Abstract
X-box-binding protein 1-transfected neural stem cells were transplanted into the right lateral ventricles of rats with rotenone-induced Parkinson's disease. The survival capacities and differentiation rates of cells expressing the dopaminergic marker tyrosine hydroxylase were higher in X-box-binding protein 1-transfected neural stem cells compared to non-transfected cells. Moreover, dopamine and 3,4-dihydroxyphenylacetic acid levels in the substantia nigra were significantly increased, α-synuclein expression was decreased, and neurological behaviors were significantly ameliorated in rats following transplantation of X-box-binding protein 1-transfected neural stem cells. These results indicate that transplantation of X-box-binding protein 1-transfected neural stem cells can promote stem cell survival and differentiation into dopaminergic neurons, increase dopamine and 3,4-dihydroxyphenylacetic acid levels, reduce α-synuclein aggregation in the substantia nigra, and improve the symptoms of Parkinson's disease in rats.
Keywords: X-box-binding protein 1, neural stem cells, Parkinson's disease, α-synuclein; dopamine
Abbreviations:
PD, Parkinson's disease; XBP1, X-box-binding protein 1; NSCs, neural stem cells; TH, tyrosine hydroxylase; PBS, phosphate-buffered saline; BrdU, 5-bromodeoxyuridine
INTRODUCTION
Endoplasmic reticulum stress is one of the major pathogenetic mechanisms of Parkinson's disease (PD)[1,2,3]. X-box-binding protein 1 (XBP1) is an important transcription factor in the endoplasmic reticulum, acting to clear abnormally accumulated proteins and playing critical roles in cell survival and differentiation[4,5,6]. XBP1 can delay the progression of PD through antagonizing endoplasmic reticulum stress[7]. XBP1-transfected neural stem cells (NSCs) with stabilized XBP1 overexpression grew more rapidly and had a higher survival rate after exposure to hypoxia compared to normal NSCs. Moreover, XBP1-NSCs showed stronger ventricular migration and differentiation ability, as well as promoting Bcl-2 expression in infarct areas, inhibiting Bax expression, and significantly ameliorating cerebral infarction in rats with ischemia/reperfusion injury. These results indicate that transplantation of XBP1-NSCs into the lesioned site can maintain stable and continuous XBP1 expression, antagonize endoplasmic reticulum stress in PD, and play a therapeutic role. In the present study, we therefore transplanted XBP1-NSCs into the brains of rats with rotenone-induced PD, to assess survival of transplanted cells and secretion of related proteins
RESULTS
Quantitative analysis of experimental animals
A total of 45 rats were injected with rotenone microspheres to establish PD models. Seven rats died within 10 days, and 11 rats died as a result of reduced body mass or diarrhea up to 20 days following injection. Of the 34 surviving rats, 27 were identified as successful models, and were randomly assigned to model, NSC, or XBP1-NSCs groups (n = 9 for each), which were injected or transplanted with phosphate-buffered saline (PBS), normal NSCs or XBP1-NSCs, respectively, into the right lateral ventricle. Three rats in the model group died because of reduced food consumption, and the remaining rats were included in the final analysis.
XBP1-NSC transplantation improved PD rat behaviors
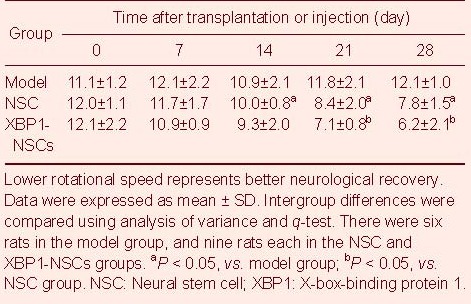
Rotation frequency was slightly decreased in NSC group rats, indicating improved rotational behavior (P < 0.05), and rotation frequency was significantly decreased in XBP1-NSCs group rats with increasing time, compared to NSC rats, with a significant difference between days 21 and 28 in the XBP1-NSCs group (P < 0.05; Table 1).
Table 1.
