Abstract
Objective:
To examine the effect of cost, a traditionally “inactive” trait of intervention, as contributor to the response to therapeutic interventions.
Methods:
We conducted a prospective double-blind study in 12 patients with moderate to severe Parkinson disease and motor fluctuations (mean age 62.4 ± 7.9 years; mean disease duration 11 ± 6 years) who were randomized to a “cheap” or “expensive” subcutaneous “novel injectable dopamine agonist” placebo (normal saline). Patients were crossed over to the alternate arm approximately 4 hours later. Blinded motor assessments in the “practically defined off” state, before and after each intervention, included the Unified Parkinson's Disease Rating Scale motor subscale, the Purdue Pegboard Test, and a tapping task. Measurements of brain activity were performed using a feedback-based visual-motor associative learning functional MRI task. Order effect was examined using stratified analysis.
Results:
Although both placebos improved motor function, benefit was greater when patients were randomized first to expensive placebo, with a magnitude halfway between that of cheap placebo and levodopa. Brain activation was greater upon first-given cheap but not upon first-given expensive placebo or by levodopa. Regardless of order of administration, only cheap placebo increased activation in the left lateral sensorimotor cortex and other regions.
Conclusion:
Expensive placebo significantly improved motor function and decreased brain activation in a direction and magnitude comparable to, albeit less than, levodopa. Perceptions of cost are capable of altering the placebo response in clinical studies.
Classification of evidence:
This study provides Class III evidence that perception of cost is capable of influencing motor function and brain activation in Parkinson disease.
In most randomized controlled clinical trials, the therapeutic efficacy of interventions is measured against the response elicited by a placebo. Such response, driven by heightened expectations of efficacy, is referred to as the placebo response. The placebo response may be influenced by nonintrinsic attributes of interventions such as price,1 brand,2 and other “inactive” traits.3
It is well recognized that expectation of a response to treatment is a great modulator of behavior.4 Thus, probing the neurobiological basis of the placebo response is important to understand the complex response to various medical and surgical interventions and for devising strategies aimed at harnessing its benefits. While the majority of studies examining the response to placebo have focused on pain disorders,4 there is a large magnitude of placebo response in Parkinson disease (PD),5 whose pathophysiology remains virtually unknown. We hypothesized that at least some improvement in motor function in PD is attributable to the perceived cost of medication as a proxy of (expectation of) efficacy. In this study, we examined the placebo response of perceived cost on motor function and blood oxygen level–dependent fMRI activity after the administration of normal saline as an “expensive” or “cheap” “injectable dopamine agonist” in patients with moderately advanced PD by using standardized measures of motor performance and a visual-motor associative learning processing fMRI task.
METHODS
Study patients.
Twelve patients (75% male; age 62.4 ± 7.9 years; disease duration 11 ± 6 years) with PD meeting UK Brain Bank criteria6 were enrolled in a double-blind crossover study that purportedly explored the motor benefits of a “new injectable dopamine agonist” compared with a lower-priced drug of similar presumed efficacy. Patients were documented to have excellent response to levodopa (“on” Schwab and England [S&E] score ≥85), with associated motor fluctuations but a relatively functional “off” state (“off” S&E score ≥70 but <85; Hoehn and Yahr score <3). This population was selected to provide a range of detectable changes during the measurement of the placebo response.7 The exclusion criteria were as follows: (1) significant tremor (>2 in the motor part of the Unified Parkinson's disease Rating Scale [UPDRS-III],8 tremor subscale) to reduce fMRI interference; (2) musculoskeletal disorders that may confound assessment of motor function; (3) previous deep brain stimulation; (4) history of stroke; (5) cerebellar, vestibular, or sensory ataxia; (6) Mini-Mental State Examination score <24; (7) women of childbearing potential; and (8) any contraindication to 4-tesla MRI.
Standard protocol approvals, registrations, and patient consents.
The institutional review board (IRB) approved this study per guidance on the use of deception in human subjects research, after 8-month deliberations including faculty from the School of Psychology experienced in research using intentional deception.9 It determined that the protocol involved no more than minimal risk and the deception would have no adverse effects on the rights or welfare of the participants. The informed consent document contained as much information about the study as possible (without revealing its true nature) and the 8 required elements as described in 45CFR46.116(a). The IRB determined that the study's value justified not disclosing information on its true nature and that the research met criteria for waiver or alteration of informed consent as described in 45CFR.116(c)(2)(d). Given the risks of affecting the trust of patients, violating the principle of respect for patient autonomy, and ultimately eroding the patient-physician relationship, the IRB also required that all participants were to be selected from the clinic of the principal investigator (A.J.E.). Participants were interviewed about their expectation of global clinical response and were fully debriefed about the study nature at study completion, per IRB requirement. This study was not registered as a clinical trial to prevent disclosure of the true nature of the study to any prospective participants.
Study design and interventions.
