Abstract
In the present study, human umbilical cord blood mesenchymal stem cells were injected into a rat model of traumatic brain injury via the tail vein. Results showed that 5-bromodeoxyuridine-labeled cells aggregated around the injury site, surviving up to 4 weeks post-transplantation. In addition, transplantation-related death did not occur, and neurological functions significantly improved. Histological detection revealed attenuated pathological injury in rat brain tissues following human umbilical cord blood mesenchymal stem cell transplantation. In addition, the number of apoptotic cells decreased. Immunohistochemistry and in situ hybridization showed increased expression of brain-derived neurotrophic factor, nerve growth factor, basic fibroblast growth factor, and vascular endothelial growth factor, along with increased microvessel density in surrounding areas of brain injury. Results demonstrated migration of transplanted human umbilical cord blood mesenchymal stem cells into the lesioned boundary zone of rats, as well as increased angiogenesis and expression of related neurotrophic factors in the lesioned boundary zone.
Keywords: angiogenesis, basic fibroblast growth factor, brain-derived neurotrophic factor, human umbilical cord blood mesenchymal stem cells, nerve growth factor, traumatic brain injury, vascular endothelial growth factor
Abbreviations:
TBI, traumatic brain injury; BDNF, brain-derived neurotrophic factor; NGF, nerve growth factor; bFGF, basic fibroblast growth factor; MSC, mesenchymal stem cell; hUCB, human umbilical cord blood
INTRODUCTION
Traumatic brain injury (TBI) has a high incidence rate worldwide, and severe TBI may result in neurological deficits[1]. An ischemic effect is the main mechanism of secondary lesions. Angiogenesis surrounding brain injury areas is the foundation for improved blood supply and plays an important role in synapse connection between neurons and functional reconstruction[2,3]. Therefore, therapeutic angiogenesis provides a promising strategy for treating cerebral ischemia. The microenvironment surrounding brain injury areas is important for neuronal survival, differentiation, propagation, and regeneration[2]. In addition, the microenvironment contains factors, such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and basic fibroblast growth factor (bFGF), which are broad-spectrum neurotrophic factors that have been shown to induce axonal growth, as well as increase survival and growth of neurons, glial cells, and endotheliocytes[3,4,5,6]. Moreover, these growth factors have been shown to attenuate hypoxia, excitatory amino acids, and free radical-induced injury, as well as promote neuronal survival under pathological condition[7].
Despite aggressive clinical management, neurons rarely undergo self-repair and there are few therapeutic methods to reverse neural injury. In recent years, bone marrow-derived mesenchymal stem cell (MSC) transplantation has provided a novel treatment for brain injury repair. MSCs provide potential therapeutic benefits in a wide range of neurological diseases, such as amyotrophic lateral sclerosis and Parkinson's disease[8,9,10,11,12,13,14,15]. Compared with bone marrow-derived MSCs, human umbilical cord blood (hUCB) MSCs (hUCB-MSCs) are extensively available, convenient to sample, and free of ethical issues[16,17,18]. Studies have suggested that hUCB-MSCs exhibit favorable effects on nervous system injuries[16,17,18]. Nevertheless, the related mechanisms remain poorly understood. The present study transplanted hUCB-MSCs in a TBI rat model to observe migration and distribution of hUCB-MSCs in the brain. In addition, the cerebroprotective effects of hUCB-MSCs transplantation were analyzed through the use of histology, behavioral analysis, angiogenesis measurements, and levels of cytokine secretion.
RESULTS
Quantitative analysis of experimental animals
A total of 90 rats were randomly assigned to three groups: sham-surgery (sham surgery), model (TBI model), and transplantation (hUCB-MSCs transplantation via tail vein following TBI model establishment). Six rats from each group were selected at 3, 7, 14, 21, and 28 days after transplantation, respectively. In total, 90 rats were included in the final analysis.
