Abstract
Despite drug formulation improving circulation times and targeting, efficacy is stymied by inadequate penetration into and retention within target tissues. This review highlights the barriers restricting delivery to the connective tissue interstitium, lymphatics, and lymph nodes as well as advances in engineering drug carriers to overcome these delivery challenges. Three-dimensional tissue physiology is discussed in the context of providing material design principles for delivery to these tissues; in particular the influence of interstitial and lymphatic flows as well as differential permeabilities of the blood and lymphatic capillaries. Key examples of materials with different characteristics developed to overcome these transport barriers are discussed as well as potential areas for further development.
Keywords: drug delivery, interstitium, lymphatics, lymph node
Introduction
Drug efficacy after administration is restricted by its capacity to appreciably accumulate at the desired site of action at bioactive doses in order to mediate its effect(s). The concept of drug targeting to enhance therapeutic benefit and minimize toxicities largely brings to mind the directed retention of drugs or imaging agents at and within cells and tissues of interest using affinity-based molecular interactions or, in the case of solid tumors, exploiting the leaky vasculature to facilitate their preferential accumulation. Innovation in carrier design has also improved the penetration of delivered agent deeply to within healthy and diseased tissues, as well as to tissue targets of emerging interest for their diagnostic and therapeutic utility – lymphatic vessels and the lymph nodes to which they drain.
This review describes the current state of interstitial-, lymphatic-, and lymph node-targeted drug delivery and gives perspective for their future directions. Its focus is on delivery strategy and carrier design and not related applications including cancer therapy [1], immunomodulation [2-4], or regenerative medicine [5], which have been reviewed in depth elsewhere. We also do not discuss sustained release formulations [6,7] or cell transplantation approaches [8], which clearly have significant promise, nor the significant delivery barrier of the mucosa. We discuss the emerging concepts pertinent to drug delivery to these tissues and design strategies that have facilitated advances in effective targeting.
The interstitium, lymphatics, and lymph nodes as targets for delivery
The extravascular fluid, solute, matrix, and cellular environment within tissues constitutes the interstitium. Interstitial targets therefore include stromal, cancer, resident immune, and other cells as well as components of the protein- and proteoglycan-rich extracellular matrix (ECM). Accordingly, the interstitium is a central target in a plethora of diseases, including cancer, arthritis, and chronic wounds, amongst others.
The lymphatic vasculature transports interstitial fluid and solutes to lymph nodes in a unidirectional fashion, regulating tissue fluid balance, immune cell homing, and lipid transport from the gut. Both initial lymphatics and the larger collecting lymphatic vessels to which they drain are comprised of lymphatic endothelial cells connected to the extracellular matrix by anchoring filaments and underlying smooth muscle, respectively. Lymphedema, which is the loss of lymphatic function that results in tissue fluid accumulation and swelling, represents an underlying pathology in a variety of disease states. Restoration or prevention of the loss of lymphatic function is of major interest for management of malignancies, particularly of the breast, as well as for a variety of infectious diseases. In particular, therapeutic lymphangiogenesis to increase lymphatic function has been explored in transplantation applications since lymphatic transport also contributes to immune regulation [9*,10].
Lymph nodes are lymphoid tissues comprised primarily of immune cells with a supporting stroma whose primary function is to orchestrate cell-cell contact in the mounting of adaptive immune reactions. They are thus central targets in immunotherapy applications, such as vaccines [11] or immune suppressive regimens [12]. Lymph node targeting may also have utility in improving the treatment of B and T cell malignancies. The lymph nodes draining solid tumors, referred to as sentinel lymph nodes, have well-appreciated diagnostic utility in staging disease since they are a common site of metastasis. Sentinel lymph nodes are also considered to be functionally involved in directing antitumor immune response and suppression [9*,13*]. Consequently, both chemotherapeutic [14] and immunotherapeutic [15**] interventions targeting lymph nodes have been explored.
Drug delivery approaches and design for interstitial-, lymphatic-, and lymph node-targeting
We now consider the material design to achieve targeting of the tissues described above, focusing on the relevant tissue physiology as a guide for the engineering of the therapeutic or imaging agent formulation. We contemplate barriers to delivery including the extracellular matrix after injection in connective tissue such as skin or extravasation from the vasculature, uptake by cells resident in the interstitium, as well as the subcapsular sinus in the lymph node. Delivery challenges such as vascular permeability effects that divert agent from its intended target tissue are also discussed. Materials with different design principles and characteristics are being developed to overcome these transport barriers.
The interstitium as a barrier and target
Delivery of therapeutic agents to the interstitium can be achieved by either direct injection in connective tissues or after extravasation from the vasculature post intravenous infusion. The extravasation process clearly presents a significant barrier to delivery and has been well-reviewed elsewhere [16]. We will instead primarily focus on penetration of the agent into the interstitium, which is restricted by the gel-like ECM that is composed of fluid, solutes, fibrillar proteins, and proteoglycans. Accordingly, macromolecular size, shape, and charge are major determinants of transport characteristics through both healthy and diseased tissues.
