Abstract
Ubiquitination is a post translational modification which mostly links with proteasome dependent protein degradation. This process has been known to play pivotal roles in the number of biological events including apoptosis, cell signaling, transcription and translation. Although the process of ubiquitination has been studied extensively, the mechanism of polyubiquitination by multi protein E3 ubiquitin ligase, SCF complex remains elusive. In the present study, we identified UbcH5a as a novel stimulating factor for poly-ubiquitination catalyzed by SCFhFBH1 using biochemical fractionations and MALDI-TOF. Moreover, we showed that recombinant UbcH5a and Cdc34 synergistically stimulate SCFhFBH1 catalyzed polyubiquitination in vitro. These data may provide an important cue to understand the mechanism how the SCF complex efficiently polyubiquitinates target substrates. [BMB Reports 2015; 48(1): 25-29]
Keywords: E2 ubiquitin conjugating enzyme, hFBH1, Polyubiquitination, SCF, Ubiquitin
INTRODUCTION
Ubiquitin is a conserved 76 amino acid polypeptide conjugated to a lysine residue of target substrates through isopeptide bond, which is called ubiquitination (1-3). Ubiquitination is a post-translational modification process that consists of consecutive enzymatic steps. First, ubiquitin is activated by E1 ubiquitin activating enzyme (E1), resulting in a thioester linkage between the C-terminal carboxyl group of ubiquitin and the E1 cysteine residue (1). This process requires ATP as an energy source. Then, activated ubiquitin is transferred from E1 to the active site cysteine residue of ubiquitin conjugating enzyme (E2) (2). At the final step, ubiquitin ligase enzyme (E3) recognizes the substrate for ubiquitination and mediates the specific interaction with E2 and substrate. By doing so, the C-terminal glycine of ubiquitin is attached on a lysine residue of the target protein which is mediated by E3 (1-3). In general, polyubiquitinated target proteins are captured and degraded by the proteasome (4). In most organisms, E1, E2 and E3 enzymes exist in a hierarchical way. A single E1 enzyme activates ubiquitin for the entire array of downstream conjugating enzyme (1, 2). All E2s share a conserved core domain consisting of about 150 amino acids. Despite their similarity to one other, different E2s execute distinct biological functions and the reflection of the specificity of E2 may be derived from the specific interaction with E3 (2, 3). Ubiquitination has been demonstrated to be involved in almost all biological processes such as cell cycle progression, signal transduction and transcriptional regulation (5).
Human F-box helicase 1 (hFBH1) is an F-box containing DNA helicase. It has been shown to act as DNA helicase and E3 ubiquitin ligase along with Skp1, Cul1 and Roc1, making an SCF complex (6). In the SCF complex, the interchangeable F-box protein governs the substrate specificity through physical interaction with target substrates. The best characterized F-box proteins are β-TRCP and SKP2. Both of them play roles in the recognition and degradation of IκBα and p27 respectively (7, 8). Recently, it has been reported that hFBH1 is required for the apoptosis induced by DNA damage in response to DNA replication stress and that the level of hFBH1 is regulated by its own F-box domain, indicating hFBH1 ubiquitinates itself (9, 10). However, the target substrates for SCFhFBH1 and its mechanism of actions remain elusive.
In the present study, we identified UbcH5a as a stimulating factor for SCFhFBH1 mediated polyubiquitination using biochemical fractionation followed by MALDI-TOF. Moreover, we demonstrated that UbcH5a and Cdc34 can synergistically catalyze polyubiquitination in vitro. These data may render an important cue to understand the mechanism of ubiquitination by SCF ubiquitin ligase complex.