Survival and migration of transplanted hUCB-MSCs in the brain
Immunohistochemical staining revealed no bromodeoxyuridine (BrdU)-labeled cells in the sham-surgery or model groups. However, BrdU-labeled cells were observed in the transplantation group and were evenly distributed around the injury site, but very few positive cells were seen in the contralateral hemisphere. In addition, positive cells survived up to 28 days following transplantation. The number of BrdU-labeled cells (/400-fold field of view) was 76.17 ± 8.30, 62.33 ± 8.40, 54.33 ± 7.50, 45.50 ± 6.59, and 28.67 ± 2.73, respectively at 3, 7, 14, 21, and 28 days following hUCB-MSC transplantation, revealing a decreasing trend (Figure 1).
Figure 1.

Bromodeoxyuridine expression in the injured cerebral cortex at 14 days after human umbilical cord blood mesenchymal stem cell transplantation (immunohistochemistry, light microscope, × 200): bromodeoxyuridine-positive cells (arrows) are distributed around the injury area.
hUCB-MSC transplantation ameliorated pathological changes in brain tissues of TBI rats
Light microscopy revealed normal cellular structures in rat brains of the sham-surgery group (Figure 2A). Breakdown of tissue, neuronal degeneration, shrunken cell bodies, cell swelling, lysis, disrupted cell membranes, and clumped cells with condensed nuclei were observed in the cerebral cortex of the model group (Figure 2B). Pathological changes were less in the transplantation group compared with the model group (Figure 2C).
Figure 2.
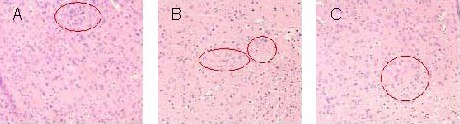
Pathological changes in the cerebral cortex of rats with traumatic brain injury at 3 days after human umbilical cord blood mesenchymal stem cell transplantation (hematoxylin-eosin staining, light microscope, × 200). Cells in the circles represent cortical brain cells.
(A) Sham-surgery group displays normal cell structure.
(B) In the model group, a large number of necrotic cells are observed, accompanied by shrunken nuclei and absent cell structures.
(C) In the transplantation group, cells are slightly swollen, and the pyknosis is less than in the model group.
hUCB-MSC transplantation inhibited cell apoptosis in brain tissues of TBI rats
Compared with the model group at each time point, the number of terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-positive cells surrounding brain injury areas significantly decreased following hUCB-MSCs transplantation (P < 0.05; Figure 3, supplementary Figure 1 online).
Figure 3.
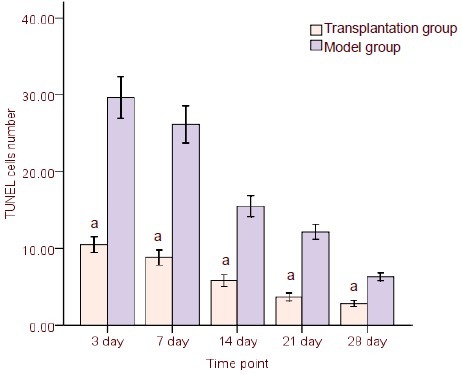
Comparison of apoptotic rate of cells between transplantation and model groups at different time points (terminal deoxynucleotidyl transferase dUTP nick-end labeling, TUNEL).
Cell number (/200-fold field of view) is presented as mean ± SD from six rats/group at each time point.
aP < 0.05, vs. model group (t-test was used to specify differences between two groups at corresponding time points).
hUCB-MSC transplantation increased neurotrophic factor expression surrounding injury areas in brain tissue of TBI rats
Immunohistochemistry and in situ hybridization results revealed significantly increased NGF protein, BDNF protein, and mRNA expression in injury areas and surrounding tissues of the transplantation group compared with the model group at each time point (P < 0.05, respectively). In addition, the number of positive cells in model and transplantation groups peaked at 14 days post-transplantation and decreased gradually thereafter. Nevertheless, the number of positive cells remained greater in the transplantation group compared with the model group at 21 and 28 days (Figure 4, supplementary Figures 2–4 online). Very few NGF- and BDNF-positive cells were observed in the sham-surgery group and were not included in the statistical analysis.