Quantification of molecular transport within living tissues has provided significant and nuanced insight into interstitial solute transport after direct injection [17,18]. For example, in mouse tail skin, transport of uncharged solutes larger than 70 kDa is hampered by the ECM (Figure 1), whereas below this molecular weight threshold, transport is dominated by size exclusion principles [17]. However, this behavior is likely altered between tissue types as a result of differences in ECM pore size [19] and composition [20]. Molecular rigidity and globular structure also restrict interstitial penetration (Figure 1), since size-matched nanospheres and proteins demonstrate reduced measured rates of interstitial convection relative to more flexible chain-like dextrans [17]. Electrostatic repulsion is also a potential mechanism reducing hindrance [17] by the negatively charged ECM [21], though computational approaches suggest this may actually restrict diffusion [22]. Importantly, following onto seminal work by Jain and colleagues measuring the diffusivity of albumin and immunoglobulin G in vivo [20,23], two-photon fluorescence correlation microscopy distinguished that transport within tumors is two-phase in nature, comprised namely by fast and slow diffusion that likely result from the aqueous and viscous components of the ECM [18]. Modification of the tumor ECM using hyaluronidase or collagenase reduced the extent of rapid and slow diffusion, respectively [18], indicating distinct functional roles for each ECM component on restricting intratumoral solute transport and antitumor activity of therapeutic drugs [24-26]. Furthermore, slow diffusion appears more pronounced with increasing molecular size [18]. Formulations that are somewhat stealth, so as to not adsorb to the interstitial biopolymer network [27], therefore have the potential for enhanced penetration through the interstitium.
Figure 1.
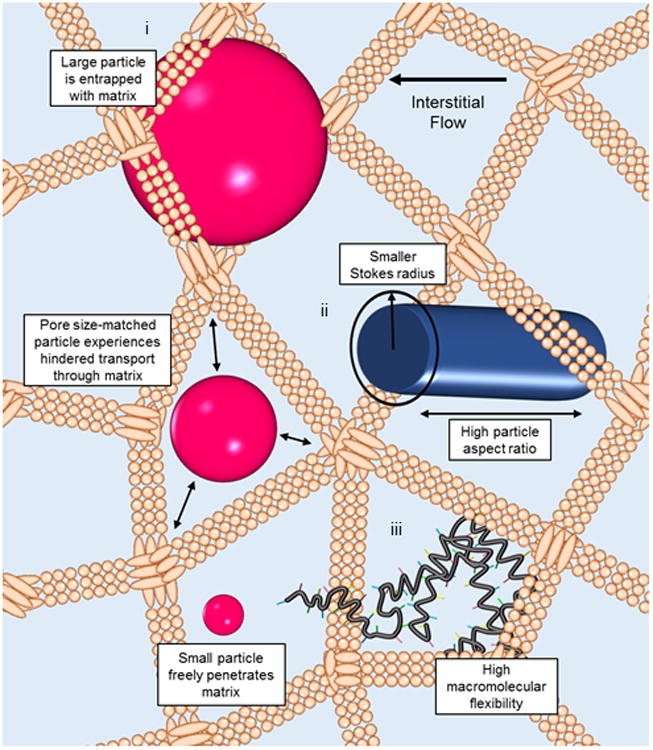
Within the interstitium under the influence of slow interstitial fluid flow, macromolecular transport is restricted by the extracellular matrix (ECM). i) Large (>70 kDa) but not small molecular species become entrapped by the gel-like ECM [17]. At similar hydrodynamic radii, penetration through the ECM is increased by ii) high particle aspect ratios, which correspond to a smaller Stokes radius along one carrier dimension [31], and iii) flexibility of the macromolecular assembly, such as that exhibited by biopolymers [17].
Since intravenous infusion is the most common delivery strategy for anticancer therapy or imaging applications, a challenge is optimizing transvascular transport, tumor penetration, and agent circulation time, for which carrier design can have opposing effects on efficiency of delivery (Figure 2A). For example, anionic charge improves transvascular transport in tumors [28] while neutral charge is ideal for elongating circulation times [29]. Furthermore, whereas increasing size prolongs agent circulation time [30], transvascular transport [31] and interstitial penetration [30] are dramatically attenuated. To overcome the opposing effects of carrier size on intratumoral delivery and penetration after intravenous infusion, a multistage delivery system (Figure 2B) in which 100 nm nanoparticles that demonstrate improved circulation times [30] “shrink” to 10 nm quantum dots after extravasation via the proteolytic activity of matrix metalloproteinase within the tumor microenvironment has been proposed [32]. This system was able to overcome perivascular entrapment by the dense collagenous tumor ECM to facilitate deep interstitial tumor penetration. Since increased aspect ratios not only improve particle circulation times [33] but also increase nanorod diffusion through pores and porous media in vitro (Figure 1), another scheme utilized nanorods to improve intratumoral penetration in vivo after intravenous infusion relative to nanospheres of similar hydrodynamic radii and plasma half-lives [31].
Figure 2.