RESULTS AND DISCUSSION
HeLa nuclear extract stimulates SCFhFBH1 catalyzed polyubiquitination
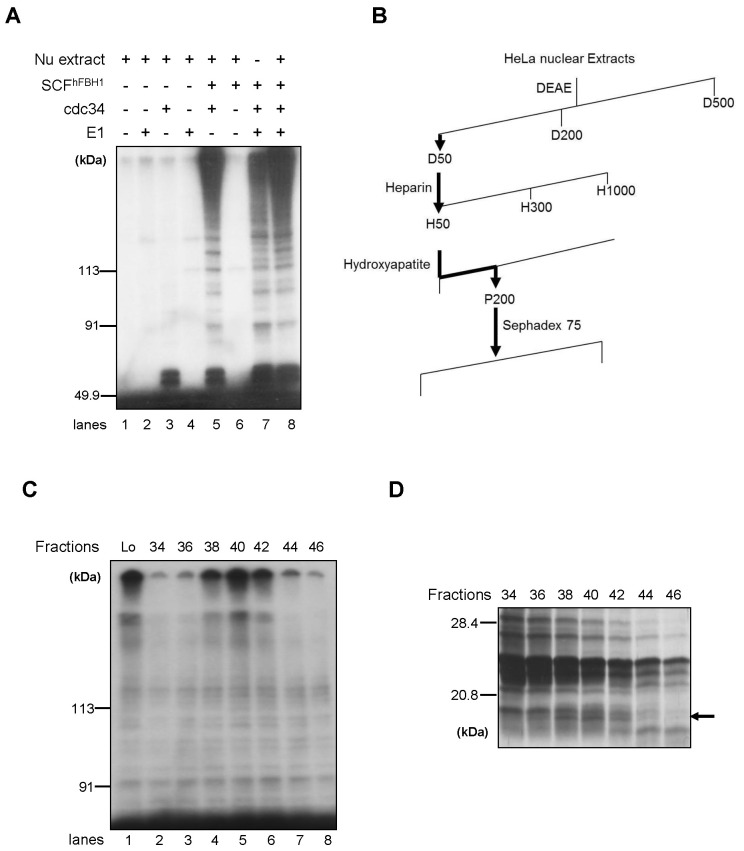
In pursue of polyubiquitination substrates for SCFhFBH1 complex, we developed the in vitro ubiquitination assay using purified SCFhFBH1, ubiquitin activating enzyme E1 and ubiquitin conjugating enzyme Cdc34 which has been well-known as E2 enzyme for SCF complex. It has been previously demonstrated that polyubiquitin is formed in the presence of E1, E2, ubiquitin ligase enzyme (E3) and ATP even in the absence of specific substrates for ubiquitination (11). Since hFBH1 is mainly localized in the nucleus, we assumed that the potential substrate would be also localized in the nucleus. Thus, using HeLa nuclear extract, the in vitro ubiquitination assay was performed. Surprisingly, HeLa nuclear extract stimulated the formation of polyubiquitination (Fig. 1A), compared with the reaction without nuclear extract (Fig. 1A, lane 7 and lane 8). When either E2 or E3 was omitted, the polyubiquitin chain was not formed (lanes 1, 2, 3, 4 and 6), indicating that the polyubiquitin chain formation is dependent on both E2 and E3. Noting that the reaction without E1 produced the polyubiquitin chains as much as the reaction with E1, we suggest that HeLa nuclear extracts contained sufficient E1 enzyme to produce polyubiquitin chains. Next, we decided to purify protein responsible for this stimulation by biochemical fractionations. Starting with HeLa nuclear extracts, we performed consecutive purifications by following the stimulating activity (Fig. 1B). Using biochemical assay with fractions from Sephadex 75, the stimulating activity peaked in fraction number 40 (Fig. 1C). Thus, in order to pinpoint this protein, fractions from Sephadex 75 were analyzed by SDS-PAGE and silver staining (Fig. 1D). Comparing the stimulating activity and protein bands in SDS-PAGE, the stimulating activity was coincident with about 18KDa protein (Fig. 1D).
Fig. 1. Purification of a stimulating factor for SCFhFBH1 catalyzed polyubiquitination from HeLa nuclear extracts. (A) Ubiquitin ligase assay was performed with or without HeLa nuclear extracts as described in Materials and Methods (B) Flowchart of purification procedure. D: DEAE fractions, H: Heparin fractions, P: Hydroxyapatite fractions. (C) Stimulation of polyubiquitin chain formation by Sephadex 75 fractions. Ubiquitin ligase assay was carried out using each Sephadex 75 fractions. The fraction number is shown as the top of each lane. Lo, Load on fraction. (D) The Sephadex 75 fractions were analyzed by SDS-PAGE and silver staining. The fraction number is shown as the top of each lane. Molecular size markers are indicated at the left of the panel.