Figure 4.
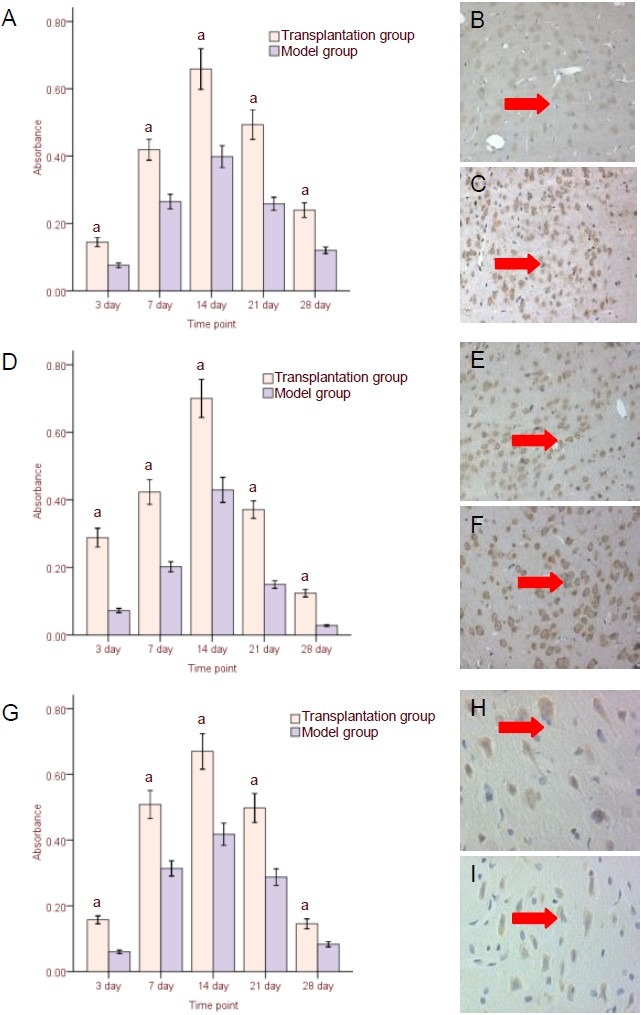
Neurotrophic factor expression in surrounding injured brain tissues following human umbilical cord blood mesenchymal stem cell transplantation.
(A–C) Nerve growth factor (NGF) protein expression (immunohistochemistry, × 200); (D–F) brain-derived neurotrophic factor (BDNF) protein expression (immunohistochemistry, × 200); (G–I) BDNF mRNA expression (in situ hybridization, × 400).
(A, D, G) Results (absorbance) are expressed as mean ± SD from six rats in each group at each time point.
aP < 0.05, vs. model group (t-test was used to specify differences between two groups at the corresponding time points); (B, E, H) model group at 14 days after transplantation; (C, F, I) transplantation group at 14 days after transplantation. Arrows: NGF protein-, BDNF protein-, and BDNF mRNA-positive cells.
hUCB-MSC transplantation promoted angiogenesis and related cytokine expression surrounding injured brain tissue in TBI rats
Microvessel density was assessed by CD34 immunostaining. A large number of microvessels were observed in the injured cortex at 3 days post-injury, and peaked at 14 days. Microvessel density in the injured cortex was significantly greater in the transplantation group compared with the model group at each time point (P < 0.05; Figure 5, supplementary Figure 5 online). Very few CD34-positive cells were observed in the sham-surgery group and were not included in the statistical analysis.
Figure 5.
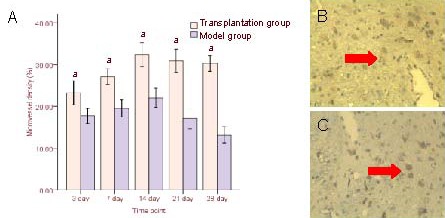
CD34-positive cells after human umbilical cord blood mesenchymal stem cell transplantation.