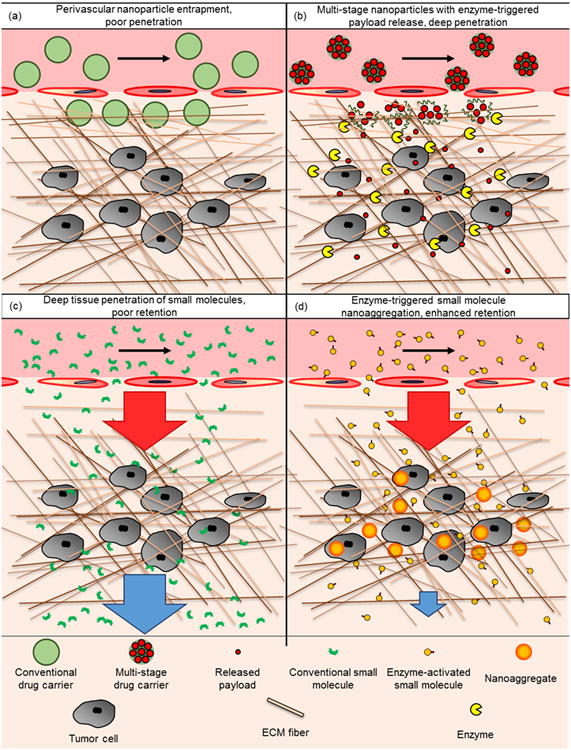
Multistage carrier design innovations overcome the opposing influence of macromolecular/particle size on interstitial penetration and retention. (A) Nanoformulation sizes (∼100 nm in diameter) optimum for intratumoral accumulation by the enhanced permeability and retention effect become trapped perivascularly as the result of remodeling of the tumor interstitium extracellular matrix, resulting in poor tumor penetration of encapsulated drug. (B) Release of payloads of smaller hydrodynamic radii from multistage delivery systems as the result of matrix metalloproteinase 2 activity within the tumor microenvironment significantly improves tumor penetration [32]. (C) Conventional small molecules penetrate rapidly and efficiently into the tumor interstitium but are poorly retained. (D) Engineered small molecules that aggregate as a result of caspase-3 activity into larger nanoaggregates are retained specifically within the tumor [35**]. Arrow orientation and size represent direction and magnitude of molecular flux. Red and blue represent molecular transport inward and outward from tumor, respectively.
Clearance from the interstitium also presents a major delivery challenge that diminishes efficacy by reducing agent residence at the site of action over the necessary timescales to achieve its desired effect(s) (Figure 2C). Thus diffusion and convection as a benefit versus detriment to interstitial delivery must be balanced. Perrault and Chan [34] proposed an elegant strategy to overcome this via serial intravenous infusion of poly(ethylene glycol) (PEG)-grafted nanoparticles bearing biotin followed by streptavidin-conjugated molecular contrast agent, both of which are highly diffusive and permeable to the tumor vasculature, thus facilitating rapid and extensive penetration within the solid tumor ECM. Upon colocalization, assembly occurs, favorably influencing retention within the tumor [34]. This approach leads to sustained high levels of intratumoral contrast agent within three hours post infusion that are on par with those seen 24 hours post infusion of the assembled macromolecular complex [34]. Another innovative approach developed to reduce agent clearance in a target-specific manner harnesses a novel nanoaggregation chemistry that selectively increases intratumoral retention in vivo to monitor apoptosis resulting from chemotherapy [35**]. These engineered molecules undergo macrocyclization in the presence of caspase, resulting in the in situ formation of 170 nm nanoaggregates [35**] (Figure 2D).
Lymphatic targeting
Due to the physiology of the lymphatic system, typical intravenously administered molecules and nano-formulations do not result in appreciable delivery to lymphatics. A considerable portion of administered agent accumulates instead in the lung, kidney, and liver. Even in the case of tumors where accumulation can be significant by virtue of the leaky tumor vasculature, agent penetration is limited to the perivascular space [30]. As a result, direct injection techniques are typically utilized for lymphatic targeted delivery strategies [15**,36]. Within the interstitium, fluid flow, which always drains to a lower pressure, is directed towards the lymphatics by virtue of their lower pressure relative to the blood and interstitial tissue. The potential for infused solutes to be directed towards and drained through the loose junctions of initial lymphatics after injection within connective tissues is thus dependent on their capacity to be subjected to this convective transport and are therefore restricted by the barriers of interstitial penetration as discussed above.
Several key aspects of connective tissue physiology influence the feasibility of lymphatic and lymph delivery despite the focusing influence of interstitial flow on directing injected agent towards draining lymphatics. Since non-fenestrated blood capillaries are permeable to molecular species less than 5 nm in hydrodynamic diameter [37], they are rapidly cleared after injection [38], reducing their potential for lymphatic exposure. Molecular species roughly 5 nm or larger, on the other hand, are largely impermeable to the blood capillaries within healthy tissues [38] and can therefore only be cleared by lymphatic drainage mechanisms. Furthermore, since the pore size of the ECM limits the movement of macromolecular species through the interstitium, infused nanoformulations ∼100 nm or larger become entrapped [36] and are thus poorly suited to lymphatic-targeted drug delivery applications. Lymphatic targeting is therefore optimized by agents or formulations roughly tens of nanometers in hydrodynamic diameter, for example synthetic formulations such as ∼30 nm Pluronic-stabilized poly(propylene sulfide) nanoparticles [36]. Lymphatic partitioning of drug after subcutaneous infusion can also be improved by PEG grafting, thereby increasing drug hydrodynamic radius. With increasing PEG molecular weight, grafted polylysine dendrimers demonstrate decreased absorption into the blood and a significant increase in dendrimer recovery in lymph [38]. Moreover, incorporation into PEGylated polylysine dendrimers can additionally increase the fraction of administered doxorubicin dose with lymph exposure from roughly zero for the free drug to 40% of injected dose [39]. PEGylation also increases passive lymph node targeting of cationic liposome-formulated vaccines [40].
