 in Mice Subjected to Endotoxemia.
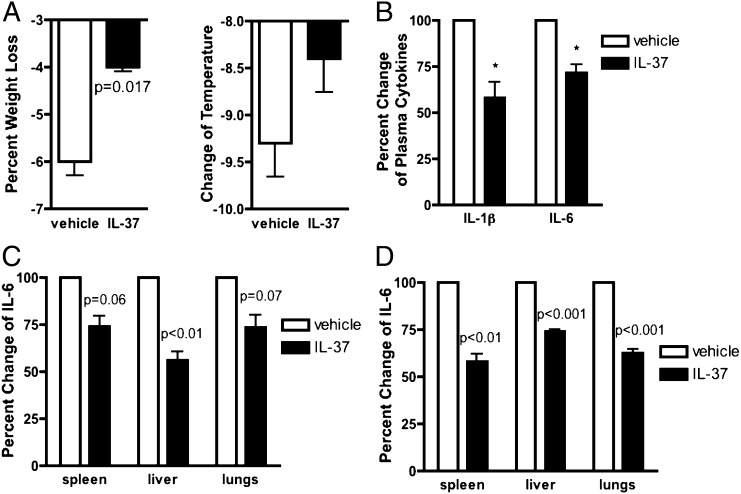
We next assessed the effect of recombinant IL-37b injected into mice 1 h before LPS challenge. A 1-h IL-37 pretreatment was effective in other models of inflammation (10, 11). As shown in Fig. 2A, IL-37b pretreatment protected the mice from endotoxin-induced weight loss and hypothermia. Plasma IL-1β and IL-6 were also reduced 24 h after LPS (Fig. 2B). We next examined IL-6 levels in whole-organ homogenates at 24 h. In the spleen, IL-6 was reduced by 26%, in the liver by 44%, and in the lungs by 27% (Fig. 2C). Using a lower dose of LPS, after 4 h IL-6 content in the spleen decreased by 42%, in the liver by 26%, and in the lung by 38% (Fig. 2D). Plasma TNFα levels were also measured after 4 h; there was a 32% decrease in circulating TNFα (P < 0.05) and a 26% decrease in plasma IL-6 (Fig. S3A). TNFα content in the spleen was reduced by 32% (P < 0.01) and in the lung by 23% (P = 0.01) but not in the liver.
Fig. 2.
Effects of recombinant IL-37 on endotoxin-induced weight loss, hypothermia, and proinflammatory cytokine production. (A) Mean ± SEM percentage change in weight loss (Left) and temperature (Right) in mice pretreated with either vehicle or 1 µg IL-37b precursor 24 h before the intraperitoneal administration of 10 mg/kg of LPS to the mice (n = 8 in each group). (B) Mean ± SEM percentage change of plasma IL-1β and IL-6 levels in mice pretreated 24 h before the intraperitoneal administration of 10 mg/kg of LPS to the mice (n = 3 groups). (C) Mean ± SEM percentage change of IL-6 level in organ lysates of mice pretreated with 1 µg of IL-37b 24 h before the intraperitoneal administration of 10 mg/kg of LPS to the mice (n = 3 groups). (D) Mean ± SEM percentage change of IL-6 level in organ lysates of mice pretreated with either vehicle or 1 µg IL-37b 2 h before the intraperitoneal administration of 2.5 mg/kg of LPS to the mice (n = 3 groups, 9 mice in total).
In addition to these studies using the recombinant precursor form of IL-37, we examined IL-37b with an N terminus at amino acid 46 as determined by Edman degradation of cells transfected with full-length IL-37b (12). Mice received three i.p. injections 24, 16, and 1 h before LPS. After 24 h, plasma IL-6 as well as spontaneous ex vivo levels of IL-6 from cultured whole blood was determined. As shown in Fig. S4A, mean plasma levels decreased from 4,000 pg/mL in vehicle-treated mice to 1,000 pg/mL. Whole-blood IL-6 production fell from 5,500 pg/million white blood cells to 1,000 pg/million white blood cells (Fig. S4B). IL-6 production from the number of cells infiltrating the peritoneal cavity decreased by 60% (P = 0.07) (Fig. S4A).
