Abstract
Alzheimer's disease severely compromises cognitive function. One of the mechanisms to explain the pathology of Alzheimer’s disease has been the hypotheses of amyloid-pore/channel formation by complex Aβ-aggregates. Clinical studies suggested the moderate alcohol consumption can reduces probability developing neurodegenerative pathologies. A recent report explored the ability of ethanol to disrupt the generation of complex Aβ in vitro and reduce the toxicity in two cell lines. Molecular dynamics simulations were applied to understand how ethanol blocks the aggregation of amyloid. On the other hand, the in silico modeling showed ethanol effect over the dynamics assembling for complex Aβ-aggregates mediated by break the hydrosaline bridges between Asp 23 and Lys 28, was are key element for amyloid dimerization. The amyloid pore/channel hypothesis has been explored only in neuronal models, however recently experiments suggested the frog oocytes such an excellent model to explore the mechanism of the amyloid pore/channel hypothesis. So, the used of frog oocytes to explored the mechanism of amyloid aggregates is new, mainly for amyloid/pore hypothesis. Therefore, this experimental model is a powerful tool to explore the mechanism implicates in the Alzheimer’s disease pathology and also suggests a model to prevent the Alzheimer’s disease pathology. [BMB Reports 2015; 48(1): 13-18]
Keywords: Amyloid, Channel, Ethanol, Oocytes, Pore
INTRODUCTION
Alzheimer's disease is described like pathology with high impact and present a public health problem of global dimensions (1, 2). A describe triggering agent for this the pathology is an aggregate form of β-amyloid peptides (Aβ-aggregates), present studies indicate the soluble amyloid oligomers are impact neuronal physiology and are describe like agent of symptoms of the disease (3). An unfinished task in the field of Alzheimer’s disease is to determine the mechanism of Aβ-aggregate for generated the disease. It has been established that this compound altered the synaptic activity (4) disrupting neurotransmission mediated by cholinergic and NMDA receptors (5) and the recycling of vesicles, in the synapsis (6, 7). Aβ-aggregates showed different effects on synaptic transmission (8), and the aggregate induce modifying on long-term potentiation in hippocampus has been widely suggested (9). Has been reported, Aβ-aggregates showed toxic for neurons (10, 11), several transgenic models of Alzheimer’s disease (12), fibroblasts (13) and cell lines (14-16).
AMYLOID PORE HYPOTHESIS
Aβ-aggregates induce a “channel activity” in neuron culture, immortalized cell lines (17) and hippocampal neurons (18). The β-amyloid pore idea is present in other models, forming a group of pathology called misfolding disease, and include amyloid, synuclein, prion and other peptides (19, 20). Recently, it was shown in neurons that Aβ-aggregates act like a poreforming neurotoxin, increasing intracellular calcium, leading to depletion of synaptic vesicles (8, 21), however, the dysregulation the calcium is not the only effect of the amyloid pore. The change in intracellular calcium concentration is an important event in the development of Alzheimer’s disease (23). Another work described that pore formation can alter the homeostasis of macromolecules such as ATP (18). Part of this is explained by in silico work on the size of amyloid channel (24), describing the change in the pore size in the model for a fluid movement of the Aβ-aggregate in the membrane. The latter supports the observation made in the conductance of the amyloid channel in the bilayer (25). One of the consequences of the interaction between Aβ-aggregates and neurons is the increase in the intracellular calcium concentration that could, when large enough, create an imbalance of the calcium ho-meostasis. This effect has been proposed to mediate neurotoxicity (26). It has also been proposed that calcium influx occurs when Aβ-aggregates change the activity of calcium channels or directly interact with components of the plasma membrane (27). Proposed mechanisms include: A) direct interaction of Aβ-aggregates with the membrane components, such as lipids, to destabilize the membrane structure, or B) the insertion of Aβ-aggregates into the membrane to form a “cation-conducting” pore. This channel-like activity can be observed in both planar lipid bilayers (22, 28), and cultured cellular lines (17). Analysis of secondary structure suggested the possibility of ion channel formation by membrane-bound amyloid β-proteins (29, 30). The amyloid-pore/channel hypothesis was first proposed by biophysical studies performed by Rojas in 1994 using artificial membrane (22), were the Aβ1-40 generate pores with channel-like activity in the model, with highly cation-selective properties, allowing permeation of Ca2+, Na+ and Cs+ (28, 29). These studies in synthetic membranes were validated using hypothalamic cell line membranes (17). Interestingly, cholesterol and phospholipid levels favored the formation of Aβ-aggregate pores with channel-like activity in artificial and hypothalamic membranes (31, 