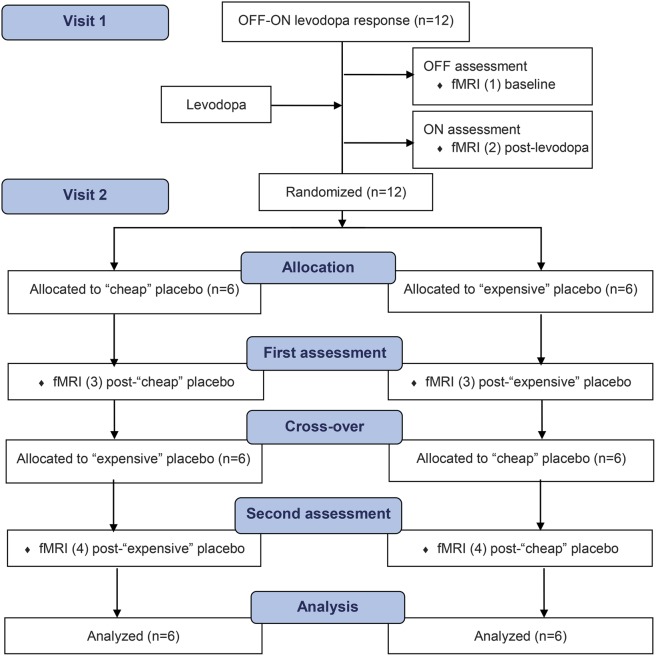
On visit 1, after ≥12 hours from the last dose of medication (“practically defined ‘off’ state”10) and when intake of their standard dopaminergic medications had become clinically effective (“on” state), subjects underwent a baseline “off-on” motor assessment to determine the magnitude of their dopaminergic benefit. Within a week, during visit 2, in the practically defined “off” state, subjects were randomized to receive the “expensive” or “cheap” injectable “dopamine agonist,” then crossed over to the alternative intervention in approximately 4 hours, once the perceived benefits were perceived to have worn off. The same motor and fMRI protocol was conducted before (off) and after (on) administration of levodopa in visit 1 and before (“baseline”) and after (post-“expensive” or post-“cheap” placebo) study interventions. A random-number sequence for the 12 subjects using a simple block randomization technique with block size 2 was generated to allocate 6 subjects in the “cheap-first” or “expensive-first” placebo arms. The random-number sequence was concealed in consecutively sealed opaque envelopes. At visit 2, subjects were informed that they would receive subcutaneous injections of 2 formulations of the same injectable dopamine agonist with the second injection after “wearing off” from the first (for standardized instructions given to patients, see appendix e-1 on the Neurology® Web site at Neurology.org). Participants were further told that although the formulations were believed to be of similar efficacy, their manufacturing allowed for expensive and cheap versions. They were made to understand that the study was intended to prove that these drugs, while differently priced, were of similar efficacy. Participants were blinded to actual study purpose and debriefed only after study completion. The assessment team, including the rating and fMRI investigators, was blinded to treatment allocation. The randomized phase of the study was conducted during a 6-hour day (figure 1).
Figure 1. CONSORT flow diagram.
At visit 1, subjects underwent an off-on motor assessment to determine their baseline magnitude of dopaminergic benefit. At visit 2, subjects in the practically defined off state were randomized to receive the cheap or expensive injectable dopamine agonist and crossed over in approximately 4 hours. CONSORT = Consolidated Standards of Reporting Trials.
Motor measurements and analyses.
Main endpoint was motor impairment measured by UPDRS-III (0–108, lower is better). Secondary clinical and device-assisted measurements included S&E activities of daily living scale (0–100, higher is better)11 and Hoehn and Yahr staging (1–5, higher is worse),12 patient Clinical Global Impression (CGI) scale of change using a 1 to 7 Likert severity scale (1 = marked improvement, 7 = marked deterioration), a tapping apparatus (higher is better), and the Purdue Pegboard Test (higher is better).
Clinical variables were summarized using means ± SDs. Paired t test was used to compare UPDRS-III scores between off and on states at visit 1. Repeated-measures analysis of variance was used to compare the 3 conditions, baseline, post–expensive treatment, and post–cheap treatment, with interaction term between intervention group and sequencing of intervention. Sequencing or order effect of intervention was examined using stratified analysis. Bonferroni correction was used for multiple comparisons. Wilcoxon signed rank test was used to compare the improvement from baseline in motor function between cheap and expensive placebos. For each sequence of randomization, a paired t test was used to compare UPDRS-III scores from baseline between expensive and cheap placebo groups. An unpaired t test was used to compare the differences in UPDRS-III scores in the first and second period of the study. The results were summarized with median or mean difference with 95% confidence interval or p value. McNemar test was used to compare the changes in proportion of improvement on CGI scales between placebo groups. Finally, we compared the changes from baseline between on-levodopa and expensive placebo, and on-levodopa and cheap placebo. Our planned crossover randomized clinical trial design with a predefined primary motor outcome but a stratified analysis met Level II classification of evidence.