In addition to achieving lymph accumulation via transluminal transport, delivery of therapeutic or imaging agents directly to the lymphatic endothelium is of interest. Directed targeting has been explored by affinity interactions facilitated via antibody-mediated recognition of lymphatic endothelial cell markers Lyve-1 [41] and podoplanin [42]. Emerging evidence supports the concept that lymphatic endothelial cells actively scavenge protein antigens in vivo [43,44**] in both inflammatory [43] and steady-state conditions [44**] (Figure 3A). The ramifications of this activity have largely been explored for its immunological consequences [43,44**], however, these data and in vitro observations of synthetic nanoparticle uptake by lymphatic endothelial cells [44**] support the concept that endogenous uptake mechanisms may be exploited for delivery of drugs to lymphatic endothelial cells. If and the extent to which solute scavenging by lymphatic endothelial cells is a detriment to penetration into lymph and delivery to lymph nodes and whether this can be overcome using formulation design has yet to be determined.
Figure 3.
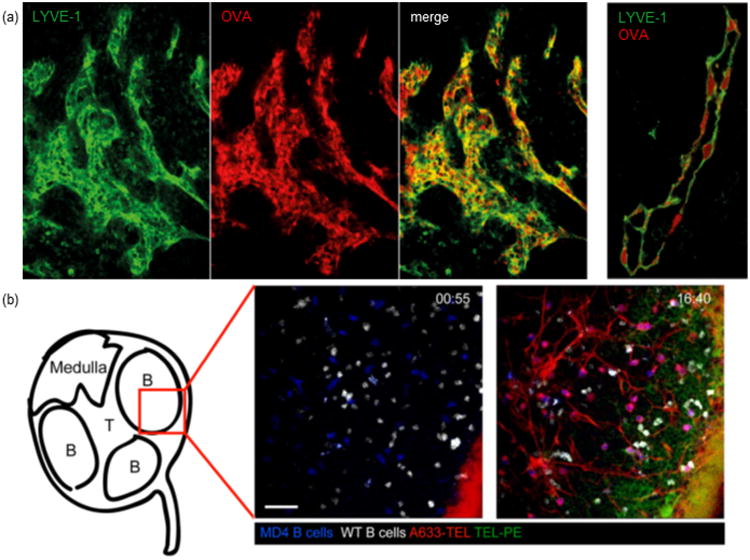
Emerging principles of in vivo molecular transport in the lymphatic system. (A) Lymphatic endothelial cells scavenge proteins in vivo. Left, brachial mouse lymph node showing lymphatic endothelial cell (Lyve-1, green)-associated AF647-conjugated ovalbumin (OVA, red). Right, intracellular localization of OVA (red) within lymphatic endothelial cells (green) of a Lyve-1+ lymphatic vessel. Reproduced with permission from [44**]. (B) Proteins are distributed within lymph node B cell follicles after injection at different rates and locations as a function of molecular size. Intravital images of the distribution of 14 kDa (A633-conjugated turkey egg lysozyme (TEL), red) and ∼250 kDa (phycoerytherin-conjugated TEL, green) protein-conjugates in a B follicle after footpad injection (white, wildtype B cells; blue, MD4 transgenic B cells). Top right corner indicates time post injection (min:sec). Scale bar, 30 μm. Reproduced with permission from [53].
Lymph node targeting
Direct lymph node injection has been proposed for transplantation applications [45*] and shown promise for immunotherapy [46], but requires laborious and invasive surgical manipulations impractical for most drug delivery scenarios. Since lymph nodes are bathed in lymph drained from the periphery in a steady state fashion, design principles optimizing drug targeting for penetration through the interstitium and lymphatics to reach lymph using more conventional and less intrusive drug administration techniques, such as subcutaneous injection, are attractive for lymph node delivery (Figure 4). Indeed, since interstitial flow always follows a decreasing pressure gradient toward the lymphatic capillary and subsequently to the draining lymph node, such an approach for targeting the lymph node after direct injection in the periphery is highly facile yet robust: the particles must mainly be small enough so as to not be entrapped by the tissue interstitium, yet large enough not to be cleared into the circulation after injection. This corresponds to roughly 10-100 nm in hydrodynamic radius and is optimum at an ideal size of 20-40 nm [36,38,39,47]. This targeted delivery strategy is also advantageous by enabling dose sparing to help minimize the potential for undesirable off-target effects and/or toxicities [48**]. This principle has been explored for lymph node targeting using both synthetic materials, including nanoformulations comprised of Pluronic-stablized poly(propylene sulfide) [36,49], polymer dendrimers [38,39] and poly(γ-glutamic acid)-l-phenylalanine ethylester [47], as well as biologically derived biopolymers, such as hyaluronic acid [50] and albumin [48**]. Such approaches have been used to improve lymph node delivery and bioactivity of co-formulated small molecules [15**,39], oligonucleotides [15**,48**,51], as well as proteins and peptides [11,48**,52]. Of particular interest is an innovative, recently described “albumin hitchhiking” approach based on adjuvant and antigen conjugation to albumin-binding lipids to improve response to vaccination by coopting albumin drainage out of the interstitium to lymph nodes [48**]. Additionally, we recently reported the benefit of toll-like receptor ligand formulation into 30 nm polypropylene sulfide) nanoparticles in improving drug delivery to sentinel (tumor-draining) lymph nodes for cancer immunotherapy [15**].