Mean rotational speed (rotations/min) in apomorphine-induced rotation test
Survival and differentiation of transplanted NSCs
NSCs were labeled with 5-bromodeoxyuridine (BrdU) prior to transplantation. Cells that differentiated into dopaminergic neurons expressed tyrosine hydroxylase (TH)[8]. At 28 days following transplantation, immunofluorescence staining of BrdU and TH identified BrdU+, TH+, and BrdU+/TH+ cells in the substantia nigra in the NSC and XBP1-NSCs groups. The numbers of BrdU+ and TH+ cells were greater in the XBP1-NSCs group compared to the NSC group (P < 0.05). Moreover, the ratio of BrdU+/TH+ co-labeled cells to BrdU+ cells was significantly greater in the XBP1-NSCs group compared to the NSC group (P < 0.01; Figure 1, Table 2).
Figure 1.

Distribution of neural stem cells in the substantia nigra at 28 days following transplantation (immunohistochemical staining, ×400).
Tyrosine hydroxylase (TH)+ cells were stained green following staining with glial fibrillary acidic protein; 5-bromodeoxyuridine (BrdU)+ cells were stained red following staining with phycoerythrin; and BrdU+/TH+ cells were stained green.
XBP1: X-box-binding protein 1; NSCs: neural stem cells.
Table 2.
Numbers of tyrosine hydroxylase (TH)+ and 5-bromodeoxyuridine (BrdU)+ cells in the substantia nigra

XBP1-NSCs transplantation increased dopamine and 3,4-dihydroxyphenylacetic acid levels in the substantia nigra of PD rats
The dopamine content in the substantia nigra was significantly increased in the NSC group at 28 days following transplantation, compared to the model group, as measured by high performance liquid chromatography (P < 0.05), while the 3,4-dihydroxyphenylacetic acid content remained unchanged (P > 0.05). The dopamine and 3,4-dihydroxyphenylacetic acid levels in the substantia nigra were significantly higher in the XBP1-NSCs group compared to the NSC group (P < 0.05; Table 3).
Table 3.
Dopamine and 3,4-dihydroxyphenylacetic acid levels (ng/mg) in the substantia nigra of rats

XBP1-NSCs transplantation decreased α-synuclein expression in the substantia nigra of PD rats
α-synuclein levels were similar in the NSC and model groups at 28 days following transplantation, as shown by western blot analysis (P > 0.05). However, α-synuclein expression was significantly reduced in the XBP1-NSCs group compared to the NSC and model groups (P < 0.01; Figure 2).
Figure 2.

α-synuclein expression in the substantia nigra of Parkinson's disease rats.
Measurement data were expressed as mean ± SD (absorbance ratio). Intergroup differences were compared using analysis of variance and q-test. There were six rats in the model group, and nine rats each in the NSC and XBP1-NSCs groups. aP < 0.01, vs. model group; bP < 0.01, vs. NSC group. NSC: Neural stem cell; XBP1: X-box-binding protein 1.
DISCUSSION
The rotenone-induced rat model provides a good simulation of PD-related characters in terms of pathogenic mechanisms, pathology, biochemistry and behaviors[9,10]. In the present study, rotenone was delivered to rats using delayed-release microspheres, which can establish a model and maintain a constant blood-drug level in rats. The final success rate of model establishment was 53.3%, indicating that this method provides a good experimental model for studying PD. XBP1 can promote cell differentiation and has been regarded as a crucial transcription factor for the growth, maturation and differentiation of hepatocytes and plasmocytes[11,12]. Moreover, XBP1 can promote NSC differentiation into nerve cells in a rat model of ischemia/reperfusion. The present study marked NSCs with BrdU prior to transplantation to assess the ability of XBP1 to promote NSC survival and differentiation into dopaminergic neurons in a PD environment[13]. There were more BrdU+ cells in the XBP1-NSCs group compared to the NSC group, indicating a stronger survival ability of XBP1-NSCs under conditions of PD-induced stress, compared to normal NSCs. TH is a specific marker of dopaminergic neurons[8]. Co-labeling of BrdU and TH can thus identify dopaminergic neurons differentiated from transplanted NSCs. The ratio of co-labeled BrdU+/TH+ to BrdU+ cells was greater in the XBP1-NSC group compared to the NSC group, indicating that XBP1 promoted NSC differentiation into dopaminergic neurons. In addition, the number of TH+ cells was also significantly greater in the XBP1-NSCs group compared to the NSC group, which could also reflect the ability of XBP1 to protect existing dopaminergic neurons in the substantia nigra against apoptosis[7].