Magnetic resonance imaging.
Anatomical and functional brain images were obtained using a 4-tesla MRI/magnetic resonance spectroscopy system (Varian Inc., Palo Alto, CA). The behavioral experiment was programmed in E-Prime, version 1 (www.pstnet.com). All participants wore magnetic resonance–compatible VGA goggles and headphones (Resonance Technologies, Inc.). For each imaging session, when the participant was positioned in the scanner, a 3-plane scout scan was performed followed by a high-resolution T1-weighted 3-dimensional anatomical scan using modified equilibrium Fourier transform sequence: repetition time/echo time 13/6 milliseconds, magnetization preparation time for MDEFT sequence 1.1 seconds, field of view 256 × 192 × 192 mm, matrix 256 × 192 × 96 mm, slice thickness 1 mm, and flip angle 20°.13 Next, a multiecho reference scan was performed to correct for geometric distortion and Nyquist ghost artifacts.14 Finally, echo-planar imaging was performed while subjects underwent 2 runs of the behavioral paradigm using a T2*-weighted, gradient-echo, echo-planar imaging pulse sequence: repetition time/echo time 3,000/29 milliseconds, field of view 256 × 256 mm, matrix 64 × 64 mm, slice thickness 4 mm, flip angle 75°. The MRI system triggered the behavioral paradigm to ensure precise timing of the task regarding image acquisition. The feedback-based visual-motor associative learning paradigm was modified from previous research15 to accommodate for slower responses. A total of 182 volumes were obtained during each fMRI run. Participants responded by pressing with their right thumb 1 of 2 buttons on a button box, with task and behavioral data (e.g., timing of stimulus and feedback presentations, responses, and outcomes of each trial) recorded.
Imaging paradigm.
The visual-motor association task consisted of control and feedback-based visual-motor associative learning conditions.15 In the control condition, participants saw the digit “1” or “2” presented and had to press the corresponding button 1 or 2. In the learning condition, participants viewed easily named color pictures (e.g., drum, vase),16 and learned by trial and error to associate each picture with button 1 or 2. Each trial began with the presentation of a fixation cross (+) in the center of the screen for 0.5 to 3.5 seconds (variable delay), then a picture or digit appeared on screen for 4.125 seconds while subjects responded. A blank screen was presented for 0.5 to 3.5 seconds (variable delay) followed by performance feedback: + indicating success (positive feedback or reward), 0 indicating an error (negative feedback), or ? indicating failure to respond in time. Before entering the scanner, participants were given oral and written instructions and training with pictures different from those used during scanning. Each participant performed 2 runs of the task in each fMRI session (i.e., each participant performed 8 runs of this task throughout the study). In each run, participants had to learn responses to 4 pictures, with each picture presented 8 times in pseudorandom order.