32). Single channel measurements showed that conductance of the Aβ1-40 induced channel was exceptionally complex, showing dependence of Cs+ concentration and conductance of 50-500 pS (25). Additionally, it was found that Zn2+, binded to Aβ-aggregates in solution (33), blocked ion current flow (25). This data suggests that Aβ-aggregates did not form a single type of ion channel, but rather, that it is possible to generate multiple complex conducting pores (34). Moreover, using “oligomer enriched” Aβ-aggregates, an increase was shown in lipid bilayer conductance and unitary events, like single channel signal are recording, adding to the complexity of the behavior of the peptide in the membrane (35). In conclusion, it is evident that Aβ-aggregates are able to increase the conductance in artificial membranes and the work of Kawahara in 1997 the effect in cell lines was confirmed (8, 18). However, this cellular model is not the only one in which the amyloid effect has been observed.
OOCYTES USED FOR AMYLOID STUDY
Membrane formation altered the relation between Aβ-aggregates and lipids. For example in aging membranes, the composition changes and induces more interaction with Aβ-aggregates (36, 37). The review suggested a relation between lipids in the membrane and Aβ-aggregate interaction. The question proposed here is if oocytes have adequate lipid composition to be a study model of Alzheimer's disease. The oocytes, present a lipid composition similar to other cells (38, 39) and, during the aging process the composition can affect their function (40). This evidence supports the idea of using oocytes for exploring the effects of Aβ-aggregates in cell membranes. It is well known that Xenopus laevis frog oocytes have been widely used for studying ion channels in a controlled in vivo environment since the system was initially development for this purpose by Miledi and coworkers (41). There have been at least four major types of studies using oocytes to examine ion channel functions. The earliest use was to examine the properties of specific ion channels in a living cell, free from other responses. The second major type of study for which the Xenopus laevis oocytes has been, and continues to be, particularly useful is the correlation of molecular structure with electrophysiological function of a specific channel. The general approach in utilizing oocytes is to determine the functional effects of mutations that cause human diseases. Other methods include using Xenopus oocytes to screen potential drugs to determine their relative efficacies against specific types of ion channels or receptors (42, 43).
Evidence suggests that amyloid-pore/channel could be a mechanism in the generation of the Alzheimer's disease, which alters biological membranes. Therefore, considering the size and different biological properties of the oocyte, this cell can be used as a biological model to test new drugs in Alzheimer’s disease. Recently, a work of Parodi in 2012, showed the ability of the peptide aggregates to disrupt contact between the oocyte and follicular cells, thus uncoupling their electrochemical communication (44). To arrive at this observation, amplitude generated by follicles exposed was compared to known membrane-perforating agents with those induced by Aβ-aggregates. The blips recorded in follicles exposed to gramicidin, amphotericin B, or Aβ-aggregates had similar amplitudes: 7.8, 6.3, and 6.8 nA, respectively that were clearly larger than those recorded in control follicles (4.5 nA). These results suggested change in the membrane properties and the intracellular calcium regulation of the oocytes. This conclusion was corroborated by the fact that the reduction of extracellular calcium reduces the blips generated by the Aβ-aggregate. Demuro and Parker in 2013 presented a similar report when using another amyloid (1-42 fragments). This work describes the effect on the membrane stability in oocytes injected or exposed to the amyloid, which generated two types of signal in the oocytes, local channel like transients and calcium waves and presented evidence of IP3 modulation in the oocytes when exposed to amyloid aggregates (45). Previous reports explored the changes of the intracellular calcium concentration in oocytes exposed to amyloid 1-40 fragments, describing the pore/channel formation by imaging of calcium influx (46). Also, there is other evidence of the pore/channel hypothesis in Alzheimer disease (47). This observation may help to understand how the Aβ-aggregates modify the neuronal structure and neurotransmission properties of patients affected by Alzheimer's. On the other hand, this evidence suggests that amyloid aggregates alter biological membranes causing a change in electrical noise and generating new conductance that not were previously present in the cellular membranes. These new membrane behaviors could be in response to the change in the intracellular calcium homeostasis. These data seem to agree with experiments presented by the group of Rojas in the 90's and new evidence of the group of Aguayo in 2014, newest and model different to neurons (44, 45).
