Abstract
Transient Receptor Potential, Melastatin-related, member 4 (TRPM4) channels are Ca2+-activated Ca2+-impermeable cation channels. These channels are expressed in various types of mammalian tissues including the brain and are implicated in many diverse physiological and pathophysiological conditions. In the past several years, the trafficking processes and regulatory mechanism of these channels and their interacting proteins have been uncovered. Here in this minireview, we summarize the current understanding of the trafficking mechanism of TRPM4 channels on the plasma membrane as well as heteromeric complex formation via protein interactions. We also describe physiological implications of protein-TRPM4 interactions and suggest TRPM4 channels as therapeutic targets in many related diseases. [BMB Reports 2015; 48(1): 1-5]
Keywords: Glycosylation, Heteromerization, Phosphorylation, SUMOylation, Trafficking, TRPM4, 14-3-3γ
TRPM4 (Transient Receptor Potential, subfamily M (Melastatinrelated), member 4) channels are Ca2+-activated nonselective cation channels (CANs) permeable only to monovalent ions (K+ and Na+). TRPM4 forms a functional channel as a tetramer and each monomer comprises six predicted transmembrane domain helices (TM1-TM6) with a pore region between TM5 and TM6, and cytoplasmic N- and C-terminal domains (1-3). TRPM4 channels are expressed in a wide range of human tissues and are involved in various physiological processes such as T cell activation, myogenic vasoconstriction, allergic reactions and neurotoxicity (4-9). In the brain, expression and/or channel activities of TRPM4 have been detected in the prefrontal cortex, hippocampus, cerebellar Purkinje cells, preBӧtzinger complex in the brainstem, magnocellular cells in supraoptic and paraventricular nuclei, and substantia nigra pars compacta (10-15).
TRPM4 channels have three isoforms resulting from alternative splicing: (1) the full-length TRPM4 (TRPM4b), (2) an N-terminal 174 amino acid deletion isoform of TRPM4 (TRPM4a), and (3) a TRPM4 isoform lacking 537 amino acids (TRPM4c) (3, 16, 17). Although the specific functions of these splicing variants in vivo are unknown, a recent study of splicing alterations in a myotonic dystrophy suggested that each TRPM4 splicing variant may have distinct functions (18).
Human mutations in the TRPM4 gene and their consequent dysfunction have been linked to cardiac diseases (19-21). Increases in the level of TRPM4 channels have been reported in vascular endothelium following hypoxia/ischemic stroke, and in the endothelial cells of capillary vessels following spinal cord injury (22, 23). TRPM4 has also been implicated in experimental autoimmune encephalomyelitis and human multiple sclerosis tissues (9). Therefore, understanding the regulatory mechanism of TRPM4 channels on the plasma membrane may open the therapeutic window for intervention in TRPM4-related diseases.
TRAFFICKING MECHANISM OF TRPM4
In addition to the regulation present at the transcription and translation levels, membrane proteins, including ion channels, are tightly regulated with regards to the number on the plasma membrane by their exocytic (forward) trafficking - retention and exit from the endoplasmic reticulum and insertion into the plasma membrane - and endocytotic (reverse) trafficking processes − internalization for sorting into either recycling or degradative pathways (24). Although there are numerous interacting molecules and post-translational modifications have been identified that affect the trafficking process of ion channels, the trafficking mechanism of TRPM4 channels remained elusive until two recent key findings (17-19).
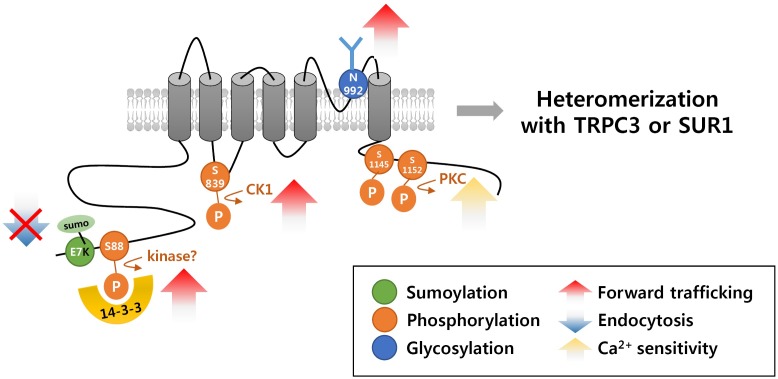
We have reported that membrane targeting of TRPM4 was mediated by interactions with 14-3-3γ (17). Based on the observation that a shorter isoform (TRPM4a) resides mostly within intracellular compartments and that a longer isoform (TRPM4b with an additional N-terminal fragment of 174 amino acids) reaches the plasma membrane, we identified 14-3-3γ as a trafficking chaperone using the N-terminal fragment (N174) of TRPM4b by yeast two-hybrid screening (16, 17). We also found that Ser88 at the N-terminus of TRPM4b is critical for 14-3-3γ binding, presumably in a phosphorylation-dependent manner and that the TRPM4b-S88A mutant failed to reach the plasma membrane (Fig. 1). Co-expression of 14-3-3γ and TRPM4b in HEK293T cells results in increased TRPM4 current density compared to TRPM4b alone. Specific gene silencing via short hairpin RNAs (shRNAs) of either 14-3-3γ or TRPM4b reduced the glutamate-induced current amplitude of TRPM4 channels endogenously expressed in a neuronal cell line, HT-22 (17). Interfering with the forward trafficking of TRPM4b channels using 14-3-3γ shRNA efficiently blocked glutamateinduced neurotoxicity in HT-22 cells, which is comparable to the effect of 9-phenanthrol, a TRPM4b specific antagonist. These results clearly showed that the interaction of TRPM4b with 14-3-3γ influences glutamate-mediated neurotoxicity through its function in controlling forward trafficking to the plasma membrane.