Image processing.
Reconstruction and analysis of the raw data (Analysis of Functional NeuroImages: http://afni.nimh.nih.gov/afni)17 are presented in detail in appendix e-2.
RESULTS
Motor results.
Compared with the off state at visit 1, levodopa significantly decreased UPDRS-III scores and increased Purdue Pegboard and tapping scores, as expected for a cohort of subjects with levodopa-responsive PD (table 1).
Table 1.
Effect of levodopa at visit 1 and effect of placebo at visit 2 (overall effect and analysis of order effect)
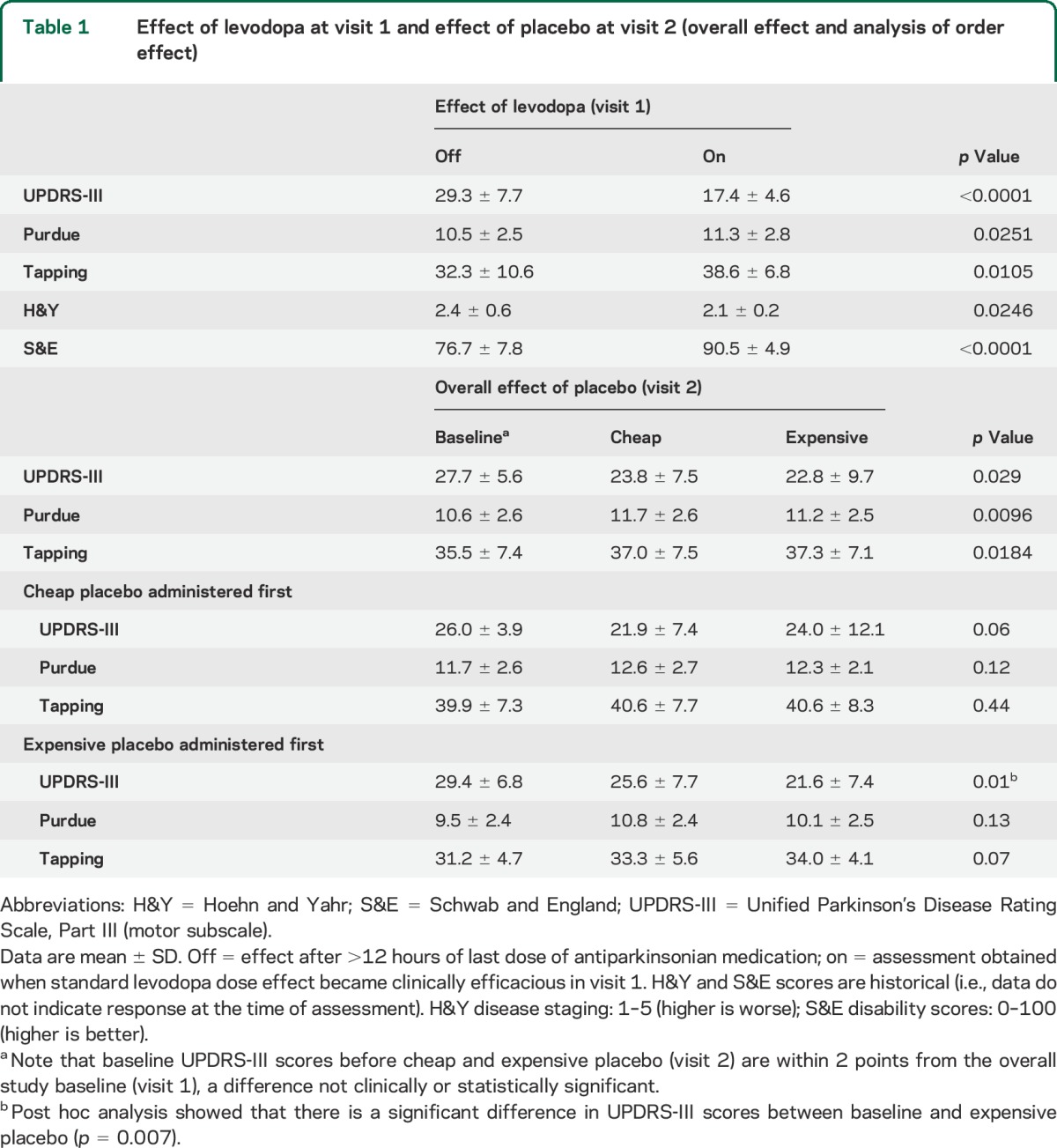
Both placebos improved motor function compared with their respective baseline. Overall, there were significant differences among the 3 groups (baseline, cheap placebo, and expensive placebo) at visit 2 (table 1). We found 9% overall improvement with expensive placebo as compared with cheap placebo (p = 0.27). When ignoring the second study period to eliminate the order effect, we observed a 10% improvement with expensive placebo as compared with cheap placebo (p = 0.28) (table e-1).
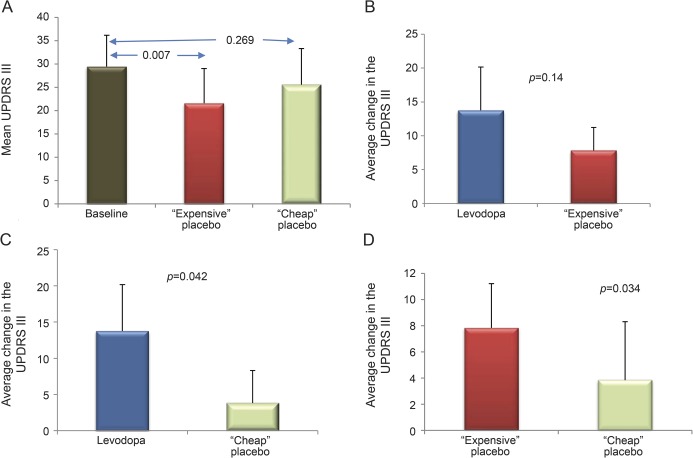
In stratified analyses, UPDRS-III score significantly decreased and tapping score increased only when patients were allocated to expensive placebo first (table 1). Overall, 14% improvement was obtained when expensive placebo was administered first compared with cheap placebo (table e-1). First-given cheap placebo reduced UPDRS-III score to a lesser extent than expensive placebo (p = 0.034) and levodopa (p = 0.042; figure 2). There was no significant order effect for the Purdue Pegboard. Expensive placebo yielded a higher overall proportion (66.7%) of “very good” and “marked improvement” on patient CGI scales compared with cheap placebo (58.3%), although the difference was not statistically significant.
Figure 2. UPDRS-III changes when expensive placebo was administered first.
The expensive placebo group yielded the strongest reduction in UPDRS-III score, by 28% (A, p = 0.007), a magnitude of change not significantly different from levodopa (B, p = 0.14). The average change of cheap placebo, a 13% reduction in UPDRS-III score compared with baseline, was significantly less than levodopa (C, p = 0.042) and of lower magnitude than the change attained with expensive placebo (D, p = 0.034). Vertical error bars indicate SD. UPDRS-III = Unified Parkinson's Disease Rating Scale, Part III.