ETHANOL AND PROTECTION
Recent evidence, present in clinical trial indicates the consumption of low doses of ethanol reduce symptoms associated with neurodegeneration pathology (48, 49). It is important the attention to the effect occurred at low doses. Moreover, nontoxic effect are observed in cells, the low doses of ethanol can altered the receptors function (50) but not the viability and in cells lines exposed to low doses of ethanol, not change in viability are observed (51), direct relation or correlation between ethanol and any protective effect with Alzheimer’s disease has been present in clinical trial. In recent work, the meta-analyses present a correlation between the moderate consumption of alcohol and protection brain from the effects of aging as well as of Alzheimer’s disease, indicating that moderate drinking (wine and another alcoholic presentations) reduce the neurodegeneration diseases, maybe for contain protective or preventive compounds (48). In the red wine, natural agents can prevent Alzheimer’s disease (52-54). Some diseases, such as heart disease due to high cholesterol, reduced oxidative stress, defects of vascular response and other can be reduced when the pacient present a moderate consumption of alcohol and in addition moderate drinking of different alcohol presentation (wine, beer and other) in several cognitive tests showed better response (55). In the wine polyphenolic molecules that are present, they act in different ways on cellular activity (52). Model of mice with Alzheimer’s (Tg2576, in example) when are exposed to moderate amounts of red wine (drinking in water); reduced the number of amyloid plaques in the brain with a reduction in the process of aggregation (52). The algae Ecklonia cava present polyphenolic molecules and can be obtained from the algae, this extract of antioxidant molecules reduced the in vitro process aggregation of β-amyloid reduced neuronal death of cortical neurons and prevent the neurodegeneration in general (56). A specific flavonoid has been describe in the red wine, the morin, this molecules present effect over β-aggregate, reduced the aggregation process and is one explain for benefits effect of the red wine (57). Other natural molecules, the fulvic acid, altered the aggregation mechanism of tau proteins, the tau are critical for stabilizing microtubules and axonal transport and can be involved in Alzheimer´s disease (58). Current evidence present a correlation moderate ethanol consumption (low dinking) and protection against neurodegeneration include Alzheimer's disease; however the molecular mechanism of the protective effect is not known and at the cellular level recently working has been showed protection and reduction of toxic structure formation. Aβ structures formation (aggregation process) is a dynamic process and can generates several complex molecules, this process has been recently approached, described a key event, peptide nucleation, his is important event for the formation of metastable Aβ-aggregate (59). The β-amyloid monomers showed specific properties and this properties help to interact monomers with monomer and initiate the nucleation, generate the first dimers thanks to this monomer interaction and this event important for self-assembly of more complex structures (60); for the dimer formation, in β-amyloid has been describe key amino acid to generate hydrosaline bridges and these residues in the dimer help to generate more complex structure, the amino acid have been clearly described in previous report (residues Asp 23 and Lys 28) and the important for dimer formation has been probed using mutations in several of these residues, with alteration in aggregation mechanism and lead to reduce Aβ toxicity (61, 62). Atomic force microscopy in lipid environments and molecular dynamic analysis have shown the presence of molecular entities with inner diameters in the 1.5-2.6 nm range (10, 11) which were similar to these generated by other peptidergic molecules known to form pores in cell membranes, such as amylin and α-synuclein (12). For several years it has been recognized that several peptides, with differing structures such as gramicidin, amphotericin and α-latrotoxin can alter membrane permeability generating ion pores (13, 14). Ormeño et al, present evidence for support the idea of ethanol reduce toxicity of the Aβ mediated by alteration of molecular structure. Thus, blocking dimerization reduces aggregation and reduces peptide toxicity in cell lines model (51).