Recombinant IL-37 Binds to Immobilized IL-18R and the Extracellular Domain of IL-1R8.
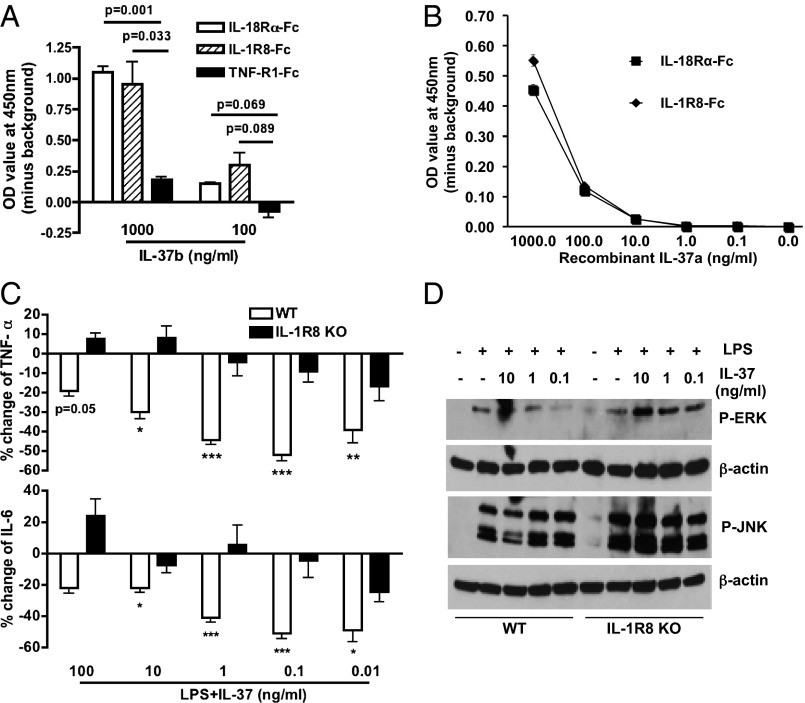
Although previous studies reported that IL-37 binds to IL-18Rα, no study provided evidence that IL-37 acts as a receptor antagonist for IL-18 (12–14). We confirmed that the recombinant IL-37 precursor binds to immobilized IL-18Rα Fc (Fig. 3A). However, we hypothesized that, upon binding to the IL-18Rα chain, IL-37 does not recruit the IL-18Rβ chain because IL-37 reduces inflammation. Instead, IL-37 would have to recruit a coreceptor that possessed anti-inflammatory properties such as IL-1R8 (15, 16). As shown in Fig. 3 A and B, recombinant IL-37 binds also to IL-1R8.
Fig. 3.
Recombinant IL-37 binds to immobilized IL-18Rα-Fc and IL-1R8-Fc and requires IL-1R8. (A) Mean ± SEM. OD of IL-37b precursor binding to the extracellular domains of IL-18Rα-Fc, IL-1R8-Fc, and TNF-R1-Fc (n = 4). (B) Binding of IL-37a to immobilized receptors as in A. (C) Mean ± SEM percentage change in LPS-induced TNFα (Upper) and IL-6 (Lower) with decreasing concentrations of IL-37 in BMDCs from WT or IL-1R8–deficient mice (n = 10, except for 0.01 ng/mL where n = 4). ***P < 0.001, **P < 0.01, and *P < 0.05, compared with LPS alone. (D) LPS-induced MAPK activation in BMDCs from WT or IL-1R8–deficient mice. Representative of two independent experiments.
IL-1R8 deficiency reverses the anti-inflammatory effects of recombinant IL-37. To define a functional role of IL-1R8 for IL-37, we examined the effect of recombinant IL-37 in bone marrow-derived dendritic cells (BMDCs) from the IL-1R8–deficient mice. Mouse DCs but not macrophages express IL-1R8, and their responsiveness to LPS is increased when IL-1R8 is absent (16, 17). Similar to the responses in human M1 cells, IL-37 pretreatment reduced LPS-induced TNFα in BMDCs from wild-type (WT) mice by 52% (Fig. 3C, Upper, P < 0.001); IL-6 was similarly reduced (Fig. 3C, Lower) by 51% (P < 0.001). However, these reductions did not occur in BMDCs derived from IL-1R8–deficient mice (Fig. 3C). We also observed a significant reduction of LPS-induced IL-1β mRNA synthesis by recombinant IL-37 precursor at 1 ng/mL in WT BMDCs (Fig. S5).