Pathologically, α-synuclein can aggregate rapidly to form Lewy bodies, inducing cell apoptosis or death[14,15]. Activation of the XBP1 gene can clear abnormally accumulated α-synuclein to play a neuroprotective role in PD[16]. In the present study, α-synuclein levels in the substantia nigra were similar in the NSC and model groups, suggesting that normal NSCs have no effect on α-synuclein synthesis or degradation. However, transplantation of XBP1-NSCs significantly reduced α-synuclein levels, indicating that NSCs overexpressing XBP1 were able to clear α-synuclein. Moreover, dopamine and 3,4-dihydroxyphenylacetic acid levels in the substantia nigra were significantly greater, and neurological function was significantly improved in the XBP1-NSCs group compared to the model and NSC groups. Overall, the results of this study indicate that transplantation of XBP1-NSCs is more effective than transplantation of normal NSCs for the treatment of PD.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The experiment was performed in the Laboratory of Neurology, Medical Transformation Center, Norman Bethune First Hospital of Jilin University, China, from March to September 2011.
Materials
Animals
A total of 45 healthy male Sprague-Dawley rats, aged 8–10 weeks, weighing 220–250 g, were provided by the Laboratory Animal Center of Jilin University (License No. SCXK (Ji) 2003-0002). They were separately housed under an artificial 12-hour day/night cycle at 21 ± 2°C and a humidity of 30–35%, with free access to food and water. There were no significant differences in exposure factors among the groups. The experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[17].
Cells
Rat NSCs and XBP1-transfected NSCs were provided by the Laboratory of Neurology, Medical Transformation Center, Norman Bethune First Hospital of Jilin University, China.
Methods
Establishment of PD model
A PD model was established by subcutaneous injection of rotenone microspheres, as previously described[18]. Briefly, rats were maintained at 20–25°C and monitored for blood gas and blood glucose. Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (0.32 mL/100 g) between 8:00 a.m. and 12:00 a.m., followed by subcutaneous injection of rotenone microspheres (60 mg/kg; Hengyuan Biotechnology, Shanghai, China) in the abdominal region. All rats were then subcutaneously injected in the neck region with apomorphine (Hengyuan Biotechnology) after 20 days, to induce rotation. Rats with a mean rotational speed greater than 7 rotations/min were included.
NSC labeling and transplantation
NSCs and XBP1-NSCs were labeled with 10 μM BrdU for 3 days prior to transplantation. NSCs at a density of 1 × 105/μL were transplanted using a microinjection pump (RWD Life Science, Shenzhen, Guangdong Province, China) using a Kopf rat stereotactic apparatus, at a rate of 5 μL/minute, as described previously[19]. Cells were transplanted into the right lateral ventricle, i.e. A: –0.5 mm, R: 3.5 mm, D: 5.0 mm. Model group rats were injected with 20 μL PBS; the NSC group was transplanted with 20 μL NSC suspension; and the XBP1-NSCs group was transplanted with 20 μL XBP1-NSCs suspension.
Behavior detection
All rats were injected with apomorphine at 7, 14, 21 and 28 days following transplantation, to induce rotation[19]. The mean rotational speeds of the rats were recorded. Low rotational speed represented improved neurological function.
Sampling
Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (3.5 mL/kg) at 28 days following transplantation, followed by cardiac perfusion. Brains were harvested and post-fixed with 4% paraformaldehyde phosphate buffer at 4°C for 24 hours. Serial 4-μm-thick coronal sections were prepared from the olfactory bulb, with one section every 100 μm throughout the substantia nigra.