UbcH5a is identified as the stimulating factor
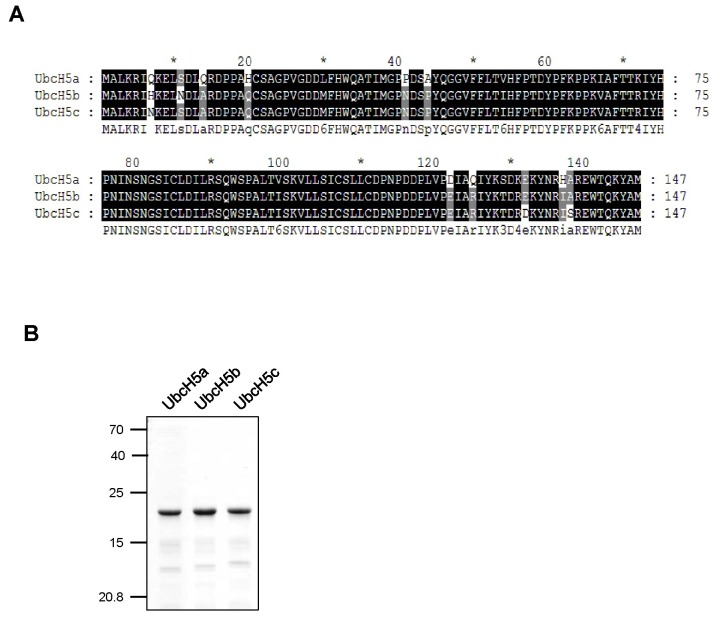
The respective gel band was excised and analyzed by MALDI-TOF to identify 18kDa protein. Two peptides (IYH PNINSNGSICLDILR and VLLSICSLLCDPNPDDPLVPDIAQIYK) corresponding to UbcH5a were determined. UbcH5 belongs to an evolutionally conserved subfamily of E2s involved in the ubiquitination of tumor suppressor p53 and hypoxia inducible transcription factor, HIF1α (12, 13). In mammals, there are 3 UbcH5 isotypes; UbcH5a, UbcH5b and UbcH5c, sharing a highly homology of amino acid sequences (the identity of UbcH5a and UbcH5b; 89%, the identity of UbcH5a and UbcH5c; 88% and the identity of UbcH5b and UbcH5c; 97%) (Fig. 2A). We then wondered whether UbcH5a as well as UbcH5b and UbcH5c can stimulate SCFhFBH1 mediating the formation of polyubiquitin chains. To this end, recombinant UbcH5a, UbcH5b and UbcH5c were purified using E. coli expression system. The recombinant UbcH5a, UbcH5b and UbcH5c used in this study are shown in Fig. 2B.
Fig. 2. Sequence alignment of UbcH5s and purification of recombinant UbcH5s. (A) Amino acids sequences of human UbcH5s were aligned using CLUSTRALW. (B) Purified recombinant UbcH5s were analyzed by SDS-PAGE. Molecular size markers are indicated at the left of the panel.