CONCLUSION
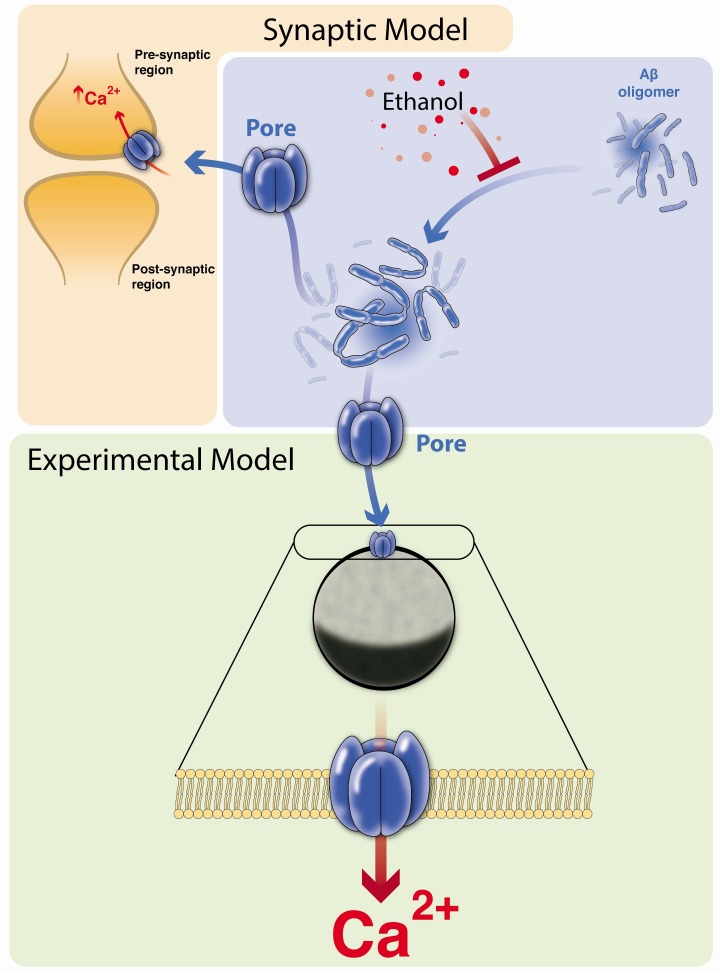
Moderate drinking in several studies, suggested the benefits of moderate ethanol in reducing heart disease and neurodegeneration (48, 49, 63). The ethanol, presences can reduces of aggregates in vitro, and the possible mechanism has been suggested for inhibition of aggregation process at molecular level (51). Fig. 1 presents the changes in the aggregation process. In this sense, in the wine are present different antioxidant molecules like flavonoids, polyphenols, and other compounds and moderate consumption prevents neurodegeneration, maybe for the presences of these molecules (64, 65). Be the ethanol, direct responsible of the benefits effect? This question should not be excluded. Recent work by Ormeño et al describe the ethanol effect on aggregation process and reveal the alteration of critical hydrophobic bridge, reduction the formation of amyloid dimers and more complex and toxic structures (51), preventing the formation of complex aggregates and formation of amyloid pore in the membrane and reduce the observation of toxic or functional effect in cells (8, 18). The observations of Sepulveda in the 2010 are important because describing the pore/channel hypothesis in neurons and Parodi in 2010 when describing the synaptic effect of amyloids. Moreover, similar experiments are present in 2014, by Aguayo group but despite this, there is not new evidence. Work from Parodi and Sepulveda in 2010, supports the idea that pore/channel amyloid is an important mechanism to explain Alzheimer’s disease. The uses of oocytes are a pharmacological tool for exploring the mechanism of amyloid action in the membrane. The work of Parodi in 2012 and Demuro in 2013, shows the uses of oocytes to explore the idea of amyloid pore/channel in a biological membrane. In the future we can use the model to continue exploring the ethanol effect or other molecules to disrupt aggregation formation. In Fig. 1 we described an overview of the Aβ-aggregate effect on the membrane and the ethanol effect on aggregation.