Fig. 1. Post-translational modifications and binding proteins of human TRPM4b.
Understanding of the retrograde trafficking of TRPM4 channels is still limited. A missense mutation at the cytoplasmic N-terminus (Glu7Lys) of TRPM4 in patients with a progressive cardiac bundle branch disease was found to cause de novo SUMOylation and consequential defect in endocytosis of the channel, which led to increased levels of the channels on the plasma membrane (19) (Fig. 1). Additional mutations (Arg164Trp, Ala432Thr, and Gly844Asp - all likely facing the intracellular side) were found in a cardiac conduction disease which also caused impaired deSUMOylation/endocytosis, resulting in increased current density of TRPM4 although the residues were not directly SUMOylated (20). SUMOylation is a post-translational modification that modulates protein function by binding a member of the SUMO (small ubiquitin-like modifier) family to the target protein (25).
The balance between SUMOylation and deSUMOylation plays an important role in regulating ion channels and neurotransmitter receptors, modulating synaptic transmission and plasticity by mainly affecting endocytosis in the brain (26). Therefore, elucidating the regulatory mechanism of TRPM4 via SUMOylation may be critical for understanding the trafficking of these channels. Although TRPM4 has been shown to be sensitive to SENP1 (sentrin-specific protease 1) and Ubc9, a SUMO conjugation enzyme, the SUMOylation site(s) of TRPM4 channels have not been identified (19). Therefore, the endocytotic mechanism of TRPM4 remains largely elusive although there is a possibility that it is dynamin-dependent (19, 20).
GLYCOSYLATION OF TRPM4
In addition to these two findings on the trafficking of TRPM4 channels, the level of TRPM4 has been shown to be affected by glycosylation (27, 28). N-glycosylation is important for maturation and proper targeting of ion channels to the plasma membrane (29).
Woo et al., showed that rat TRPM4 on the plasma membrane is sensitive to peptide:N-glycosidase F, and the Asn988 residue was identified as a glycosylation site. They reported that a mutation at this residue did not affect surface expression or channel activities. The study also suggested that N-glycosylation may play a role in stabilizing the surface expression of TRPM4, based on comparisons of the number of wild-type and Asn988Gln mutant channels in the presence of cyclohexamide, a protein synthesis inhibitor (27).
Syam et al. identified Asn992 (the homologous site of Asn988 in rat TRPM4) of human TRPM4 as a glycosylation site and a mutation at this residue decreased current density without affecting the number of channels at the plasma membrane (28) (Fig. 1). They also reported that application of tunicamycin, an inhibitor of protein glycosylation, to HEK293 cells expressing wild type TRPM4 increased the current density of TRPM4, which was in contrast to the results from Woo et al (27, 28). The discrepancy in trafficking effects of glycosylation at a conserved site of TRPM4 remains to be explained: whether it is merely species-specific or whether additional regulatory mechanism(s), including additional protein-protein interaction(s), are required.