Figure 4.
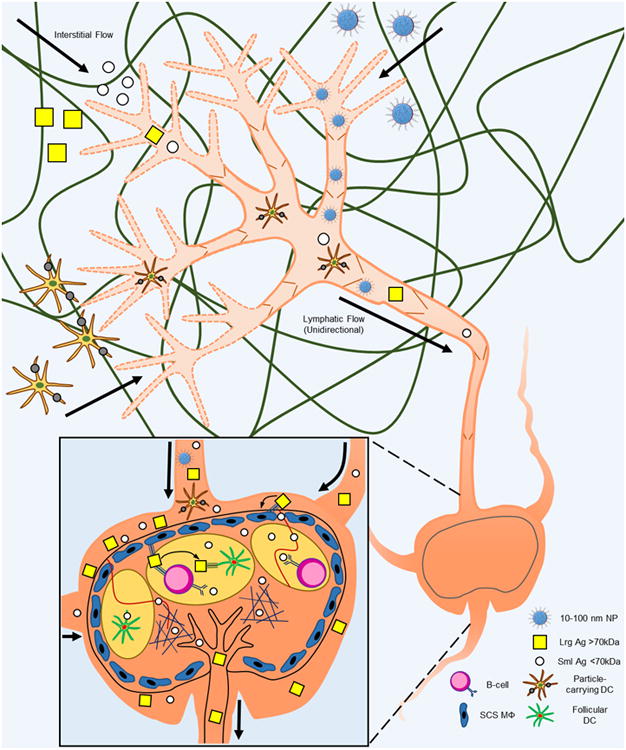
Principles of lymph node targeting including the major transport barriers of the interstitium and the lymph node subcapsular sinus. Antigen presenting cells such as dendritic cells take up large particles (particle-carrying DC) and actively migrate to lymph nodes. Synthetic carriers such as nanoparticles (NP) 10-100 nm in diameter (optimum for penetration of the interstitial extracellular matrix) as well as protein antigens are carried from the interstitium by interstitial and lymphatic flows to draining lymph nodes. Lymph-borne solutes are distributed within lymph nodes in a size-dependent fashion. For example, large protein antigens (Lrg Ag) are taken up by subcapsular macrophages (SCS MΦ) and transferred to follicular dendritic cells (DC) either directly or as mediated by B cells. Small protein antigens, however, are efficiently taken up by follicular conduits and directly transported to B cell follicles or the T cell cortex. Thick arrows, direction of flow. Thin arrows, direction of antigen transfer to and between lymph node-resident cells.
Once arriving in the lymph node, lymph-borne solutes are filtered at the subcapsular sinus depending on size [53] and opsonization [54] (Figure 4). A fraction of lymph solutes will penetrate into the lymph node follicle and cortex; the remainder will leave via the efferent lymph to reach the blood [49]. Larger sinus-penetrating molecules or particles are taken up by subcapsular macrophages and transferred to B cells [53-55], whereas smaller molecules (less than roughly 3-5 nm in diameter) can penetrate more deeply into the lymph node to enter the B- or T-cell zones through conduits [53] to be sampled directly by B cells [53] or taken up by dendritic cells [56]. Drug and macromolecular formulation therefore have the potential to play a significant role in regulating the distribution within the lymph node as a function of macromolecular size [36] and complement deposition [52].
The complex siphoning taking place in the interstitium and lymph node subcaspsular sinus plays a critical role in the rate, extent, and distribution of agent accumulation within lymph nodes that may influence drug efficacy, required dose, and associated toxicities. For example, Roozendaal et al. [53] found that when administered simultaneously in the periphery of immunized animals, small molecules are taken up by lymph node B cells more rapidly than larger ones (Figure 3B). Though faster, small molecule uptake by lymph node B cells is not as efficient as that of larger molecules [53]. Small molecules also penetrate more deeply into lymph nodes as the result of enhanced shunting through lymph node conduits relative to larger molecules [53]. These data support the concept that despite nanoformulations of an intermediate nano-scale size being ideal for lymphatic partitioning after injection in the periphery, small molecules have increased potential to accumulate deeply within the lymph node. This model might be utilized to optimally target lymph node-resident cells via controlled release strategies of co-formulated drug such as multi-stage release strategies that improve intratumoral drug penetration [32].