Fig. 1. Model effect of ethanol in aggregation process, oocytes membrane use for pharmacological solution exploration. The model, show the summary of Aβ-aggregate review, in the right upper panel, the effect of the ethanol in the aggregation process, left panel show the Aβ-aggregate effect over synapsis in particular over presynaptic membrane. The lower panel presents the use of oocytes for explored Aβ-aggregate effect, pore formation and future research in easy membrane model.
Acknowledgments
Jorge Parodi was supported by MECESUP UCT0804. We used www.journalrevisions.com for manuscript editing. Felipe Serrano for model design graphique-science.blogspot.com.
References
- 1.Uc EY, Rizzo M. Driving and neurodegenerative diseases. Curr Neurol Neurosci Rep. (2008);8:377–383. doi: 10.1007/s11910-008-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler-Graham K, Brookmeyer R, Johnson E, Arrighi HM. Worldwide variation in the doubling time of Alzheimer's disease incidence rates. Alzheimers Dement. (2008);4:316–323. doi: 10.1016/j.jalz.2008.05.2479. [DOI] [PubMed] [Google Scholar]
- 3.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. (2007);8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 4.Lacor PN, Buniel MC, Chang L, et al. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J Neurosci. (2004);24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourin M, Ripoll N, Dailly E. Nicotinic receptors and Alzheimer's disease. Curr Med Res Opin. (2003);19:169–177. doi: 10.1185/030079903125001631. [DOI] [PubMed] [Google Scholar]
- 6.Rowan MJ, Klyubin I, Wang Q, Anwyl R. Synaptic plasticity disruption by amyloid beta protein: modulation by potential Alzheimer's disease modifying therapies. Biochem Soc Trans. (2005);33:563–567. doi: 10.1042/BST0330563. [DOI] [PubMed] [Google Scholar]
- 7.Bell KF, Claudio Cuello A. Altered synaptic function in Alzheimer's disease. Eur J Pharmacol. (2006);545:11–21. doi: 10.1016/j.ejphar.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 8.Parodi J, Sepulveda FJ, Roa J, Opazo C, Inestrosa NC, Aguayo LG. Beta-amyloid causes depletion of synaptic vesicles leading to neurotransmission failure. J Biol Chem. (2010);285:2506–2514. doi: 10.1074/jbc.M109.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh DM, Klyubin I, Fadeeva JV, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. (2002);416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura I, Uetsuki T, Dani SU, et al. Degeneration in vivo of rat hippocampal neurons by wild-type Alzheimer amyloid precursor protein overexpressed by adenovirus-mediated gene transfer. J Neurosci. (1998);18:2387–2398. doi: 10.1523/JNEUROSCI.18-07-02387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yankner BA, Lu T. Amyloid beta-protein toxicity and the pathogenesis of Alzheimer disease. J Biol Chem. (2009);284:4755–4759. doi: 10.1074/jbc.R800018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oddo S, Caccamo A, Tran L, et al. Temporal profile of amyloid-beta (Abeta) oligomerization in an in vivo model of Alzheimer disease. A link between Abeta and tau pathology. J Biol Chem. (2006);281:1599–1604. doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- 13.Zhu YJ, Lin H, Lal R. Fresh and nonfibrillar amyloid beta protein (1-40) induces rapid cellular degeneration in aged human fibroblasts: evidence for AbetaPchannel-mediated cellular toxicity. FASEB J. (2000);14:1244–1254. doi: 10.1096/fasebj.14.9.1244. [DOI] [PubMed] [Google Scholar]
- 14.Uetsuki T, Takemoto K, Nishimura I, et al. Activation of neuronal caspase-3 by intracellular accumulation of wild-type Alzheimer amyloid precursor protein. J Neurosci. (1999);19:6955–6964. doi: 10.1523/JNEUROSCI.19-16-06955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DS, Kim JY, Han YS. Alzheimer's disease drug discovery from herbs: neuroprotectivity from beta-amyloid (1-42) insult. J Altern Complement Med. (2007);13:333–340. doi: 10.1089/acm.2006.6107. [DOI] [PubMed] [Google Scholar]