(A) Comparison of CD34 expression at different time points in transplantation and model groups. Results (microvessel density) are expressed as mean ± SD from six rats in each group at each time point. aP < 0.05, vs. model group (t-test).
CD34-positive cells (arrows) from immunohistochemistry in model (B) and transplantation groups at 14 days after transplantation (× 200).
Immunohistochemistry and in situ hybridization revealed numerous cells expressing vascular endothelial growth factor (VEGF) protein and mRNA, as well as bFGF protein, in injured brain tissues of the model group. The number of cells peaked at 3 and 7 days post-transplantation, respectively. However, the number of positive cells gradually decreased at 21 and 28 days in the model group. VEGF protein and mRNA, as well as bFGF protein, expression significantly increased following hUCB-MSC transplantation compared with the model group at each time point (P < 0.05, respectively; Figure 6, supplementary Figures 6–8 online). Very few positive cells were detected in the sham-surgery group and were not included in the statistical analysis.
Figure 6.
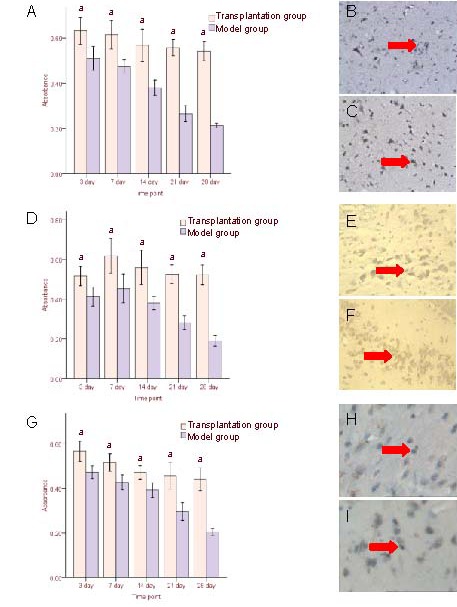
Basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) expression in the cortex surrounding the injury following human umbilical cord blood mesenchymal stem cell transplantation.
(A–C) bFGF protein expression (immunohistochemistry, × 200); (D–F) VEGF protein expression (immunohistochemistry, × 200); (G–I) VEGF mRNA expression (in situ hybridization, × 200).
(A, D, G) Results (absorbance) are expressed as mean ± SD from six rats in each group at each time point. aP < 0.05, vs. model group (t-test).
(B, E, H) Model group at 3 (B, H) and 7 (E) days after cell transplantation; (C, F, I) transplantation group at 3 (C, I) and 7 (F) days after cell transplantation.
Arrows: bFGF protein-, VEGF protein-, and VEGF mRNA- positive cells.
hUCB-MSC transplantation improved neurological function in TBI rats
TBI is typically accompanied by characteristic behavioral deficits. The sham-surgery group did not exhibit neurological deficits based on neurological severity scores (0 score). At 3 days post-hUCB-MSC transplantation, neurological severity scores were similar between transplantation and model groups (P > 0.05). At 7, 14, 21, and 28 days, scores significantly decreased in the transplantation group compared with the model group (P < 0.05; Figure 7).
Figure 7.
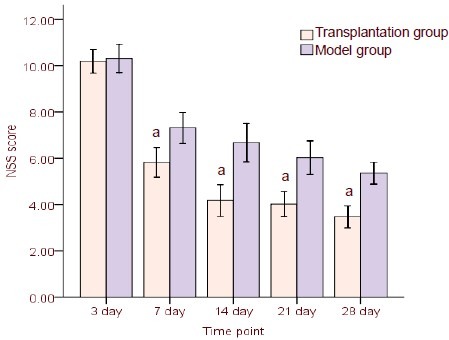
Neurological severity scores in rats following human umbilical cord blood mesenchymal stem cell transplantation. High scores represent severe injury.
Results are presented as mean ± SD from six rats in each group at each time point. aP < 0.05, vs. model group (t-test).