UbcH5a and UbcH5c but not UbcH5b facilitate SCFhFBH1 catalyzed polyubiquitination
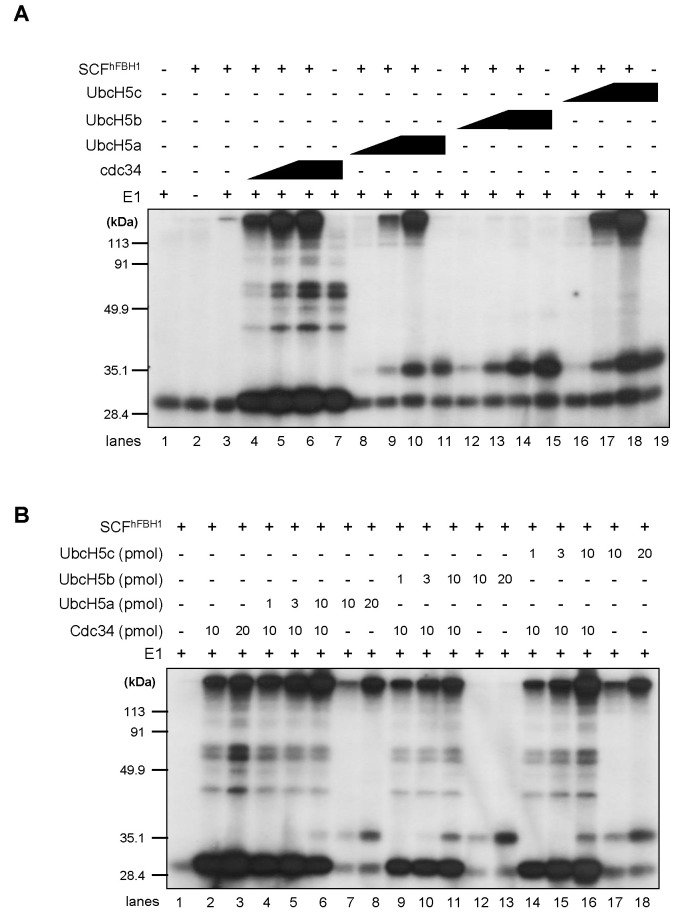
Since UbcH5 is an E2 ubiquitin conjugating enzyme per se, it is possible that sole UbcH5 can promote the formation of polyubiquitin in the absence of Cdc34, a well-known E2 for the SCF complex. To test this, an increasing amount of either Cdc34, UbcH5a, UbcH5b or UbcH5c was incubated with ubiquitin, E1, and SCFhFBH1 in the presence of ATP. As shown in Fig. 3A, UbcH5a and UbcH5c promoted SCFhFBH1 catalyzed polyubiquitination less efficiently when compared to Cdc34, suggesting that UbcH5a and UbcH5c can act as E2 for SCFhFBH1. Although there are only 4 amino acids difference between UbcH5b and UbcH5c, UbcH5b did not promote the polyubiquitination (Fig. 3A, lanes 12-15). Note that different E2 showed different patterns of mono- and di-ubiquitination. As UbcH5a initially was identified as a stimulating factor for SCFhFBH1 catalyzed polyubiquitination, we tested whether UbcH5s could stimulate the polyubiquitination or not. For this purpose, purified UbcH5a, UbcH5b or UbcH5c was additionally added to the reaction containing Ubiquitin, E1, Cdc34 and SCFhFBH1. When increasing amounts of UbcH5a or UbcH5c were added, more polyubiquitin chains were formed compared with the reaction with either the same amounts of UbcH5 or Cdc34 (Fig. 3B, lanes 2-8 and lanes 14-18). However, similar to the previous result, UbcH5b did not show a stimulating activity even in the presence of Cdc34 (Fig. 3B, lanes 9-13). It should be noted that the formation of poly-ubiquitin chains was totally dependent on SCFhFBH1.
Fig. 3. The formation of ubiquitin polymers by SCFhFBH1 was catalyzed by either UbcH5a or UbcH5c but not by UbcH5b. (A) The ubiquitin ligase assay was performed with various E2 enzymes (Cdc34, UbcH5a, UbcH5b or UbcH5c). (B) The ubiquitin ligase assay was performed under addition of indicated E2 enzyme.