PHOSPHORYLATION OF TRPM4
Phosphorylation has been studied extensively for the purpose of regulating ion channels and neurotransmitter receptors, especially with respect to their trafficking and protein-protein interactions and influencing cellular excitability and synaptic functions (30-33). As Ca2+-activated channels, PKC-mediated phosphorylation of TRPM4 at Ser1145 and Ser1152 has been shown to affect their Ca2+ sensitivity, and Ca2+/calmodulun binding sites have been identified at the C-terminus of TRPM4 (34) (Fig. 1). In addition to the presumably phosphorylationdependent, 14-3-3γ-dependent forward trafficking described above, PKCδ activation increased the surface expression of TRPM4 channels in vascular smooth muscle cells, and casein kinase 1 (CK1) phosphorylation of TRPM4 at Ser839 has been reported as responsible for its basolateral localization (8, 35) (Fig. 1). However, whether TRPM4 channels can be modulated by PKA and/or other kinases (and resulting protein-protein interactions) is unknown, although multiple putative PKA phosphorylation sites are predicted at both the N- and C-termini of the channels (34).
HETEROMERIZATION OF TRPM4 WITH TRPC3 AND SUR1
Intriguingly, TRPM4 has been shown to associate with different types of ion channel subunits (36, 37). First, TRPM4b has been shown to bind to TRPC3, and TRPM4b activation suppresses TRPC3-mediated current density in HEK293T cells (36) (Fig. 1). TRPC3, a calcium-activated, calcium-permeable TRP family member, may play an important role in physiological and pathophysiological functions due to the wide spectrum of its calcium signaling mechanism (38). However, the physiological significance of the TRPM4-TRPC3 heteromer has not been examined in cells with simultaneous expression of these two channels. Therefore, it remains to be seen how TRPM4-TRPC3 heteromeric channels function in vivo.
Another interesting finding is the functional heteromerization of SUR1 (sulfonylurea receptor 1) with TRPM4 (37) (Fig. 1). SUR1 is known to associate with Kir6.2 (or Kir6.1) to form KATP channels that link metabolic signals to excitability in various types of cells (39). In traumatic brain and spinal cord injury or ischemic stroke, increased levels of TRPM4 and SUR1 have been found in astrocytes, neurons and capillaries (37, 40). Co-expression of TRPM4 and SUR1 showed functional distinction compared to the properties of the individual channels: pharmacological properties of SUR1 − acquired sensitivity to diazoxide and glibenclamide, an activator and an inhibitor, respectively - and biophysical properties of TRPM4 - doubled affinity for calmodulin and doubled sensitivity to intracellular calcium (37). It remains to be seen how interactions of SUR1 with TRPM4 and Kir6.2 functionally affect both TRPM4 and KATP activities in the same cells (10). The bigger question is whether different combinations of heteromeric TRPM4 channels (with other types of ion channels) require additional protein-protein interactions or new regulatory modes for trafficking.
As mentioned previously, understanding of the trafficking mechanism of TRPM4 is still in the early stages. For example, in Brugada syndrome, an associate risk of cardiac arrhythmia, mutations in TRPM4 that resulted in either increased (Thr873Ile and Leu1075Pro) or decreased (Pro779Arg and Lys914X) expression on the plasma membrane were found without changing the biophysical properties of the channels (41). Therefore, there may be additional mechanism(s) affecting the trafficking of TRPM4 on the plasma membrane, especially where polar expression patterns exist in subcellular locations (e.g., axon and dendrites of the pyramidal neurons). In addition, other post-translational modifications (e.g., ubiquitylation and protein lipidation) that have been shown to modulate many other ion channels need to be examined for trafficking regulation of TRPM4 channels (42-44).
CONCLUSIONS
Here, we briefly summarized the regulation of TRPM4 channels via protein-protein interactions. Trafficking of TRPM4 channels often requires post-translational modifications (phosphorylation, and SUMOylation) to achieve protein-protein interactions, however, other types of modifications yet remain to be examined. Heteromerization of TRPM4 channels with SUR1 and/or TRPC3 need to be studied further for their physiological significance in diverse tissues. This will lead to a better understanding of the dysfunctional contributions of these channels and open the therapeutic door for diseases related to the channels.
Acknowledgments
This work was supported by the National Research Foundation (NRF) of Korea (NRF-2014R1A2A1A01007039).