DISCUSSION
Traumatic injuries to the central nervous system are attributed to acute and progressive loss of neurons and glial cells. Central nervous system regeneration and repair remain difficult to accomplish.
Following TBI, brain tissue extracts revealed significantly increased MSC migration across the membrane compared with non-TBI tissue, suggesting that MSCs are targeted by inflammatory chemotactic agents and cytokines, such as interleukin-8, monocyte chemotactic protein-1, and macrophage inflammatory protein-1 alpha, which surrounds injured brain tissue[19]. Previous studies have shown that stromal cell-derived factor-l promotes homing of circulating MSCs to actively remodeling neovasculature. MSCs expressing CXCR4 were shown to home to sites of active neovascularization, but not to normal non-immune tissues. In addition, antagonists of stromal cell-derived factor-l block in vitro and in vivo adhesion of these cells to endothelia, as well as homing to neovasculature[20,21]. In the present study, transplanted cells were found in multiple brain areas. The highest concentration of labeled cells survived in the lesion site and migrated 5 mm from injured tissues. However, there were few cells in the contralateral cortex, indicating that hUCB-MSCs crossed the blood-brain barrier and migrated into damaged regions. Histological analysis showed surviving MSCs up to 4 weeks post-transplantation in the brain. However, the number of cells was less than at 1 week post-transplantation. Previous clinical studies have described the migration of MSCs to injury sites and subsequent survival and differentiation of these cells into neurons and astrocytes, subsequently resulting in improved neurological functions[22,23,24]. Doubts remain regarding the ability of MSCs to transdifferentiate into neurons, as well as the frequency of transdifferentiation. MSCs have been shown to differentiate into neural cells in vitro[16,25], although donor cells do not frequently transform into neuronal cells post-transplantation[26,27]. The beneficial effects of MSCs following TBI were not likely due to cellular replacement, because only a small percentage of transplanted MSCs express parenchymal cell phenotypes[28]. At 1 month post-transplantation, the number of MSCs that exhibit astrocytic or neuronal differentiation is low (6–13%), but at 3 months, no transplanted MSCs exhibit astrocytic or neuronal differentiation[23]. The ability of these transplanted cells to establish functional synaptic connections with host tissue remains uncertain.
Recent evidence has shown that the latent therapeutic benefits of MSCs therapy is due to the following mechanisms: secretion of growth factors[29]; stabilization of damaged cells via gene/protein transfer through cell-to-cell contact or fusion; induction of angiogenesis[12]; and effects on immune regulation[30]. Endogenous MSCs are likely activated after TBI and participate in neural recovery, although the levels might be insufficient to induce remarkable recovery. With an additional exogenous MSC supply, this recovery process could be amplified and subsequently accelerate neurological improvement.
Widely distributed neuronal damage is well documented in the cerebral cortex and hippocampus of the rat CNS during the first few hours after TBI. The acute phase of neuronal damage is followed by a delayed phase of neuronal loss in the affected areas within the first 4 weeks after TBI[31,32]. Neurons induced to undergo apoptosis in vitro can be rescued by resupplying nerve growth factor within 12 hours after growth factor deprivation[22]. Compared with the model group, the number of TUNEL-positive cells in surrounding brain injury areas significantly decreased in rats following hUCB-MSC transplantation.
The important role of neurotrophic growth factors in neural repair and regeneration has been well established[19,33,34,35,36,37]. MSCs cultured with supernatant of homogenized ischemic brain tissue from a rat model of middle cerebral artery occlusion showed an increase in BDNF, NGF, VEGF, epidermal growth factor, fibroblast growth factor 2, and insulin-like growth factor 1 compared with the model group[38]. Increased levels of nerve growth factors could stimulate resident tissue cells to undergo repair and could account for the functional benefits of MSC therapy. Neurotrophic factors control intercellular and intracellular signaling pathways that sculpt neuronal circuits during brain development and fundamentally regulate plasticity, as well as cell survival, in the brain. These factors not only enhance survival, but also rescue neural cells from apoptosis[22]. In addition, trophic factors enhance angiogenesis and vascular stabilization in the lesion boundary area, where large numbers of surviving MSCs are located in the brain. Microcirculation disturbances after TBI result in nervous system perfusion defect, which induce irreversible degeneration and necrosis of brain tissue. In addition to neuronal regeneration and reconstruction of synapses, it has been hypothesized that neuroprotection, angiogenesis, and functional recovery could provide beneficial effects for reconstruction and function of endothelial cells. The promotion of neurogenesis and remodeling to provide sufficient nutrition and regulation of regional microenvironments should remain a therapeutic goal.