UbcH5a and cdc34 synergistically stimulates SCFhFBH1 catalyzed polyubiquitination
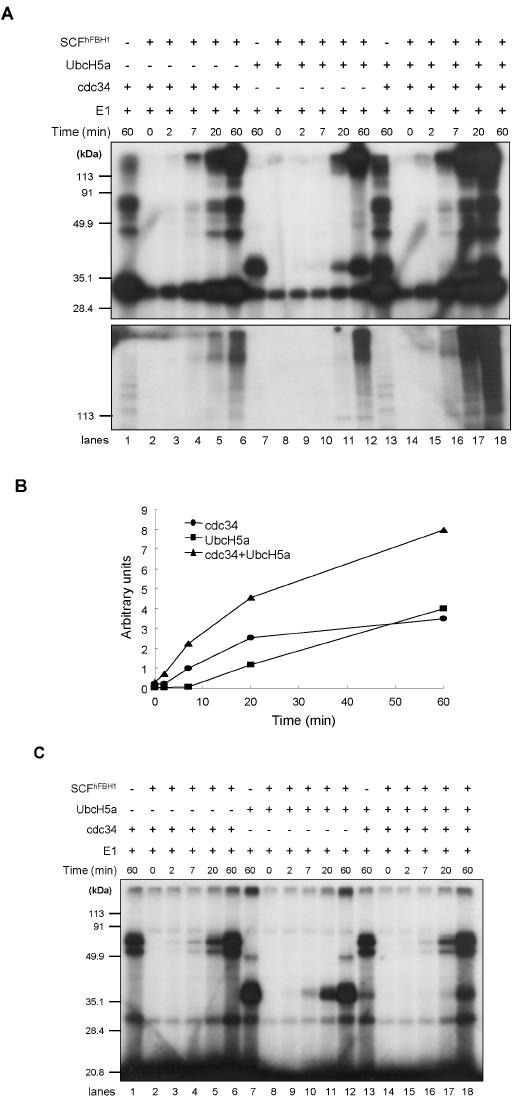
We carried out the time course experiments to observe the effect of UbcH5a in a time dependent manner. Since we did not observe any differences between UbcH5a and UbcH5c, UbcH5a was tested. The rate of poly-ubiquitin chain formation by UbcH5a was slow with a lag period, whereas that by Cdc34 was rapid and saturated at 20 minutes (Fig. 4A and 4B). When UbcH5a and Cdc34 were both added, the poly-ubiquitin formation proceeded with a short lag period (less than 2 minutes), and was still actively catalyzed at 60 minutes. The levels of ubiquitin polymers produced by both UbcH5a and cdc34 were significantly increased, implying that UbcH5a and cdc34 synergistically act on the formation of poly-ubiquitin chains (Fig. 4A and 4B). There is a literature describing that UbcH5a catalyzes non K48-linked poly-ubiquitination together with HECT E3 (14). Therefore, the question was raised if synergistically catalyzed ubiquitin polymers could be non K48-linked polyubiquitination. To test this possibility, the time course experiment was performed using mutant Ubiquitin where lysine 48 was mutated to arginine (K48R mutant). As a result, the formation of poly-ubiquitin chains was greatly reduced with the use of K48R Ubiquitin (Fig. 4C), even though UbcH5 and cdc34 were charged by K48R Ubiquitin. This result suggests that ubiquitin polymers synergistically produced by UbcH5a and cdc34 are lysine 48 linked poly-ubiquitination. Interestingly, our results somewhat support a previous report that a single ubiquitin is initially rapidly transferred by UbcH5 to IκBα and then Cdc34 catalyzes SCFβTrCp2 catalyzed poly-ubiquitination of IKBα (15). Ryu and his colleagues showed E2 dependent target specificity and processivity of p27 ubiquitination (16). The difference between previous reports and our results may be caused by the window we observed here that the formation of polyubiquitin chains was neither mono- nor di-ubiquitination. In addition, we utilized an in vitro reconstituted system to examine the SCF catalyzed polyubiquitination. Considering the amino acid sequences and the structural similarity of UbcH5s, the other interesting finding was that UbcH5a and UbcH5c can stimulate polyubiquitination but UbcH5b did not. It would be intriguing to know if UbcH5b/c chimeric mutant were generated and examined to stimulate polyubiquitin formation or not. This iterative mutation experiments could render which amino acid is critical to differentiate the specificity among UbcH5a, b and c. In summary, we present the synergistic effect of two E2 enzymes in the formation of polyubiquitin chains. These results may provide a mechanistic cue to understand the process of SCF catalyzed polyubiquitination.