Immunohistochemical staining for NSC distribution and differentiation in the substantia nigra
Paraffin sections of the substantia nigra were dewaxed, hydrated, and re-warmed at room temperature for 0.5 hours, washed three times with 0.01 M PBS (5 minutes each) at 37°C, followed by HCl (2 M) for 0.5 hours. The sections were washed three times with 0.01 M PBS (5 minutes each), mixed with 0.5% Triton X-100 for 30 minutes, followed by a further three washings with 0.01 M PBS (5 minutes each), and blocking with 5% nucleic-acid-stabilizing solution. They were then incubated with mouse anti-rat phycoerythrin-BrdU monoclonal antibody IgG (1:400; BD, San Diego, CA, USA) overnight at 4°C, dried in the dark, incubated with mouse anti-rat glial fibrillary acidic protein-TH monoclonal antibody IgG (1:400; BD) at 37°C for 2 hours, washed three times with PBS (5 minutes each), followed by goat anti-mouse IgM (1:100; BD) at room temperature for 2 hours. The sections were dried and observed under a fluorescence microscope (Olympus, Tokyo, Japan). The mean numbers of TH+, BrdU+ and BrdU+/TH+ cells in five 100 × magnification fields of view surrounding an area of substantia nigra ischemia were quantified, and the ratio of BrdU+/TH+ to BrdU+ cells was calculated.
High performance liquid chromatography analysis of dopamine and 3,4-dihydroxyphenylacetic acid levels in the substantia nigra
Dopamine and 3,4-dihydroxyphenylacetic acid levels in the substantia nigra were assessed by HPLC coupled to electrochemical detection, according to a previously published method[20]. Briefly, substantia nigra tissues were placed in 1.5-mL eppendorf tubes (Eppendorf, Hamburg, Germany), weighed, mixed with cold HClO4 (4 mM; Zhengcheng Chemical Product, Tianjin, China), placed in an ice bath, exposed to ultrasound for 10 seconds (1 Hz), and left for 1 hour. The homogenate was centrifuged at 12 000 r/min at 4°C for 10 minutes. The supernatant was transferred to a clean eppendorf tube, mixed with potassium citrate (20 mM), K2HPO4 (30 mM) and ethylenediamine tetraacetic acid·2Na (2 mM; Zhengcheng Chemical Product) and stored at 4°C. The maximum column pressure for high performance liquid chromatography (J-H Instruments, Shanghai, China) was set to 17 327.5 Pa, with a flow rate of 1.0 mL/minute, and electrochemical detection voltage of 0.65 V. A standard curve was mapped and a linear regression equation was obtained. The supernatant from brain tissues was detected for 35 minutes.
Western blot assay for substantia nigra α-synuclein expression
Substantia nigra tissue (50 mg) was cut into pieces, mixed with ethylenediamine tetraacetic acid-treated tissue lysate on ice for 30 minutes, homogenized for 30 seconds, and centrifuged at 12 000 r/min for 20 minutes. The supernatant was obtained and diluted with lysate. Total protein concentration was determined using the bicinchoninic acid assay[21]. Total protein (15 μg) from each sample was harvested, mixed with 4 μL 6 × loading buffer, placed in boiling water for 5 minutes, and subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 80 V for 40 minutes and 110 V for 90 minutes. It was then electrotransferred to a membrane at 200 mA for 60-90 minutes, blocked with bovine serum albumin (Boster, Wuhan, China) and defatting milk for 2 hours, and eluted six times (10 minutes each) with Tris-buffered saline Tween-20 (Boster). The product was incubated with mouse anti-rat α-synuclein (1:2 000), β-actin (1:2 000) monoclonal antibodies (R&D Systems, Inc., Minneapolis, MN, USA) overnight at 4°C and eluted six times (10 minutes each) with Tris-buffered saline Tween-20. The next day, the samples were incubated with horseradish peroxidase-labeled rabbit anti-mouse polyclonal antibody (1:200; R&D Systems) at room temperature for 2 hours, followed by enhanced chemiluminescence. Results were represented by the absorbance ratio of the target protein to β-actin.
Statistical analysis
Data were analyzed using SPSS version 17.0 (SPSS, Chicago, IL, USA). All data were expressed as mean ± SD, except the ratio of TH+/BrdU+ to BrdU+ cells, which was represented by relative number. Intergroup differences were compared using analysis of variance and q-tests. Differences were considered significant at an alpha level of 0.05.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Ethics Committee of Jilin University, China.