LPS triggering of TLR4 leads to downstream MAPK activation and JNK phosphorylation but is enhanced and prolonged in IL-1R8–deficient cells (15). Next, we compared LPS-induced MAPK activation in BMDCs from both WT and IL-1R8–deficient mice. Consistent with the reduction in cytokine production, the ability of recombinant IL-37 to reduce LPS-induced MAPK activation was also reversed in the BMDCs from IL-1R8–deficient mice (Fig. 3D). These data reveal that IL-1R8 has a functional role in the anti-inflammatory properties of IL-37.
IL-1R8 Is Abundantly Expressed in M1 Macrophages and Increases Its Cell-Surface Localization in Response to LPS.
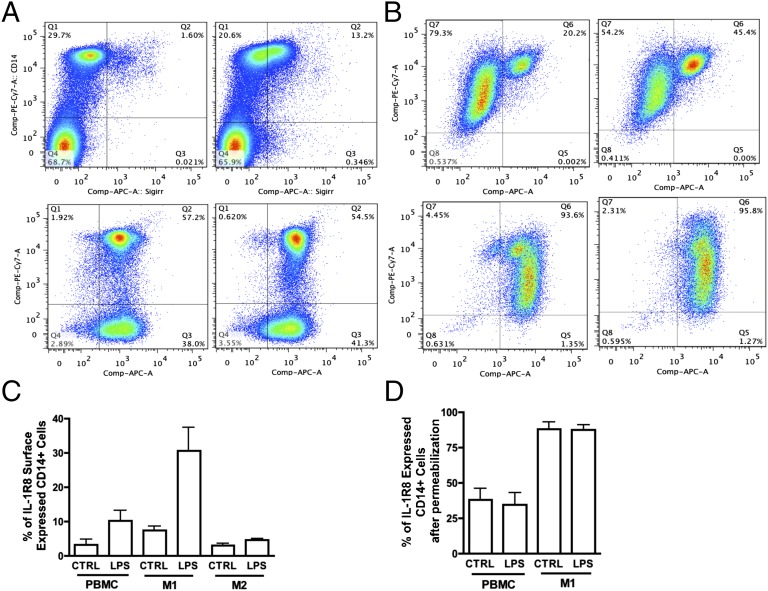
We next measured surface expression of IL-1R8 in both resting and LPS-stimulated cells. In resting PBMCs, few CD14+ monocytes express IL-1R8 (Fig. 4A, Upper Left) and nor do CD68+ macrophages or CD3+ lymphocytes (Fig. S6). However, following LPS stimulation, there is significantly greater surface IL-1R8 (Fig. 4A, Upper Right), whereas the intracellular levels of IL-1R8 remain unchanged (Fig. 4A, Lower Left and Lower Right). In M1 cells, we observed greater numbers of IL-1R8–positive CD14+ macrophages than in freshly obtained PBMCs (Fig. 4B, Upper Left). LPS stimulation increased IL-1R8 surface expression (Fig. 4B, Upper Right), but total intracellular IL-1R8 remained unchanged (Fig. 4B, Lower panels). The mean percentage change in surface and intracellular IL-1R8 in PBMC, M1, and M2 macrophages is summarized in Fig. 4 C and D. Overall, CD14+ M1 cells have greater IL-1R8 surface expression compared with resting PBMCs and show a robust increase of surface IL-1R8 level with LPS (mean change 8–30%, Fig. 4C). IL-1R8 surface expression on CD14+ M2 cells is nearly unchanged (Fig. 4C).
Fig. 4.