Fig. 4. UbcH5a stimulates SCFhFBH1 mediated polyubiquitination. (A) Ubiquitin ligase assay was performed in different incubation time periods. The top and bottom panels were analyzed by 13.5% and 7.5% SDS-PAGE, respectively. (B) The quantitation of the results is obtained in (A). The intensity of high molecular ladders was measured using a Phosphorimager. (C) The ubiquitin ligase assay was performed using K48R ubiquitin.
MATERIALS AND METHODS
Purification of stimulating factor of SCFhFBH1 catalyzed polyubiquitination
HeLa nuclear extract was prepared as described previously (17). Nuclear extracts were dialyzed with T50. Dialyzed extract (75 ml, 365 mg) was loaded onto DEAE dextrose (50 ml). Flow-through fractions (85 ml, 97 mg) were fractionated over heparin sepharose (15 ml) which was preequilibrated with Buffer T50 (25 mM Tris-HCl pH 7.5, 50 mM NaCl, 10% glycerol, 1 mM DTT and 1 mM EDTA). The flow-through fraction of heparin sepharose (90 ml, 54 mg) was dialyzed with Buffer H (50 mM HEPES-KOH pH6.0, 50 mM NaCl, 10% glycerol, 1 mM DTT) and resulting fractions were subjected onto a Hydroxyapatite column (2 ml). Bound proteins were eluted with buffer p200 (200 mM Potassium phosphate pH6.0, 50 mM NaCl, 10% glycerol, 1 mM DTT) (0.5 ml, 4 mg). Eluted proteins were loaded onto Sephadex 75 HR 10/30 (GE). The peak activity was exhibited by approximately 12 ml of eluted fraction. Each fraction was precipitated by TCA. Precipitants were analyzed by SDS-PAGE and silver staining.
Purification of recombinant UbcH5s
The full length cDNAs of UbcH5s were cloned by PCR using HeLa cDNA library. Amplified cDNA was subcloned to pET28 (Life Technologies, NY, USA). All DNA sequences of UbcH5s were confirmed by sequencing. E. coli BL21 (DE3) harboring pET28-UbcH5s (0.5 L) were grown at 37℃ and induced with IPTG for 2 hours. The cell pellets were resuspended in buffer containing 25 mM Tris-HCl pH 7.5, 200 mM NaCl, 10% glycerol 5 mM imidazole and disrupted by sonication (3 cycles of 30 second pulses and a 2 minutes cooling interval). The extracts were cleared by centrifugation at 40,000 rpm for 20 minutes in a Beckman 70 Ti rotor and the supernatant was loaded onto 1 ml of Ni-chelating Hitrap (GE, PA, USA). After extensive washing with buffer containing 50 mM imidazole, the column was eluted with buffer containing 200 mM imidazole. The peak fractions were pooled and dialyzed with buffer containing 25 mM Tris-HCl pH7.5, 100 mM NaCl, 20% glycerol, 1 mM DTT, 1 mM EDTA and protease inhibitors.
Ubiquitin ligation assay
Recombinant E1, Cdc34, SCFhFBH1, and pk-Ub proteins were isolated as previously described (6). Briefly, pk-Ub (7 ug) was phosphorylated in a reaction mixture containing 20 mM Tris-HCl pH 7.4, 12 mM MgCl2, 2 mM NaF, 50 mM NaCl, 25 uM ATP, 5 uCi of [r-32P] ATP, 0.1 mg/ml BSA, and 1 U of cAMP kinase (Sigma). To inactivate the reaction, the mixture was boiled at 70℃ for 5 minutes. The reaction mixture (30 μl) contained 50 mM Tris-HCl pH7.4, 5 mM MgCl2, 2 mM NaF, 10 nM okadaic acid, 2 mM ATP, 0.6 mM DTT, 5 μg of 32P-Ub, E1 (2 pmol), Cdc34 (10 pmol) were incubated at 37℃ for 60 minutes, added with 10 μl of 5XSDS loading buffer and boiled for 3 minutes prior to loading to SDS-PAGE for analysis. The amounts of polyubiquitin chains larger than E2 were measured using a Phosphorimager (BAS, FUJIFILM, Tokyo, Japan).