References
- 1.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. (2002);109:397–407. doi: 10.1016/S0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 2.Fleig A, Penner R. The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharmacol Sci. (2004);25:633–639. doi: 10.1016/j.tips.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Vennekens R, Nilius B. Insights into TRPM4 function, regulation and physiological role. Handb Exp Pharmacol. (2007);179:269–285. doi: 10.1007/978-3-540-34891-7_16. [DOI] [PubMed] [Google Scholar]
- 4.Xu XZ, Moebius F, Gill DL, Montell C. Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci U S A. (2001);98:10692–10697. doi: 10.1073/pnas.191360198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet JP. TRPM4 regulates calcium oscillations after T cell activation. Science. (2004);306:1374–1377. doi: 10.1126/science.1098845. [DOI] [PubMed] [Google Scholar]
- 6.Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res. (2006);26:159–178. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- 7.Vennekens R, Olausson J, Meissner M, et al. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol. (2007);8:312–320. doi: 10.1038/ni1441. [DOI] [PubMed] [Google Scholar]
- 8.Crnich R, Amberg GC, Leo MD, et al. Vasoconstriction resulting from dynamic membrane trafficking of TRPM4 in vascular smooth muscle cells. Am J Physiol Cell Physiol. (2010);299:C682–C694. doi: 10.1152/ajpcell.00101.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schattling B, Steinbach K, Thies E, et al. TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. (2012);18:1805–1811. doi: 10.1038/nm.3015. [DOI] [PubMed] [Google Scholar]
- 10.Mironov SL, Skorova EY. Stimulation of bursting in pre-Bötzinger neurons by Epac through calcium release and modulation of TRPM4 and K-ATP channels. J Neurochem. (2011);117:295–308. doi: 10.1111/j.1471-4159.2011.07202.x. [DOI] [PubMed] [Google Scholar]
- 11.Mrejeru A, Wei A, Ramirez JM. Calcium-activated non-selective cation currents are involved in generation of tonic and bursting activity in dopamine neurons of the substantia nigra pars compacta. J Physiol. (2011);15:2497–2514. doi: 10.1113/jphysiol.2011.206631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teruyama R, Sakuraba M, Kurotaki H, Armstrong WE. Transient receptor potential channel m4 and m5 in magnocellular cells in rat supraoptic and paraventricular nuclei. J Neuroendocrinol. (2011);23:1204–1213. doi: 10.1111/j.1365-2826.2011.02211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schattling B, Steinbach K, Thies E, et al. TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. (2012);18:1805–1811. doi: 10.1038/nm.3015. [DOI] [PubMed] [Google Scholar]
- 14.Kim YS, Kang E, Makino Y, et al. Characterizing the conductance underlying depolarization-induced slow current in cerebellar Purkinje cells. J Neurophysiol. (2013);109:1174–1181. doi: 10.1152/jn.01168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei YT, Thuault SJ, Launay P, Margolskee RF, Kandel ER, Siegelbaum SA. Differential contribution of TRPM4 and TRPM5 nonselective cation channels to the slow afterdepolarization in mouse prefrontal cortex neurons. Front Cell Neurosci. (2014);8:267. doi: 10.3389/fncel.2014.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo JC, Yarishkin OV, Hwang EM, et al. Cloning and characterization of rat transient receptor potential-melastatin 4 (TRPM4). Biochem Biophys Res Commun. (2010);391:806–811. doi: 10.1016/j.bbrc.2009.11.142. [DOI] [PubMed] [Google Scholar]
- 17.Cho CH, Kim E, Lee YS, et al. Depletion of 14-3-3γ reduces the surface expression of Transient Receptor Potential Melastatin 4b (TRPM4b) Channels and attenuates TRPM4b-mediated glutamate-induced neuronal cell death. Mol Brain. (2014);22:52. doi: 10.1186/s13041-014-0052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klinck R, Fourrier A, Thibault P, et al. RBFOX1 cooperates with MBNL1 to control splicing in muscle, including events altered in myotonic dystrophy type 1. PLoS One. (2014);9:e107324. doi: 10.1371/journal.pone.0107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruse M, Schulze-Bahr E, Corfield V, et al. Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest. (2009);119:2737–2744. doi: 10.1172/JCI38292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, El Zein L, Kruse M, et al. Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet. (2010);3:374–385. doi: 10.1161/CIRCGENETICS.109.930867. [DOI] [PubMed] [Google Scholar]
- 21.Stallmeyer B, Zumhagen S, Denjoy I, et al. Mutational spectrum in the Ca(2+)--activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum Mutat. (2012);33:109–117. doi: 10.1002/humu.21599. [DOI] [PubMed] [Google Scholar]