Flow cytometric analysis of IL-1R8/SIGIRR expression in CD14+ PBMCs, M1, and M2 differentiated macrophages. (A) Representative flow panels of IL-1R8 expression in CD14+ PBMCs with or without LPS stimulation. (Upper) Nonpermeabilized cells. (Lower) Permeabilized cells. (B) Representative flow panels of IL-1R8 expression in CD14+ M1 macrophages with or without LPS stimulation. (Upper) Nonpermeabilized cells. (Lower) Permeabilized cells. (A and B, Left panels) Resting or without LPS stimulation; (Right panels) with LPS stimulation. (C) Mean ± SEM percentage of IL-1R8 cell-surface–expressed cells in CD14+ PBMCs, M1, and M2 macrophages with or without LPS stimulation (n = 7 for PBMCs and M1; n = 3 for M2). (D) Mean ± SEM percentage of IL-1R8–expressed cells in CD14+ PBMCs and M1 cells with or without LPS stimulation (n = 3).
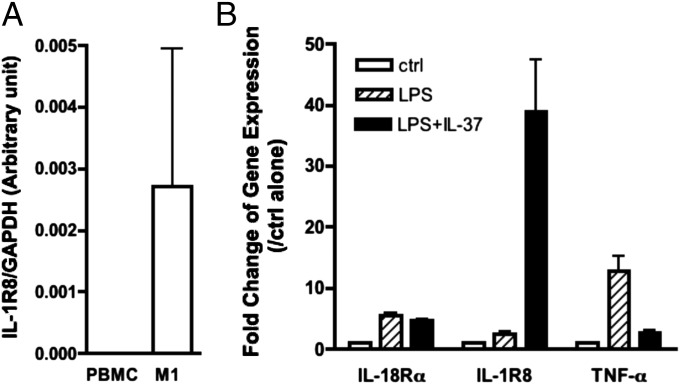
IL-37–Related Gene Expressions in PBMCs and M1 Macrophages.
Consistent with flow cytometry data, we detected high levels of IL-1R8 mRNA in M1 cells compared with PBMCs (Fig. 5A). Moreover, we found that both IL-18Rα and IL-1R8 mRNA levels increase with LPS stimulation (Fig. 5B), although the increase in IL-18Rα is modest. TNFα gene expression is also increased in M1 cells following LPS (Fig. 5B). At picomolar levels of IL-37, we observed a marked increase in IL-37–pretreated LPS-induced steady-state mRNA levels of IL-1R8 (Fig. 5B, Middle) but also a marked reduction in gene expression for TNFα. mRNA levels of IL-18Rα were similar for LPS as well as LPS plus IL-37 (Fig. 5B).
Fig. 5.
mRNA expression level of IL-37–related genes in PBMCs and M1 macrophages. (A) Mean ± SEM steady-state mRNA levels of IL-1R8 in fresh PBMCs as well as differentiated M1 cells (n = 5). (B) Mean ± SEM steady-state mRNA levels of IL-18Rα, IL-1R8, and TNFα in M1 cells with or without LPS or IL-37 + LPS (n = 11 for IL-1R8 and IL-18Rα; n = 5 for TNFα). P = 0.06 for IL -1R8 gene and P = 0.0018 for TNFα gene when comparing the combination of LPS plus IL-37 with LPS alone.
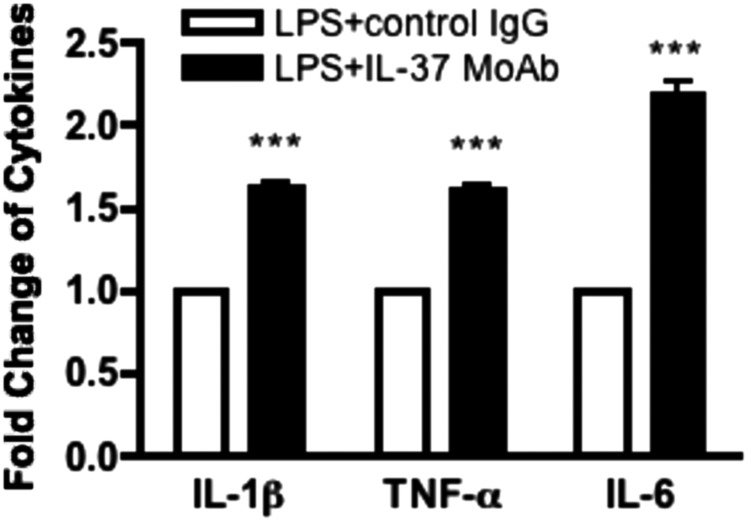
Neutralization of Endogenous IL-37 in LPS-Stimulated PBMCs Results in an Increase of Inflammatory Cytokine Production.