MALDI-TOF/MS
Corresponding silver stained bands were excised and analyzed by MALDI-TOF/MS. Mass spectrometry (MS) was performed on Yonsei proteome research center (YPRC, Korea) with a MALDI-TOF/TOF mass spectrometer (4800 ABSciex, MA, USA). Amino acid sequences of two peptides corresponding UbcH5a were determined (IYHPNINSNGSICLDILR and VLLSICSLLCDPNPDDPLVPDIAQIYK).
Acknowledgments
We thank S. Y. Cho and K. H. Bae for the critical reading of the manuscript. This work was supported by KRIBB and research grants from the National Research Foundation of Korea (2011-0008842 and NRF- 2011-0028172).
References
- 1.Schulman BA, Harper JW. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat Rev Mol Cell Biol. (2009);10:319–331. doi: 10.1038/nrm2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye Y, Rape M. Building ubiquitin chains: E2 enzymes at work. Nat Rev Mol Cell Biol. (2009);10:755–764. doi: 10.1038/nrm2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. (2009);78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 4.Chau V, Tobias JW, Bachmair A, et al. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. (1989);243:1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- 5.Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. (2012);81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Kim J, Kim DH, et al. SCFhFBH1 can act as helicase and E3 ubiquitin ligase. Nucleic Acids Res. (2004);32:2287–2297. doi: 10.1093/nar/gkh534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaron A, Hatzubai A, Davis M, et al. Identification of the receptor component of the IkappaBalpha-ubiquitin ligase. Nature. (1998);396:590–594. doi: 10.1038/25159. [DOI] [PubMed] [Google Scholar]
- 8.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. (1999);1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 9.Jeong YT, Rossi M, Cermak L, et al. FBH1 promotes DNA double-strand breakage and apoptosis in response to DNA replication stress. J Cell Biol. (2013);200:141–149. doi: 10.1083/jcb.201209002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiolo I, Saponaro M, Baryshnikova A, Kim JH, Seo YS, Liberi G. The human F-Box DNA helicase FBH1 faces Saccharomyces cerevisiae Srs2 and postreplication repair pathway roles. Mol Cell Biol. (2007);27:7439–7450. doi: 10.1128/MCB.00963-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan P, Fuchs SY, Chen A, et al. Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of I kappa B alpha. Mol Cell. (1999);3:527–533. doi: 10.1016/S1097-2765(00)80481-5. [DOI] [PubMed] [Google Scholar]
- 12.Jung JH, Bae S, Lee JY, et al. E3 ubiquitin ligase Hades negatively regulates the exonuclear function of p53. Cell Death Differ. (2011);18:1865–1875. doi: 10.1038/cdd.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paltoglou S, Roberts BJ. HIF-1alpha and EPAS ubiquitination mediated by the VHL tumour suppressor involves flexibility in the ubiquitination mechanism, similar to other RING E3 ligases. Oncogene. (2007);26:604–609. doi: 10.1038/sj.onc.1209818. [DOI] [PubMed] [Google Scholar]
- 14.Mastrandrea LD, You J, Niles EG, Pickart CM. E2/E3-mediated assembly of lysine 29-linked polyubiquitin chains. J Biol Chem. (1999);274:27299–27306. doi: 10.1074/jbc.274.38.27299. [DOI] [PubMed] [Google Scholar]
- 15.Wu K, Kovacev J, Pan ZQ. Priming and extending: a UbcH5/Cdc34 E2 handoff mechanism for polyubiquitination on a SCF substrate. Mol Cell. (2010);37:784–796. doi: 10.1016/j.molcel.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu KS, Choi YS, Ko J, et al. Direct characterization of E2-dependent target specificity and processivity using an artificial p27-linker-E2 ubiquitination system. BMB Rep. (2008);41:852–857. doi: 10.5483/BMBRep.2008.41.12.852. [DOI] [PubMed] [Google Scholar]
- 17.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. (1983);11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]