Imaging results.
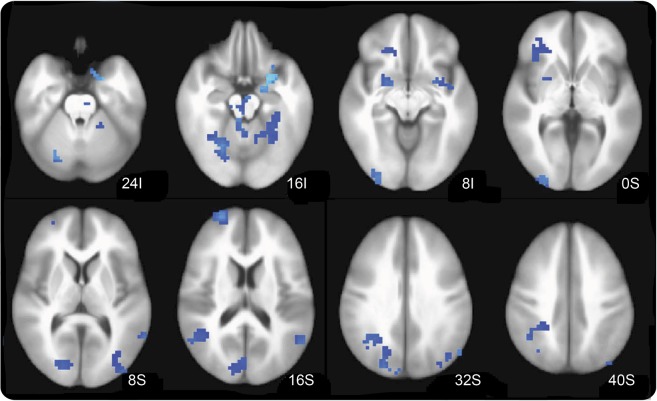
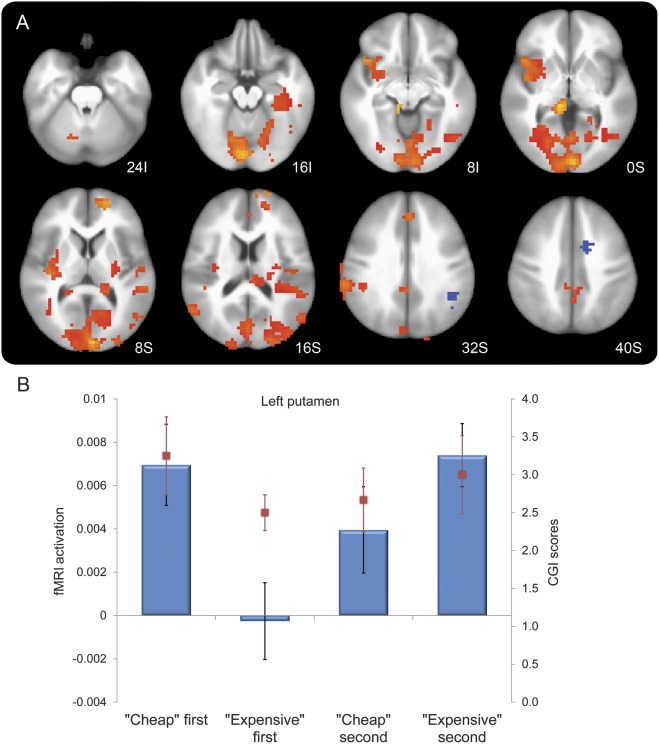
Compared with baseline off state in visit 1, levodopa led to a deactivation during task performance of selected brain regions, including the left putamen, left sensorimotor cortices, and left premotor cortex (voxel-wise p < 0.005, cluster of ≥37 voxels) (figure 3). At visit 2, cheap but not expensive placebo increased activation in the bilateral anterior and posterior cingulate cortices, left lateral sensorimotor cortex, and right parietal cortex and other regions (figure e-1, table e-2). Stratifying by order of administration, first-given cheap placebo produced stronger brain activation compared with first-given expensive placebo. The magnitude of activation of expensive placebo was robust only when given after cheap placebo (carryover effect) (figure 4A, table e-3). The order-of-administration interaction effect on levels of brain activation during the visual-motor association task was qualitatively similar in all 4 conditions in selected brain regions depending on which placebo was delivered first (figure 4B). First administration consistently increased brain activation by cheap but not by expensive placebo. The effects of the second administration were confounded by the outcomes of the first administration.
Figure 3. Effect of levodopa.
Compared with baseline, there was a reduction of activity during task performance in selected brain regions, including the left putamen, left sensorimotor cortices, and left premotor cortex (voxel-wise p < 0.005, cluster of ≥37 voxels).
Figure 4. Effect of cheap vs expensive placebo stratified by order of administration, and interaction of cost by order of administration in the left putamen.
(A) Compared with expensive placebo, cheap placebo produced stronger brain activation when given first. The magnitude of activation of expensive placebo was robust only when given after cheap placebo. Areas in red-orange show significant interaction of cost by order of administration, in a pattern similar to that shown in the supplementary material (see table e-2). (B) First administration greatly increased brain activation by cheap but not by expensive placebo. The effect of the second administration was confounded by the outcomes of the first administration. Task-induced brain activation (raw units) was greatest when cheap placebo was given first, with little change in activation when expensive was received after cheap (carryover effect). Activation was lowest when expensive placebo was given first, and the reduction was present but attenuated when cheap was received after expensive. We observed a qualitatively similar clinical response pattern shown by the directionality of the Clinical Global Impression (CGI) scores (improvement rated from 4 = unchanged to 1 = marked improvement), which are overlapped in red bars.