Studies with siRNA knockdown indicated a suppressive role for endogenous IL-37 in PBMCs (2). However, it remains unclear to what extent nuclear translocation of IL-37 accounted for the increase in cytokines with the knockdown of total endogenous IL-37 or whether the IL-37 precursor in the extracellular compartment affected the observation. We thus added a neutralizing monoclonal anti–IL-37 to freshly obtained PBMCs to prevent the extracellular function of endogenous IL-37. As shown in Fig. 6, anti–IL-37 increased IL-1β. This increase in LPS-induced cytokine production was compared with the control IgG treatment (Fig. 6). IL-6 increased more than 2.0-fold. TNFα increased 1.6-fold and IL-1β increased 1.6-fold.
Fig. 6.
Anti–IL-37 monoclonal antibody reverses the anti-inflammatory function of endogenous IL-37 in PBMCs. Mean ± SEM percentage change in LPS-induced cytokine production with or without control IgG or anti–IL-37 monoclonal antibody in PBMC culture. n = 13 for IL-1β, n = 15 for TNFα, and n = 16 for IL-6. ***P < 0.001, compared with LPS + control IgG.
Discussion
Although IL-37b translocates to the nucleus following LPS activation and is associated with decreased cytokine production (7), we sought to demonstrate an extracellular role for IL-37. We used two methods: first, we assessed the activities of recombinant IL-37 added to cells in vitro or injected into mice challenged with LPS. Second, we used a neutralizing antibody to IL-37 to prevent the effects of endogenous extracellular IL-37. Both methods revealed that IL-37 functions extracellularly to suppress cytokine production induced by LPS. These data are a confirmation of the binding of IL-37 to an extracellular receptor, in this case IL-18Rα (12, 13), but in contrast to an increase in inflammation, as would be expected from an IL-18 signal, the extracellular effects of IL-37 results in reduced inflammation. To do this, IL-37 exploits the decoy IL-1R8 because cells deficient in IL-1R8 do not exhibit reductions in cytokines when exposed to recombinant IL-37 compared with WT cells. We also show that recombinant IL-37 binds to immobilized IL-18Rα as well as to IL-1R8. Others have reported a role for the extracellular domain of IL-1R8 (18). Thus, IL-37 is ligand for the orphan receptor IL-1R8. IL-1R8–deficient mice consistently have greater disease severity in various models of inflammation (16, 19, 20).
In the present report, we studied the biological properties of the recombinant IL-37b precursor. However, it remains unclear to what extent extracellular processing of the precursor takes place in vitro or in vivo. We compared recombinant IL-37a isoform with an N terminus at lysine 27 (Lys27-Asp192) to the IL-37b precursor on M1 macrophages. The precursor was more active in suppressing LPS-induced LPS. The N terminus at Lys27 of IL-37a is nine amino acids forward from the consensus sequence, specifically IHD. In a model of hepatic ischemia reperfusion injury, the IL-37a isoform is protective (10). A recombinant form of IL-37b with the N terminus at the predicted caspase-1 site bound to the immobilized recombinant IL-18Rα chain (13). In combination with the IL-18BP, this form of IL-37 suppressed IL-18–induced IFNγ (14).
We also assessed the in vivo effect of recombinant IL-37 with an N terminus at amino acid 46. Amino acid 46 is in exon 4, and IL-37a, IL-37b, and IL-37d share exons 4–6. The amino acid valine 46 was found during Edman degradation of supernatants from cell lines transfected with full-length IL-37b. Both the full-length and the processed form (N46) were found in the supernatants (12). The recombinant N46-218 injected into WT mice suppressed circulating IL-6 levels fivefold. It is possible that the IL-37 precursor is processed in vivo to a form similar to N46-218. Nevertheless, we conclude that processing increases specific activity of IL-37.