(Edited by Yang HQ, Li XF/Su LL/Song LP)
REFERENCES
- [1].Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569(1-2):29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- [2].Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13(3):385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- [3].Holtz WA, O’Malley KL. Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. J Biol Chem. 2003;278(21):19367–19377. doi: 10.1074/jbc.M211821200. [DOI] [PubMed] [Google Scholar]
- [4].Yoshida H. Unconventional splicing of XBP-1 mRNA in the unfolded protein response. Antioxid Redox Signal. 2007;9(12):2323–2333. doi: 10.1089/ars.2007.1800. [DOI] [PubMed] [Google Scholar]
- [5].Iwakoshi NN, Pypaert M, Glimcher LH. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J Exp Med. 2007;204(10):2267–2275. doi: 10.1084/jem.20070525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Souid S, Lepesant JA, Yanicostas C. The xbp-1 gene is essential for development in Drosophila. Dev Genes Evol. 2007;217(2):159–167. doi: 10.1007/s00427-006-0124-1. [DOI] [PubMed] [Google Scholar]
- [7].Sado M, Yamasaki Y, Iwanaga T, et al. Protective effect against Parkinson's disease-related insults through the activation of XBP1. Brain Res. 2009;1257:16–24. doi: 10.1016/j.brainres.2008.11.104. [DOI] [PubMed] [Google Scholar]
- [8].Benavides-Piccione R, DeFelipe J. Distribution of neurons expressing tyrosine hydroxylase in the human cerebral cortex. J Anat. 2007;211(2):212–222. doi: 10.1111/j.1469-7580.2007.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schmidt WJ, Alam M. Controversies on new animal models of Parkinson's disease pro and con: the rotenone model of Parkinson's disease (PD) J Neural Transm Suppl. 2006;70:273–276. [PubMed] [Google Scholar]
- [10].Xiong N, Huang J, Zhang Z, et al. Stereotaxical infusion of rotenone: a reliable rodent model for Parkinson's disease. PLoS One. 2009;4(11):e7878. doi: 10.1371/journal.pone.0007878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reimold AM, Etkin A, Clauss I, et al. An essential role in liver development for transcription factor XBP-1. Genes Dev. 2000;14(2):152–157. [PMC free article] [PubMed] [Google Scholar]
- [12].Iwakoshi NN, Lee AH, Vallabhajosyula P, et al. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4(4):321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- [13].Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53(1):198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- [14].Auluck PK, Caraveo G, Lindquist S. α-Synuclein: membrane interactions and toxicity in Parkinson's disease. Annu Rev Cell Dev Biol. 2010;26:211–233. doi: 10.1146/annurev.cellbio.042308.113313. [DOI] [PubMed] [Google Scholar]
- [15].Brown DR. Oligomeric alpha-synuclein and its role in neuronal death. IUBMB Life. 2010;62(5):334–339. doi: 10.1002/iub.316. [DOI] [PubMed] [Google Scholar]
- [16].Jiang P, Gan M, Ebrahim AS, et al. ER stress response plays an important role in aggregation of α-synuclein. Mol Neurodegener. 2010;5:56. doi: 10.1186/1750-1326-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [18].Huang J, Liu H, Gu W, et al. A delivery strategy for rotenone microspheres in an animal model of Parkinson's disease. Biomaterials. 2006;27(6):937–946. doi: 10.1016/j.biomaterials.2005.07.005. [DOI] [PubMed] [Google Scholar]
- [19].Yang DB, Li YL, Yang LZ, et al. Experimental study of intraventricular neural stem cell transplantation for rat focal cerebral ischemia. Chinese Journal of Minimally Invasive Neurosurgery. 2006;11:27–29. [Google Scholar]
- [20].Aoki S, Inukai K, Sakurai M, et al. Monitoring of dopamine metabolism by in vivo voltammetry and high performance liquid chromatography in L-dopa-treated rats. Jpn J Pharmacol. 1987;43(1):98–102. doi: 10.1254/jjp.43.98. [DOI] [PubMed] [Google Scholar]
- [21].Li H, Sun NL, Wang J, et al. Circadian expression of clock genes and angiotensin II type 1 receptors in suprachiasmatic nuclei of sinoaortic-denervated rats. Acta Pharmacol Sin. 2007;28(4):484–492. doi: 10.1111/j.1745-7254.2007.00543.x. [DOI] [PubMed] [Google Scholar]