- 16.Clark EM, Vulliet R. Toxicity of beta-amyloid25-35 peptide in PC12 cells. Proc West Pharmacol Soc. (1993);36:273–276. [PubMed] [Google Scholar]
- 17.Kawahara M, Kuroda Y, Arispe N, Rojas E. Alzheimer's beta-amyloid, human islet amylin, and prion protein fragment evoke intracellular free calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell line. J Biol Chem. (2000);275:14077–14083. doi: 10.1074/jbc.275.19.14077. [DOI] [PubMed] [Google Scholar]
- 18.Sepulveda FJ, Parodi J, Peoples RW, Opazo C, Aguayo LG. Synaptotoxicity of Alzheimer beta amyloid can be explained by its membrane perforating property. PLoS One. (2010);5:e11820. doi: 10.1371/journal.pone.0011820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quist A, Doudevski I, Lin H, et al. Amyloid ion channels: a common structural link for protein-misfolding disease. Proc Natl Acad Sci U S A. (2005);102:10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Colodner KJ, Feany MB. Protein misfolding and oxidative stress promote glial-mediated neurodegeneration in an Alexander disease model. J Neurosci. (2011);31:2868–2877. doi: 10.1523/JNEUROSCI.3410-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sepúlveda FJ, Fierro H, Fernandez E, et al. Nature of the neurotoxic membrane actions of amyloid-beta on hippocampal neurons in Alzheimer's disease. Neurobiol Aging. (2014);35:472–481. doi: 10.1016/j.neurobiolaging.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 22.Arispe N, Pollard HB, Rojas E. The ability of amyloid beta-protein [A beta P (1-40)] to form Ca2+ channels provides a mechanism for neuronal death in Alzheimer's disease. Ann N Y Acad Sci. (1994);747:256–266. doi: 10.1111/j.1749-6632.1994.tb44414.x. [DOI] [PubMed] [Google Scholar]
- 23.Mattson MP, Chan SL. Dysregulation of cellular calcium homeostasis in Alzheimer's disease: bad genes and bad habits. J Mol Neurosci. (2001);17:205–224. doi: 10.1385/JMN:17:2:205. [DOI] [PubMed] [Google Scholar]
- 24.Jang H, Zheng J, Nussinov R. Models of beta-amyloid ion channels in the membrane suggest that channel formation in the bilayer is a dynamic process. Biophys J. (2007);93:1938–1949. doi: 10.1529/biophysj.107.110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawahara M, Arispe N, Kuroda Y, Rojas E. disease amyloid beta-protein forms Zn(2+)-sensitive, cation-selective channels across excised membrane patches from hypothalamic neurons. Biophys J. (1997);73:67–75. doi: 10.1016/S0006-3495(97)78048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. (2004);430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arispe N, Diaz JC, Simakova O. Abeta ion channels. Prospects for treating Alzheimer's disease with Abeta channel blockers. Biochim Biophys Acta. (2007);1768:1952–1965. doi: 10.1016/j.bbamem.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Arispe N. Architecture of the Alzheimer's A beta P ion channel pore. J Membr Biol. (2004);197:33–48. doi: 10.1007/s00232-003-0638-7. [DOI] [PubMed] [Google Scholar]
- 29.Prangkio P, Yusko EC, Sept D, Yang J, Mayer M. Multivariate analyses of amyloid-beta oligomer populations indicate a connection between pore formation and cytotoxicity. PLoS One. (2012);7:e47261. doi: 10.1371/journal.pone.0047261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connelly L, Jang H, Arce FT, et al. Atomic force microscopy and MD simulations reveal pore-like structures of all-D-enantiomer of Alzheimer's beta-amyloid peptide: relevance to the ion channel mechanism of AD pathology. J Phys Chem B. (2012);116:1728–1735. doi: 10.1021/jp2108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stockl MT, Zijlstra N, Subramaniam V. alpha-Synuclein oligomers: an amyloid pore? Insights into mechanisms of alpha-synuclein oligomer-lipid interactions. Mol Neurobiol. (2013);47:613–621. doi: 10.1007/s12035-012-8331-4. [DOI] [PubMed] [Google Scholar]