The variable responses to IL-37 in PBMCs appear to be related to the presence of surface expression of IL-1R8. The flow cytometric data reveal that M1 cells express more surface and total IL-1R8 compared with PBMCs and M2 macrophages (Fig. 4 C and D) and thus may account for the low responsiveness of these latter two cells. As only picomolar concentrations of recombinant IL-37 initiate an anti-inflammatory response (Fig. 1), the limiting factor in the response to the ligand is likely the level of surface IL-1R8 expression. IL-1R8 expression levels are variable in the different species and cell types (16).
An unexpected finding is that both the IL-37b precursor and the IL-37a processed form reduce LPS-induced IL-1β, IL-6, and TNFα in the picomolar range with the optimal reduction consistently observed at 1,000 and 100 pg/mL. Low-picomolar concentrations of IL-1β and IL-1α induce IL-6 from A549 cells, which are rich in IL-1R3 but low in IL-1R2 (21), and thus we can conclude that high surface levels of IL-1R8 on M1 macrophages account for the high level of sensitivity. Similarly, nanomolar concentrations of IL-37 in PBMCs (100 ng/mL) can increase the production of LPS-induced cytokines in some donors, which is also observed for IL-10 (22). We propose that at picomolar concentrations, IL-37 binds IL-18Rα and recruits IL-1R8, whereas at micromolar concentrations the complex of IL-37/IL-18Rα may recruit IL-18Rβ and the corresponding IL-18 signal.
Using siRNA knockdown of endogenous IL-37 in PBMCs, there was an increase in LPS- and IL-1β–induced IL-6, TNFα, and IL-1β production, revealing functional endogenous IL-37 in these cells (2). Similarly, siRNA to IL-37 in human renal tubular epithelial cells resulted in increased IL-6, IL-1β, and TNFα when stimulated by IL-18, LPS, or IFNγ (23). Despite this near-global increase in cytokines, it remained unclear whether the increase in cytokines was due to nuclear translocation of endogenous IL-37. In the present study, we treated PBMCs with an anti–IL-37 monoclonal antibody to prevent extracellular effects of endogenous IL-37. The increase in LPS-induced cytokine production (Fig. 6) was observed at concentrations as low as 1 μg/mL and 100 ng/mL of the antibody. This is consistent with the concentration range of recombinant IL-37 in vitro (Fig. 1). As only picomolar concentrations are needed to limit inflammation, a correspondingly low concentration of anti–IL-37 would be required to block extracellular endogenous IL-37.
Members of the IL-1 family bind to their respective ligand-binding receptor chain but recruit different coreceptor accessory chains. For example, the ligand-binding chain for IL-1α and IL-1β is IL-1R1 and the coreceptor is IL-1R3; IL-1R3 also serves as the coreceptor for IL-1R2, IL-1R4, and IL-1R6 (24). IL-18 binds to IL-18Rα (IL-1R5), and its coreceptor IL-1R7 results in a proinflammatory signal. Although IL-37 binds to IL-18Rα (12, 13) and IL-18BP (14), no coreceptor has been identified for IL-37. IL-37 does not act as a receptor antagonist for IL-18Rα (14). Moreover, in mice deficient in IL-18Rα or using a blocking antibody to IL-18Rα, a hyper-responsive state has been observed (25, 26). In the present studies, we provide data supporting that the recombinant IL-37 precursor binds to immobilized IL-1R8 and suggesting that IL-1R8 acts as the coreceptor for IL-37. IL-37 fails to suppress LPS-induced cytokines as well as MAPK in dendritic cells from IL-1R8–deficient mice (Fig. 3). These data are consistent with mice deficient in IL-1R8 showing greater inflammation than WT mice (15, 16, 27). In a model of inflammatory aspergillosis, recombinant IL-37 precursor is protective but not in mice deficient in IL-1R8 (11). Together, these data support the concept that the anti-inflammatory and immunosuppressive properties of extracellular IL-37 require the decoy effect of IL-1R8. This finding is also consistent with the loss of the protection of IL-37 transgenic mice deficient in IL-1R8 (28).