Debriefing.
Upon completion of the study, subjects were informed as to the nature of the study with placebo-only use. Responses ranged from disbelief to amazement regarding changes experienced, with 8 subjects endorsing having held heightened expectations of benefit whereas 4 subjects admitting no prior expectation of benefits. Subjects in the latter group experienced overall negligible changes and declared no change in the exit CGI assessment. All patients understood the importance of the study and expressed their appreciation at the magnitude of changes induced by their expectations. All patients resumed routine neurologic care with the principal investigator.
DISCUSSION
The perception of higher cost modified the placebo response in subjects with PD. A larger motor benefit was achieved by a “costlier” intervention, particularly when given first. This differential effect of cost was dependent on the order of administration, whereby expensive placebo improved motor function 2-fold over cheap placebo if given first. Effects on brain activation exhibited a corresponding pattern of order, although with a reversed direction of effect. Expensive placebo improved motor function but decreased brain activation (in a manner qualitatively similar to levodopa), whereas cheap placebo exerted a robust level of brain activation with only modest effect on motor improvement. A similar pattern of response to placebo in the sensory-motor network was recently found in an fMRI study evaluating brain activity during rest.18 Our data showed that brain activation of visual-motor associative circuits is greatest when cheap placebo is given first and lowest when expensive placebo is given first, suggesting greater brain “effort” (more activity) under placebo conditions of lowered expectations. Both the brain activation and clinical response were qualitatively similar to levodopa. This relationship between brain activation and motor scores is supported by the mirrored directionality with CGI scores shown in figure 4B.
This study is the first to examine in a randomized and blinded manner the effects of cost, as a nonintrinsic property of any intervention, on the placebo response in PD. Strengths include the use of a validated fMRI paradigm and the elimination of clinically meaningful symptomatic effects by examining patients with moderately advanced PD in the practically defined off period. Limitations include the short-term nature of the assessments (forced by the need to avoid a prolonged levodopa-deprived state) and the low number of study subjects. While it is possible that there are additional differences between treatments in brain activation, our study was only powered to detect major differences based on motor control. One important limitation is that UPDRS-III scores were obtained by an investigator who, while blinded to the order of interventions, was aware of the nature of the study and may have introduced a positive expectation bias (i.e., anticipation of improvement). However, such bias would have affected all subjects and conditions rather than selectively for certain interventions, attenuating the differences between study arms but not blunting them altogether. This potential bias was also mitigated by the additional use of rater-independent instrumental tasks, namely, the tapping apparatus, which mirrored the UPDRS-III changes, and the Purdue Pegboard, which failed to detect differences.
The placebo response in PD is mediated by the release of endogenous dopamine from both the dorsal and ventral striatum.19,20 The changes in putamen activation may have represented the neurobiological substrate of the improved motor scores in our study because it is the major target for nigral dopaminergic projections and principal generator of implicit “motor motivation.”21 Dopaminergic upregulation of the same pathways affected by PD is correlated with positive anticipation, motivation, and response to novelty in normal subjects.22 Saline injection given to subjects with PD believing to have received an active drug and, thus, demonstrating an “expectation of reward” response, is associated with dopamine release in the nucleus accumbens similar to the response to reward itself.23 The effect of a costly intervention, through heightened expectation and positive anticipation, may be expected to yield similar increases in dopamine output to the putamen.
Patients' expectations have an important role in the efficacy of medical therapies. Our findings complement the well-known patient preference of brand name over generic, as recently confirmed in a study of similar design to ours.24 A number of surgical therapies, including fetal mesencephalic grafting25 and intraparenchymal infusions of glial-derived neurotrophic factor,26 demonstrated pronounced benefits in initial open-label studies only to fail in randomized controlled trials.27,28 The antioxidant glutathione provided for a large out-of-pocket fee as IV infusion at several community practices,29 despite its failure to cross the blood-brain barrier and its demonstrated lack of efficacy,30 is another example. Patients require “booster” injections to replicate the dwindling “expectation of reward” response. The unethical commercialization of “the stem cell miracle”31 is threatening progress for a therapeutic modality with many current challenges that require additional rigorous investigations before it can be further translated to patients. At large personal expense (€6,000–30,000 per procedure), patients with a variety of neurologic disorders including PD continue to receive intrathecal bone marrow–derived mesenchymal stem cells at “stem cell clinics” in Germany, Italy, China, and elsewhere. A retrospective analysis of 17 European patients treated for parkinsonism available for follow-up to independent investigators demonstrated no improvements and, in fact, an overall trend for worsening on measures of motor impairment or disability, after a median of 10 months from the intervention.32
While the open application of expensive but unproven interventions is unethical, strategies to harness placebo responses to enhance benefits of treatment are to be encouraged.33 Pharmacologic and surgical clinical trials may be augmented by the synergistic addition of superimposed placebo responses in order to maximize benefits while reducing dosage and toxicity.34,35 Given that the benevolent use of deception to generate a placebo response is in conflict with the principle of respect for patient autonomy, preliminary data suggest that the use of “nonblind placebo” (inert content disclosed) may be effective36 and eliminate the need for deception or concealment even in a randomized clinical trial setting.37 The potentially large benefit of placebo, with or without price manipulations, is waiting to be untapped for patients with PD as well as those with other neurologic and medical diseases.