- 32.Alarcon JM, Brito J, Hermosilla T, Atwater I, Mears D, Rojas E. Ion channel formation by Alzheimer's disease amyloid beta-peptide (Abeta40) in unilamellar liposomes is determined by anionic phospholipids. Peptides. (2006);27:95–104. doi: 10.1016/j.peptides.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Bush AI, Pettingell WH, Multhaup G, et al. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. (1994);265:1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 34.Kourie JI, Henry CL, Farrelly P. Diversity of amyloid beta protein fragment [1-40]-formed channels. Cell Mol Neurobiol. (2001);21:255–284. doi: 10.1023/A:1010995121153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glabe CG, Kayed R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology. (2005);66(2 Suppl 1):S74–78. doi: 10.1212/01.wnl.0000192103.24796.42. [DOI] [PubMed] [Google Scholar]
- 36.Wood WG, Schroeder F, Igbavboa U, Avdulov NA, Chochina SV. Brain membrane cholesterol domains, aging and amyloid beta-peptides. Neurobiol Aging. (2002);23:685–694. doi: 10.1016/S0197-4580(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 37.Simakova O, Arispe NJ. The cell-selective neurotoxicity of the Alzheimer's Abeta peptide is determined by surface phosphatidylserine and cytosolic ATP levels. Membrane binding is required for Abeta toxicity. J Neurosci. (2007);27:13719–13729. doi: 10.1523/JNEUROSCI.3006-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosa KA, Kumar K, Chhikara S, et al. Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Res. (2012);21:1265–1277. doi: 10.1007/s11248-012-9600-8. [DOI] [PubMed] [Google Scholar]
- 39.Morrill GA, Kostellow AB. Progesterone release of lipid second messengers at the amphibian oocyte plasma membrane: role of ceramide in initiating the G2/M transition. Biochem Biophys Res Commun. (1998);246:359–363. doi: 10.1006/bbrc.1998.8620. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt D, MacKinnon R. Voltage-dependent K+ channel gating and voltage sensor toxin sensitivity depend on the mechanical state of the lipid membrane. Proc Natl Acad Sci U S A. (2008);105:19276–19281. doi: 10.1073/pnas.0810187105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gundersen CB, Jenden DJ, Miledi R. Choline acetyltransferase and acetylcholine in Xenopus oocytes injected with mRNA from the electric lobe of Torpedo. Proc Natl Acad Sci U S A. (1985);82:608–611. doi: 10.1073/pnas.82.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernareggi A, Duenas Z, Reyes-Ruiz JM, Ruzzier F, Miledi R. Properties of glutamate receptors of Alzheimer's disease brain transplanted to frog oocytes. Proc Natl Acad Sci U S A. (2007);104:2956–2960. doi: 10.1073/pnas.0611513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miledi R, Duenas Z, Martinez-Torres A, Kawas CH, Eusebi F. Microtransplantation of functional receptors and channels from the Alzheimer's brain to frog oocytes. Proc Natl Acad Sci U S A. (2004);101:1760–1763. doi: 10.1073/pnas.0308224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parodi J, Ochoa-de la Paz L, Miledi R, Martinez-Torres A. Functional and structural effects of amyloid-beta aggregate on Xenopus laevis oocytes. Mol Cells. (2012);34:349–355. doi: 10.1007/s10059-012-2247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demuro A, Parker I. Cytotoxicity of intracellular abeta42 amyloid oligomers involves Ca2+ release from the endoplasmic reticulum by stimulated production of inositol trisphosphate. J Neurosci. (2013);33:3824–3833. doi: 10.1523/JNEUROSCI.4367-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demuro A, Smith M, Parker I. Single-channel Ca(2+) imaging implicates Abeta1-42 amyloid pores in Alzheimer's disease pathology. J Cell Biol. (2011);195:515–524. doi: 10.1083/jcb.201104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. (2005);280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 48.Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing. (2008);37:505–512. doi: 10.1093/ageing/afn095. [DOI] [PubMed] [Google Scholar]