Materials and Methods
Generation of Recombinant IL-37b Precursor.
Full-length human IL-37b isoform was inserted in pCACTUS under a chicken β-actin promoter and N-terminal 6-histidines (11). After expression in E. coli, the recombinant molecule was purified on Talon followed by FPLC size exclusion. The fractions isolated from FPLC were pooled and applied to a C6 HPLC column, and the IL-37 peak was eluted in acetonitrile, isolated, and lyophilized. The lyophilized IL-37 was reconstituted in PBS. The Limulus amebocyte lysate assayed indicated that, at 10 µg/mL of recombinant IL-37, there was less than 0.1 ng/mL of LPS-like activity endotoxin unit equivalents. Recombinant IL-37, when incubated in PBMCs at concentrations from 100 pg to 100 ng/mL, did not induce IL-1β. Recombinant IL-37 N46-218 was supplied by Vassili Kalabokis, Bio-Techne, Minneapolis.
Cell Culture.
Venous blood from healthy consenting donors was drawn into lithium heparin-containing tubes and PBMCs were isolated as previously described (29). Adherent cells were differentiated into M1 or M2 macrophages or dendritic cells (see SI Materials and Methods for details). Cytokines were measured by specific ELISA (BioTechne) and ECL assays (Bioveris).
LPS-Induced Systemic Inflammation.
Animal protocols were approved by the University of Colorado Animal Care and Use Committee. C57BL/6 mice were obtained from Charles River. Details of mouse studies are in SI Materials and Methods. IL-1R8–deficient mice were as previously described (16).
Bone Marrow-Derived Dendritic Cells.
Mouse bone marrow-derived dendritic cells were collected from both tibias from either WT or IL-1R8–deficient mice and differentiated as described in SI Materials and Methods.
Flow Cytometry.
As described in SI Materials and Methods, PBMCs differentiated into M1 or M2 cells were washed twice with flow cytometry staining buffer (BioTechne) and incubated with an Fc-receptor blocking antibody (eBioscience) for 15 min to reduce nonspecific staining.
Steady-State mRNA Collection and Real-Time PCR.
See SI Materials and Methods for details for determining steady-state mRNA levels and primers.
Supplementary Material
Acknowledgments
We thank Vassili Kalabokis and Bio-Techne for providing IL-37 N46-218; Claudia A. Nold-Petry, Marcel F. Nold, Philip Bufler, and Frank L. van de Veerdonk for discussions and helpful advice; and Isak Tengesdal, Dr. Giulio Cavalli, and Benjamin Swartzwelter for assistance. This study was supported by National Institutes of Health Grants AI-15614 and AR-45584 (to C.A.D.); National Research Foundation of Korea Grant Ministry of Education, Science, and Technology: 2012R1A2A1A01001791 (to S.K.); the Veterans Affairs Merit Review Award and Interleukin Foundation (to M.F.). S.L. is supported by Postdoctoral Fellowship 12POST12030134 from the American Heart Association. C.P.N. is supported by NIH T32 AI007405.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424626112/-/DCSupplemental.