Supplementary Material
GLOSSARY
- CGI
Clinical Global Impression
- IRB
institutional review board
- PD
Parkinson disease
- S&E
Schwab and England
- UPDRS-III
Unified Parkinson's Disease Rating Scale, Part III
Footnotes
Editorial, page 766
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Espay: study concept and design, acquisition of data, analysis and interpretation, drafting of the manuscript, critical revision of the manuscript for important intellectual content, study supervision. Mr. Norris: analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Eliassen: analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Dwivedi: analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. Mr. Smith: analysis and interpretation, critical revision of the manuscript for important intellectual content. Mrs. Banks: acquisition of data, study supervision. Dr. Allendorfer: acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Lang: analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Fleck: analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Linke: analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Szaflarski: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
This study was funded in part by the Davis Phinney Foundation for Parkinson's Disease (co–principal investigators: A.J.E. and J.P.S.). A.J.E. is currently supported by NIH grant 1K23MH092735. J.P.S. was supported by NIH K23 NS052468 at the time of this work.
DISCLOSURE
A. Espay is supported by a K23 award (NIMH, 1K23MH092735). He has received grant support from CleveMed/Great Lakes NeuroTechnologies, Davis Phinney Foundation, and Michael J. Fox Foundation; personal compensation as a consultant/scientific advisory board member for Solvay, Abbott, Merz, Chelsea Therapeutics, Teva, Impax, and Eli Lilly; and honoraria from Novartis, the American Academy of Neurology, and the Movement Disorders Society. He serves as associate editor of Movement Disorders and Frontiers in Movement Disorders and on the editorial boards of Parkinsonism and Related Disorders and The European Neurological Journal. M. Norris, J. Eliassen, A. Dwivedi, M. Smith, and C. Banks report no disclosures relevant to the manuscript. J. Allendorfer has received funding from the Shor Foundation for Epilepsy Research. She serves as an associate editor of the journal Restorative Neurology and Neuroscience. A. Lang has served as an advisor for AbbVie, Allon Therapeutics, AstraZeneca, Biovail, Boehringer-Ingelheim, Cephalon, Ceregene, Eisai, GSK, Lundbeck A/S, Medtronic, Merck Serono, Novartis, Santhera, Solvay, and Teva, and received grants from Canadian Institutes of Health Research, Dystonia Medical Research Foundation, Michael J. Fox Foundation, National Parkinson Foundation, and Ontario Problem Gambling Research Centre, and has served as an expert witness in cases related to the welding industry. D. Fleck has received research support from NIH and honoraria from Elsevier and USAMRMC. M. Linke is the Chair of the University of Cincinnati institutional review board (IRB); however, he was not involved in the IRB review of the protocol or its postapproval monitoring. He has nothing else to disclose. J. Szaflarski received research funding from NIH, Shor Foundation for Epilepsy Research, Epilepsy Foundation of America, Food and Drug Administration, Neuren Pharmaceuticals, Compumedics Neuroscan, Inc., the University of Alabama at Birmingham, and UCB Pharma, Inc. While this research was conducted, he was supported by NIH K23 NS052468. He serves as an associate editor of the journal Restorative Neurology and Neuroscience and on editorial boards of the journals Epilepsy & Behavior and Journal of Epileptology. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Rao AR, Monroe KB. The effect of price, brand name, and store name on buyers' perceptions of product quality. J Mark Res 1989;26:351–357. [Google Scholar]
- 2.Allison RI, Uhl KP. Influence of beer brand identification on taste perception. J Mark Res 1964;1:36–39. [Google Scholar]
- 3.Lee L, Frederick S, Ariely D. Try it, you'll like it: the influence of expectation, consumption, and revelation on preferences for beer. Psychol Sci 2006;17:1054–1058. [DOI] [PubMed] [Google Scholar]
- 4.Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med 2010;16:1277–1283. [DOI] [PubMed] [Google Scholar]
- 5.McRae C, Cherin E, Yamazaki TG, et al. Effects of perceived treatment on quality of life and medical outcomes in a double-blind placebo surgery trial. Arch Gen Psychiatry 2004;61:412–420. [DOI] [PubMed] [Google Scholar]
- 6.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goetz CG, Wuu J, McDermott MP, et al. Placebo response in Parkinson's disease: comparisons among 11 trials covering medical and surgical interventions. Mov Disord 2008;23:690–699. [DOI] [PubMed] [Google Scholar]
- 8.Fahn S, Elton RL; Committee at UD. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Health Care Information; 1987:153–164. [Google Scholar]