- 22.Gerzanich V, Woo SK, Vennekens R, et al. De novo expression of Trpm4 initiates secondary hemorrhage in spinal cord injury. Nat Med. (2009);15:185–191. doi: 10.1038/nm.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loh KP, Ng G, Yu CY, et al. TRPM4 inhibition promotes angiogenesis after ischemic stroke. Pflugers Arch. (2014);466:563–576. doi: 10.1007/s00424-013-1347-4. [DOI] [PubMed] [Google Scholar]
- 24.Schwappach B. An overview of trafficking and assembly of neurotransmitter receptors and ion channels (Review). Mol Membr Biol. (2008);25:270–278. doi: 10.1080/09687680801960998. [DOI] [PubMed] [Google Scholar]
- 25.Luo J, Ashikaga E, Rubin PP, et al. Receptor trafficking and the regulation of synaptic plasticity by SUMO. Neuromolecular Med. (2013);4:692–706. doi: 10.1007/s12017-013-8253-y. [DOI] [PubMed] [Google Scholar]
- 26.Martin S, Wilkinson KA, Nishimune A, Henley JM. Emerging extranuclear roles of protein SUMOylation in neuronal function and dysfunction. Nat Rev Neurosci. (2007);8:948–959. doi: 10.1038/nrn2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woo SK, Kwon MS, Ivanov A, Geng Z, Gerzanich V, Simard JM. Complex N-glycosylation stabilizes surface expression of transient receptor potential melastatin 4b protein. J Biol Chem. (2013);288:36409–36417. doi: 10.1074/jbc.M113.530584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Syam N, Rougier JS, Abriel H. Glycosylation of TRPM4 and TRPM5 channels: molecular determinants and functional aspects. Front Cell Neurosci. (2014);8:52. doi: 10.3389/fncel.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baycin-Hizal D, Gottschalk A, Jacobson E, et al. Physiologic and pathophysiologic consequences of altered sialylation and glycosylation on ion channel function. Biochem Biophys Res Commun. (2014);453:243–253. doi: 10.1016/j.bbrc.2014.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raymond LA, Blackstone CD, Huganir RL. Phosphorylation of amino acid neurotransmitter receptors in synaptic plasticity. Trends Neurosci. (1993);16:147–153. doi: 10.1016/0166-2236(93)90123-4. [DOI] [PubMed] [Google Scholar]
- 31.Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. (1994);56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- 32.Ismailov II, Benos DJ. Effects of phosphorylation on ion channel function. Kidney Int. (1994);48:1167–1179. doi: 10.1038/ki.1995.400. [DOI] [PubMed] [Google Scholar]
- 33.Smart TG. Regulation of excitatory and inhibitory neurotransmitter-gated ion channels by protein phosphorylation. Curr Opin Neurobiol. (1997);7:358–367. doi: 10.1016/S0959-4388(97)80063-3. [DOI] [PubMed] [Google Scholar]
- 34.Nilius B, Prenen J, Tang J, et al. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem. (2005);280:6423–6433. doi: 10.1074/jbc.M411089200. [DOI] [PubMed] [Google Scholar]
- 35.Cerda O, Cáceres M, Park KS, et al. Casein kinase-mediated phosphorylation of serine 839 is necessary for basolateral localization of the Ca(2+)-activated non-selective cation channel TRPM4. Pflugers Arch. doi: 10.1007/s00424-014-1610-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JY, Hwang EM, Yarishkin O, et al. TRPM4b channel suppresses store-operated Ca2+ entry by a novel protein-protein interaction with the TRPC3 channel. Biochem Biophys Res Commun. (2008);368:677–683. doi: 10.1016/j.bbrc.2008.01.153. [DOI] [PubMed] [Google Scholar]
- 37.Woo SK, Kwon MS, Ivanov A, Gerzanich V, Simard JM. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J Biol Chem. (2013);288:3655–3667. doi: 10.1074/jbc.M112.428219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lichtenegger M, Groschner K. TRPC3: a multifunctional signaling molecule. Handb Exp Pharmacol. (2014);222:67–84. doi: 10.1007/978-3-642-54215-2_4. [DOI] [PubMed] [Google Scholar]
- 39.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. (2006);440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 40.Simard JM, Chen M, Tarasov KV, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. (2006);4:433–440. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu H, Chatel S, Simard C, et al. Molecular genetics and functional anomalies in a series of 248 Brugada cases with 11 mutations in the TRPM4 channel. PLoS One. (2013);8:e54131. doi: 10.1371/journal.pone.0054131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abriel H, Staub O. Ubiquitylation of ion channels. Physiology (Bethesda) (2005);20:398–407. doi: 10.1152/physiol.00033.2005. [DOI] [PubMed] [Google Scholar]
- 43.Bongiorno D, Schuetz F, Poronnik P, Adams DJ. Regulation of voltage-gated ion channels in excitable cells by the ubiquitin ligases Nedd4 and Nedd4-2. Channels (Austin) (2011);5:79–88. doi: 10.4161/chan.5.1.13967. [DOI] [PubMed] [Google Scholar]
- 44.Shipston MJ. Ion channel regulation by protein S-acylation. J Gen Physiol. (2014);143:659–678. doi: 10.1085/jgp.201411176. [DOI] [PMC free article] [PubMed] [Google Scholar]