In addition to lymph node drug delivery guided by passive convection, several successful schemes rely on exploiting mechanisms of endogenous cell-mediated trafficking. This principle is based on the activity of antigen presenting cells, which reside in most tissues performing immunological surveillance and migrate via lymphatics in homeostatic and inflamed conditions to lymph nodes to present antigen to resident T cells [57]. Conventional as well as more recent engineered vaccines have relied on this active trafficking mediated by antigen presenting cells after uptake of administered formulation in peripheral tissues. The utility of particle size [58] and shape [59], functionalization with dendritic cell binding moieties including mannan [60] or antibodies against C-type lectin receptors such as DEC-205 (CD205) [61] and DC-SIGN (CD209) [62], as well as attraction by delivery of chemotactic stimuli [63], to enhance uptake by these migratory cells has been demonstrated. The timing, extent, and tissue distribution of lymph node delivery facilitated by active cell-mediated trafficking is significantly altered relative to passive delivery approaches [58] and may influence potency and quality of therapeutic response.
Conclusion
Consideration of three-dimensional physiology and the corresponding fluid mechanical microenvironment within interstitial, lymphatic, and lymph node tissues provides a means to improve the design of drug formulations for enhanced delivery. The full potential of existing and even orphaned drugs may be improved by reformulation to optimize delivery to their target site of action, which in the case of lymphatics and lymph nodes as well as the tumor interstitium, are not efficiently dosed with conventional drug formulations and administration regimens. Moreover, effective targeting can spare dosage and reduce potential toxicity. Overall, engineering design to overcome several key transport barriers has addressed many of the challenges in drug delivery to improve potency and therapeutic efficacy.
Highlights.
Multistage delivery systems for interstitial penetration and retention in tumors.
Drug focusing to lympahtics and lymph nodes via interstitial and lymphatic flows.
Lymph node drug targeting using nanoparticles or “albumin hitchhiking”.
Acknowledgments
This work was supported by National Science Foundation Grant 1342194, Georgia Institute of Technology Institute of Bioengineering and Bioscience Grant 1241349, and by PHS Grant UL1TR000454 from the Clinical and Translational Science Award Program, National Institutes of Health, National Center for Advancing Translational Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest.
** of outstanding interest.
- 1.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462(7272):449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 3.Swartz MA, Hirosue S, Hubbell JA. Engineering approaches to immunotherapy. Sci Transl Med. 2012;4(148):148rv149. doi: 10.1126/scitranslmed.3003763. [DOI] [PubMed] [Google Scholar]
- 4.Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12(11):978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hastings CL, Roche ET, Ruiz-Hernandez E, Schenke-Layland K, Walsh CJ, Duffy GP. Drug and cell delivery for cardiac regeneration. Adv Drug Deliv Rev. 2014 doi: 10.1016/j.addr.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Lin CC, Anseth KS. Peg hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26(3):631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam J, Lu S, Kasper FK, Mikos AG. Strategies for controlled delivery of biologics for cartilage repair. Adv Drug Deliv Rev. 2014 doi: 10.1016/j.addr.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeWard AD, Komori J, Lagasse E. Ectopic transplantation sites for cell-based therapy. Curr Opin Organ Transplant. 2014;19(2):169–174. doi: 10.1097/MOT.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Thomas SN, Rutkowski JM, Pasquier M, Kuan EL, Alitalo K, Randolph GJ, Swartz MA. Impaired humoral immunity and tolerance in k14-vegfr-3-ig mice that lack dermal lymphatic drainage. J Immunol. 2012;189(5):2181–2190. doi: 10.4049/jimmunol.1103545. Quantified the contribution of fluid, antigen, and cell transport by lymphatics to draining lymph nodes in the regulation of B and T cell adaptive immune response to immunization and contact sensitization. First report of autoimmune outcomes associated with chronic deficiencies in lymphatic drainage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, Ruddle NH, Jain RK, Fukumura D, Padera TP. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci U S A. 2011;108(46):18784–18789. doi: 10.1073/pnas.1116152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies AJ, Marsland BJ, Swartz MA, Hubbell JA. Nanoparticle conjugation of antigen enhances cytotoxic t-cell responses in pulmonary vaccination. Proc Natl Acad Sci U S A. 2011;108(44):E989–997. doi: 10.1073/pnas.1104264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dane KY, Nembrini C, Tomei AA, Eby JK, O'Neil CP, Velluto D, Swartz MA, Inverardi L, Hubbell JA. Nano-sized drug-loaded micelles deliver payload to lymph node immune cells and prolong allograft survival. J Control Release. 2011;156(2):154–160. doi: 10.1016/j.jconrel.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 13*.Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA. Vegf-c promotes immune tolerance in b16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012;1(3):191–199. doi: 10.1016/j.celrep.2012.01.005. Demonstrates the influence of vascular endothelial growth factor C-induced tumor lymphangiogenesis and sentinel lymph nodes in promoting tumor immune tolerance. [DOI] [PubMed] [Google Scholar]