References
- 1.Dinarello CA, Bufler P. Interleukin-37. Semin Immunol. 2013;25(6):466–468. doi: 10.1016/j.smim.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Nold MF, et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11(11):1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNamee EN, et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci USA. 2011;108(40):16711–16716. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballak DB, et al. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat Commun. 2014;5:4711. doi: 10.1038/ncomms5711. [DOI] [PubMed] [Google Scholar]
- 5.Yousif NG, et al. Expression of IL-37 in mouse protects the myocardium against ischemic injury via modulation of NF-κB activation. Circulation. 2011;124(Suppl 21):A8603. [Google Scholar]
- 6.Luo Y, et al. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc Natl Acad Sci USA. 2014;111(42):15178–15183. doi: 10.1073/pnas.1416714111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S, et al. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunol. 2008;180(8):5477–5482. doi: 10.4049/jimmunol.180.8.5477. [DOI] [PubMed] [Google Scholar]
- 8.Bulau AM, et al. Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc Natl Acad Sci USA. 2014;111(7):2650–2655. doi: 10.1073/pnas.1324140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riva F, et al. TIR8/SIGIRR is an interleukin-1 receptor/Toll like receptor family member with regulatory functions in inflammation and immunity. Front Immunol. 2012;3:322. doi: 10.3389/fimmu.2012.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai N, et al. Interleukin-37 reduces liver inflammatory injury via effects on hepatocytes and non-parenchymal cells. J Gastroenterol Hepatol. 2012;27(10):1609–1616. doi: 10.1111/j.1440-1746.2012.07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moretti S, et al. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog. 2014;10(11):e1004462. doi: 10.1371/journal.ppat.1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan G, et al. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine. 2001;13(1):1–7. doi: 10.1006/cyto.2000.0799. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine. 2002;18(2):61–71. doi: 10.1006/cyto.2002.0873. [DOI] [PubMed] [Google Scholar]
- 14.Bufler P, et al. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc Natl Acad Sci USA. 2002;99(21):13723–13728. doi: 10.1073/pnas.212519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wald D, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4(9):920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 16.Garlanda C, et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci USA. 2004;101(10):3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polentarutti N, et al. Unique pattern of expression and inhibition of IL-1 signaling by the IL-1 receptor family member TIR8/SIGIRR. Eur Cytokine Netw. 2003;14(4):211–218. [PubMed] [Google Scholar]
- 18.Bulek K, et al. The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. J Immunol. 2009;182(5):2601–2609. doi: 10.4049/jimmunol.0802729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garlanda C, Riva F, Bonavita E, Gentile S, Mantovani A. Decoys and regulatory “receptors” of the IL-1/Toll-like receptor superfamily. Front Immunol. 2013;4:180. doi: 10.3389/fimmu.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garlanda C, et al. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res. 2007;67(13):6017–6021. doi: 10.1158/0008-5472.CAN-07-0560. [DOI] [PubMed] [Google Scholar]
- 21.Kim B, et al. The interleukin-1alpha precursor is biologically active and is likely a key alarmin in the IL-1 family of cytokines. Front Immunol. 2013;4:391. doi: 10.3389/fimmu.2013.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmit A, et al. Dose-effect of interleukin-10 and its immunoregulatory role in Crohn’s disease. Eur Cytokine Netw. 2002;13(3):298–305. [PubMed] [Google Scholar]
- 23.Yang Y, et al. IL-37 inhibits IL-18-induced tubular epithelial cell expression of pro-inflammatory cytokines and renal ischemia-reperfusion injury. Kidney Int. 2014 doi: 10.1038/ki.2014.295. [DOI] [PubMed] [Google Scholar]
- 24.Boraschi D, Tagliabue A. The interleukin-1 receptor family. Semin Immunol. 2013;25(6):394–407. doi: 10.1016/j.smim.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Nold-Petry CA, et al. Increased cytokine production in interleukin-18 receptor alpha-deficient cells is associated with dysregulation of suppressors of cytokine signaling. J Biol Chem. 2009;284(38):25900–25911. doi: 10.1074/jbc.M109.004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis EC, Dinarello CA. Responses of IL-18- and IL-18 receptor-deficient pancreatic islets with convergence of positive and negative signals for the IL-18 receptor. Proc Natl Acad Sci USA. 2006;103(45):16852–16857. doi: 10.1073/pnas.0607917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garlanda C, Anders HJ, Mantovani A. TIR8/SIGIRR: An IL-1R/TLR family member with regulatory functions in inflammation and T cell polarization. Trends Immunol. 2009;30(9):439–446. doi: 10.1016/j.it.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Nold-Petry C, et al. IL-37 requires IL-18Rα and IL-1R8 to carry out its multi-faceted anti-inflammatory program on innate signal transduction. Nat Immunol. 2015 doi: 10.1038/ni.3103. in press. [DOI] [PubMed] [Google Scholar]
- 29.Leoni F, et al. The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Mol Med. 2005;11(1-12):1–15. doi: 10.2119/2006-00005.Dinarello. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.