- 49.Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. (2009);17:542–555. doi: 10.1097/JGP.0b013e3181a2fd07. [DOI] [PubMed] [Google Scholar]
- 50.Yevenes GE, Peoples RW, Tapia JC, et al. Modulation of glycine-activated ion channel function by G-protein betagamma subunits. Nat Neurosci. (2003);6:819–824. doi: 10.1038/nn1095. [DOI] [PubMed] [Google Scholar]
- 51.Ormeno D, Romero F, Lopez-Fenner J, Avila A, Martinez-Torres A, Parodi J. Ethanol reduces amyloid aggregation in vitro and prevents toxicity in cell lines. Arch Med Res. (2013);44:1–7. doi: 10.1016/j.arcmed.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Ho L, Chen LH, Wang J, et al. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer's disease-type neuropathology and cognitive deterioration. J Alzheimers Dis. (2009);16:59–72. doi: 10.3233/JAD-2009-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russo A, Palumbo M, Aliano C, Lempereur L, Scoto G, Renis M. Red wine micronutrients as protective agents in Alzheimer-like induced insult. Life Sci. (2003);72:2369–2379. doi: 10.1016/S0024-3205(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Ho L, Zhao Z, et al. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer's disease. FASEB J. (2006);20:2313–2320. doi: 10.1096/fj.06-6281com. [DOI] [PubMed] [Google Scholar]
- 55.Collins MA, Neafsey EJ, Wang K, Achille NJ, Mitchell RM, Sivaswamy S. Moderate ethanol preconditioning of rat brain cultures engenders neuroprotection against dementia-inducing neuroinflammatory proteins: possible signaling mechanisms. Mol Neurobiol. (2010);41:420–425. doi: 10.1007/s12035-010-8138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang IJ, Jeon YE, Yin XF, et al. Butanol extract of Ecklonia cava prevents production and aggregation of beta-amyloid, and reduces beta-amyloid mediated neuronal death. Food Chem Toxicol. (2011);49:2252–2259. doi: 10.1016/j.fct.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 57.Noor H, Cao P, Raleigh DP. Morin hydrate inhibits amyloid formation by islet amyloid polypeptide and disaggregates amyloid fibers. Protein Sci. (2012);21:373–382. doi: 10.1002/pro.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cornejo A, Jimenez JM, Caballero L, Melo F, Maccioni RB. Fulvic acid inhibits aggregation and promotes disassembly of tau fibrils associated with Alzheimer's disease. J Alzheimers Dis. (2011);27:143–153. doi: 10.3233/JAD-2011-110623. [DOI] [PubMed] [Google Scholar]
- 59.Zhao J, Wang Q, Liang G, Zheng J. Molecular Dynamics Simulations of Low-Ordered Alzheimer beta-Amyloid Oligomers from Dimer to Hexamer on Self-Assembled Monolayers. Langmuir. (2011);27:14876–14887. doi: 10.1021/la2027913. [DOI] [PubMed] [Google Scholar]
- 60.Ding F, LaRocque JJ, Dokholyan NV. Direct observation of protein folding, aggregation, and a prion-like conformational conversion. J Biol Chem. (2005);280:40235–40240. doi: 10.1074/jbc.M506372200. [DOI] [PubMed] [Google Scholar]
- 61.Lührs T, Ritter C, Adrian M. 3D structure of Alzheimer's amyloid-beta(1-42) fibrils. Proc Natl Acad Sci U S A. (2005);102:17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edelstein-keshet L, Spiros A. Exploring the formation of Alzheimer's disease senile plaques in silico. J Theor Biol. (2002);216:301–326. doi: 10.1006/jtbi.2002.2540. [DOI] [PubMed] [Google Scholar]
- 63.Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris) (1997);153:185–192. [PubMed] [Google Scholar]
- 64.Sun AY, Simonyi A, Sun GY. The "French Paradox" and beyond: neuroprotective effects of polyphenols. Free Radic Biol Med. (2002);32:314–318. doi: 10.1016/S0891-5849(01)00803-6. [DOI] [PubMed] [Google Scholar]
- 65.Scarmeas N, Luchsinger JA, Mayeux R, Stern Y. Mediterranean diet and Alzheimer disease mortality. Neurology. (2007);69:1084–1093. doi: 10.1212/01.wnl.0000277320.50685.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]