- 14.Liu R, Gilmore DM, Zubris KA, Xu X, Catalano PJ, Padera RF, Grinstaff MW, Colson YL. Prevention of nodal metastases in breast cancer following the lymphatic migration of paclitaxel-loaded expansile nanoparticles. Biomaterials. 2013;34(7):1810–1819. doi: 10.1016/j.biomaterials.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 2014;35(2):814–824. doi: 10.1016/j.biomaterials.2013.10.003. Toll-like receptor ligands were targeted to sentinel lymph nodes co-draining solid tumors using lymphatic-targeting 30 nm synthetic nanoparticles. Adjuvanted nanoparticle delivery to sentinel lymph nodes induced dendritic cell maturation and antigen-specific immunity to endogenously produced tumor antigen that resulted in slowed tumor growth. [DOI] [PubMed] [Google Scholar]
- 16.Maeda H, Nakamura H, Fang J. The epr effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65(1):71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Reddy ST, Berk DA, Jain RK, Swartz MA. A sensitive in vivo model for quantifying interstitial convective transport of injected macromolecules and nanoparticles. J Appl Physiol (1985) 2006;101(4):1162–1169. doi: 10.1152/japplphysiol.00389.2006. [DOI] [PubMed] [Google Scholar]
- 18.Alexandrakis G, Brown EB, Tong RT, McKee TD, Campbell RB, Boucher Y, Jain RK. Two-photon fluorescence correlation microscopy reveals the two-phase nature of transport in tumors. Nat Med. 2004;10(2):203–207. doi: 10.1038/nm981. [DOI] [PubMed] [Google Scholar]
- 19.Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009;20(8):931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60(9):2497–2503. [PubMed] [Google Scholar]
- 21.Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73(1):1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Stylianopoulos T, Poh MZ, Insin N, Bawendi MG, Fukumura D, Munn LL, Jain RK. Diffusion of particles in the extracellular matrix: The effect of repulsive electrostatic interactions. Biophys J. 2010;99(5):1342–1349. doi: 10.1016/j.bpj.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chary SR, Jain RK. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc Natl Acad Sci U S A. 1989;86(14):5385–5389. doi: 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith KJ, Skelton HG, Turiansky G, Wagner KF. Hyaluronidase enhances the therapeutic effect of vinblastine in intralesional treatment of kaposi's sarcoma. Military medical consortium for the advancement of retroviral research (mmcarr) J Am Acad Dermatol. 1997;36(2 Pt 1):239–242. doi: 10.1016/s0190-9622(97)70288-3. [DOI] [PubMed] [Google Scholar]
- 25.Beckenlehner K, Bannke S, Spruss T, Bernhardt G, Schonenberg H, Schiess W. Hyaluronidase enhances the activity of adriamycin in breast cancer models in vitro and in vivo. J Cancer Res Clin Oncol. 1992;118(8):591–596. doi: 10.1007/BF01211802. [DOI] [PubMed] [Google Scholar]
- 26.McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, Bawendi MG, Boucher Y, Breakefield XO, Jain RK. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66(5):2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 27.Stroh M, Zipfel WR, Williams RM, Ma SC, Webb WW, Saltzman WM. Multiphoton microscopy guides neurotrophin modification with poly(ethylene glycol) to enhance interstitial diffusion. Nat Mater. 2004;3(7):489–494. doi: 10.1038/nmat1159. [DOI] [PubMed] [Google Scholar]
- 28.Campbell RB, Fukumura D, Brown EB, Mazzola LM, Izumi Y, Jain RK, Torchilin VP, Munn LL. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 2002;62(23):6831–6836. [PubMed] [Google Scholar]
- 29.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268(1):235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 30.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9(5):1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 31.Chauhan VP, Popovic Z, Chen O, Cui J, Fukumura D, Bawendi MG, Jain RK. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew Chem Int Ed Engl. 2011;50(48):11417–11420. doi: 10.1002/anie.201104449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A. 2011;108(6):2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrault SD, Chan WC. In vivo assembly of nanoparticle components to improve targeted cancer imaging. Proc Natl Acad Sci U S A. 2010;107(25):11194–11199. doi: 10.1073/pnas.1001367107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Ye D, Shuhendler AJ, Cui L, Tong L, Tee SS, Tikhomirov G, Felsher DW, Rao J. Bioorthogonal cyclization-mediated in situ self-assembly of small-molecule probes for imaging caspase activity in vivo. Nat Chem. 2014;6(6):519–526. doi: 10.1038/nchem.1920. Design and in vivo demonstration of small molecule macrocylization and nanoaggregation triggered by the caspase-3 activity of apoptotic tumor cells. Formed nanoaggregates exhibit improved tumor retention and enable in vivo monitoring of response to chemotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release. 2006;112(1):26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res. 2010;2:14. doi: 10.1186/2040-2384-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaminskas LM, Kota J, McLeod VM, Kelly BD, Karellas P, Porter CJ. Pegylation of polylysine dendrimers improves absorption and lymphatic targeting following sc administration in rats. J Control Release. 2009;140(2):108–116. doi: 10.1016/j.jconrel.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Ryan GM, Kaminskas LM, Bulitta JB, McIntosh MP, Owen DJ, Porter CJ. Pegylated polylysine dendrimers increase lymphatic exposure to doxorubicin when compared to pegylated liposomal and solution formulations of doxorubicin. J Control Release. 2013;172(1):128–136. doi: 10.1016/j.jconrel.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Zhuang Y, Ma Y, Wang C, Hai L, Yan C, Zhang Y, Liu F, Cai L. Pegylated cationic liposomes robustly augment vaccine-induced immune responses: Role of lymphatic trafficking and biodistribution. J Control Release. 