- 9.Sloan L, Hull J. Institutional review board management and function. In: Bankert EA, Amdur RJ, editors. Deception of Research Subjects, 2nd ed Sudbury, MA: Jones & Bartlett Publishers; 2006:210–215. [Google Scholar]
- 10.Langston JW, Widner H, Goetz CG, et al. Core assessment program for intracerebral transplantations (CAPIT). Mov Disord 1992;7:2–13. [DOI] [PubMed] [Google Scholar]
- 11.Schwab RS, England ACJ. Projection technique for evaluating surgery in Parkinson's disease. In: Gillingham FJ, Donaldson IML, editors. Third Symposium on Parkinson's Disease. Edinburgh: E & S Livingstone; 1969:152–157. [Google Scholar]
- 12.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Garwood M, Menon R, et al. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med 1995;34:308–312. [DOI] [PubMed] [Google Scholar]
- 14.Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging 2001;20:535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eliassen JC, Lamy M, Allendorfer JB, et al. Selective role for striatal and prefrontal regions in processing first trial feedback during single-trial associative learning. Brain Res 2012;1458:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart's object pictorial set: the role of surface detail in basic-level object recognition. Perception 2004;33:217–236. [DOI] [PubMed] [Google Scholar]
- 17.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 18.Esposito F, Tessitore A, Giordano A, et al. Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson's disease by levodopa. Brain 2013;136:710–725. [DOI] [PubMed] [Google Scholar]
- 19.de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science 2001;293:1164–1166. [DOI] [PubMed] [Google Scholar]
- 20.de la Fuente-Fernandez R, Stoessl AJ. The placebo effect in Parkinson's disease. Trends Neurosci 2002;25:302–306. [DOI] [PubMed] [Google Scholar]
- 21.Mazzoni P, Hristova A, Krakauer JW. Why don't we move faster? Parkinson's disease, movement vigor, and implicit motivation. J Neurosci 2007;27:7105–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duzel E, Bunzeck N, Guitart-Masip M, Duzel S. Novelty-related motivation of anticipation and exploration by dopamine (NOMAD): implications for healthy aging. Neurosci Biobehav Rev 2010;34:660–669. [DOI] [PubMed] [Google Scholar]
- 23.de la Fuente-Fernandez R, Phillips AG, Zamburlini M, et al. Dopamine release in human ventral striatum and expectation of reward. Behav Brain Res 2002;136:359–363. [DOI] [PubMed] [Google Scholar]
- 24.Faasse K, Cundy T, Gamble G, Petrie KJ. The effect of an apparent change to a branded or generic medication on drug effectiveness and side effects. Psychosom Med 2013;75:90–96. [DOI] [PubMed] [Google Scholar]
- 25.Freed CR, Breeze RE, Rosenberg NL, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson's disease. N Engl J Med 1992;327:1549–1555. [DOI] [PubMed] [Google Scholar]
- 26.Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS. Intraputamenal infusion of glial cell line–derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol 2005;57:298–302. [DOI] [PubMed] [Google Scholar]
- 27.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med 2001;344:710–719. [DOI] [PubMed] [Google Scholar]
- 28.Lang AE, Gill S, Patel NK, et al. Randomized controlled trial of intraputamenal glial cell line–derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 2006;59:459–466. [DOI] [PubMed] [Google Scholar]
- 29.Perlmutter D, Colman C. The Better Brain Book: The Best Tools for Improving Memory and Sharpness and Preventing Aging of the Brain. New York: The Penguin Group; 2004. [Google Scholar]
- 30.Hauser RA, Lyons KE, McClain T, Carter S, Perlmutter D. Randomized, double-blind, pilot evaluation of intravenous glutathione in Parkinson's disease. Mov Disord 2009;24:979–983. [DOI] [PubMed] [Google Scholar]
- 31.Rader WC. Blocked in the USA: The Stem Cell Miracle. Malibu, CA: Nanog Publishing; 2010. [Google Scholar]
- 32.Storch A, Csoti I, Eggert K, et al. Intrathecal application of autologous bone marrow cell preparations in Parkinsonian syndromes. Mov Disord 2012;27:1552–1555. [DOI] [PubMed] [Google Scholar]
- 33.Colloca L, Miller FG. Harnessing the placebo effect: the need for translational research. Philos Trans R Soc Lond B Biol Sci 2011;366:1922–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ader R, Mercurio MG, Walton J, et al. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosom Med 2010;72:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandler AD, Glesne CE, Bodfish JW. Conditioned placebo dose reduction: a new treatment in attention-deficit hyperactivity disorder? J Dev Behav Pediatr 2010;31:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aulas JJ, Rosner I. Efficacy of a non blind placebo prescription [in French]. Encephale 2003;29:68–71. [PubMed] [Google Scholar]
- 37.Kaptchuk TJ, Friedlander E, Kelley JM, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One 2010;5:e15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.