2012;159(1):135–142. doi: 10.1016/j.jconrel.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Guo Q, Liu Y, Xu K, Ren K, Sun W. Mouse lymphatic endothelial cell targeted probes: Anti-lyve-1 antibody-based magnetic nanoparticles. Int J Nanomedicine. 2013;8:2273–2284. doi: 10.2147/IJN.S45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang H, Zou LG, Zhang S, Gong MF, Zhang D, Qi YY, Zhou SW, Diao XW. Feasibility of mr imaging in evaluating breast cancer lymphangiogenesis using polyethylene glycol-goldmag nanoparticles. Clin Radiol. 2013;68(12):1233–1240. doi: 10.1016/j.crad.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Tamburini BA, Burchill MA, Kedl RM. Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat Commun. 2014;5:3989. doi: 10.1038/ncomms4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Hirosue S, Vokali E, Raghavan VR, Rincon-Restrepo M, Lund AW, Corthesy-Henrioud P, Capotosti F, Halin Winter C, Hugues S, Swartz MA. Steady-state antigen scavenging, cross-presentation, and cd8+ t cell priming: A new role for lymphatic endothelial cells. J Immunol. 2014;192(11):5002–5011. doi: 10.4049/jimmunol.1302492. First report of synthetic nanoparticle uptake by lymphatic endothelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Komori J, Boone L, DeWard A, Hoppo T, Lagasse E. The mouse lymph node as an ectopic transplantation site for multiple tissues. Nat Biotechnol. 2012;30(10):976–983. doi: 10.1038/nbt.2379. Mouse lymph nodes evaluated as potential ectopic transplantation site. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jewell CM, Lopez SC, Irvine DJ. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc Natl Acad Sci U S A. 2011;108(38):15745–15750. doi: 10.1073/pnas.1105200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shima F, Uto T, Akagi T, Baba M, Akashi M. Size effect of amphiphilic poly(gamma-glutamic acid) nanoparticles on cellular uptake and maturation of dendritic cells in vivo. Acta Biomater. 2013;9(11):8894–8901. doi: 10.1016/j.actbio.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 48**.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507(7493):519–522. doi: 10.1038/nature12978. Significant design innovation utilizing conjugation to albumin-binding lipids to enhance antigen and adjuvant accumulation in draining lymph nodes after subcutaneous injection to improve vaccine efficacy. Demonstrate that neither free drug nor stable liposomal drug formulation result in optimal lymph node accumulation nor efficacy relative to albumin-binding free lipid-drug conjugates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kourtis IC, Hirosue S, de Titta A, Kontos S, Stegmann T, Hubbell JA, Swartz MA. Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. PLoS One. 2013;8(4):e61646. doi: 10.1371/journal.pone.0061646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bagby TR, Cai S, Duan S, Thati S, Aires DJ, Forrest L. Impact of molecular weight on lymphatic drainage of a biopolymer-based imaging agent. Pharmaceutics. 2012;4(2):276–295. doi: 10.3390/pharmaceutics4020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Titta A, Ballester M, Julier Z, Nembrini C, Jeanbart L, van der Vlies AJ, Swartz MA, Hubbell JA. Nanoparticle conjugation of cpg enhances adjuvancy for cellular immunity and memory recall at low dose. Proc Natl Acad Sci U S A. 2013;110(49):19902–19907. doi: 10.1073/pnas.1313152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas SN, van der Vlies AJ, O'Neil CP, Reddy ST, Yu SS, Giorgio TD, Swartz MA, Hubbell JA. Engineering complement activation on polypropylene sulfide vaccine nanoparticles. Biomaterials. 2011;32(8):2194–2203. doi: 10.1016/j.biomaterials.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 53.Roozendaal R, Mempel TR, Pitcher LA, Gonzalez SF, Verschoor A, Mebius RE, von Andrian UH, Carroll MC. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30(2):264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node b cells. Nat Immunol. 2007;8(9):992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 55.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27(1):160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 56.Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the t cell area of the lymph node. Immunity. 2005;22(1):19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5(8):617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 58.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38(5):1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 59.Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H, Orba Y, Kawaguchi A, Hasegawa H, Kajino K, Ninomiya T, et al. Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7(5):3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 60.Wattendorf U, Coullerez G, Voros J, Textor M, Merkle HP. Mannose-based molecular patterns on stealth microspheres for receptor-specific targeting of human antigen-presenting cells. Langmuir. 2008;24(20):11790–11802. doi: 10.1021/la801085d. [DOI] [PubMed] [Google Scholar]
- 61.Kwon YJ, James E, Shastri N, Frechet JM. In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on ph-responsive microparticles. Proc Natl Acad Sci U S A. 2005;102(51):18264–18268. doi: 10.1073/pnas.0509541102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tacken PJ, de Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, Faas SJ, Punt CJ, Torensma R, Adema GJ, et al. Effective induction of naive and recall t-cell responses by targeting antigen to human dendritic cells via a humanized anti-dc-sign antibody. Blood. 2005;106(4):1278–1285. doi: 10.1182/blood-2005-01-0318. [DOI] [PubMed] [Google Scholar]
- 63.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8(2):151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]