Angiogenesis, the process of new blood vessel formation from pre-existing vessels, is critical for brain development and repair[2]. When anti-angiogenic agents are used to suppress neovascularization, neurogenesis is also impaired, suggesting that hUCB-MSCs exhibit an angiogenic effect to support endogenous neurogenesis[9]. Recent studies have shown that MSC transplantation provides therapeutic effects through angiogenesis[12,26]. In addition, an intravenous injection of MSCs following induced ischemic stroke in a rat model was shown to increase expression of VEGF and VEGF receptor 2, as well as angiogenesis in the transition zone[39]. Indeed, the present study demonstrated significantly increased angiogenesis and expression of angiogenic cytokines, including VEGF and bFGF, in TBI rats following hUCB-MSC transplantation. Increased growth factor production continued over time, at least until 4 weeks post-MSC treatment.
Results from the present study demonstrated that intravenous MSC transplantation from the human umbilical cord was beneficial in a rat model of TBI. The underlying mechanisms could involve cytokine production in surrounding injured brain tissues, which then stimulate and attract MSCs to migrate into the lesion boundary zone. Within parenchymal cells, MSCs secrete and induce neurotrophic growth factors and pro-angiogenic cytokines, such as BDNF, NGF, and VEGF, thereby further amplifying endogenous brain levels. These factors influence several neural restorative functions, such as synaptogenesis, angiogenesis, and neurogenesis. Through these mechanisms, MSCs were shown to inhibit apoptosis and repair injured brain tissue. Therefore, hUCB-MSCs could serve as a potential cell candidate for cell-based therapy of neurological disorders.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The experiments were performed in the Central Laboratory of the Affiliated Hospital of Hebei United University, China from August 2010 to July 2011.
Materials
A total of 90 male, Sprague-Dawley rats, aged 2–3 months and weighing 250–350 g, were purchased from Vital River Laboratories, Beijing, China (license No. SCXK (Jing) 2007-0001). The rats were housed under natural illumination and were allowed free access to food and water. Experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[40].
Methods
hUCB-MSC labeling
hUCB-MSCs were graciously provided by the Institute of Hematology, Chinese Academy of Medical Science. hUCB-MSC isolation, culture, and identification were performed according to previous methods[16]. Cells were cultured and the culture medium was replaced until the fourth passage. The cells were then trypsinized and washed with phosphate-buffered saline (PBS). The dividing hUCB-MSCs were labeled with BrdU (200 μM; Sigma, St. Louis, MO, USA) for 72 hours prior to transplantation. Subsequently, immunocytochemistry was used to detect cell-labeling efficiency, and the trypan blue exclusion test was used to determine cell viability. Cells with efficiency and viability rates > 90% were utilized in the following experiments.
Establishment of the TBI model
The TBI model was modified from the Feeney free-falling method[41]. Adult male, Sprague-Dawley rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate (3 mL/kg), and a 2.0-cm long incision was made through the sagittal median line to separate the subcutaneous tissue and periosteum and to expose bregma, coronal suture, biparietal suture, and bilateral parietal bone. To maintain endocranium integrity, a 5-mm diameter bone window was made using a hand drill at 4 mm behind the right coronal suture and 2 mm lateral to the median line. Moderate TBI was induced by a free-fall, 30-cm high drop using a 20-driving hammer, which impacted the endocranium. The bump-injury power was 0.048 kg·m/s. Model establishment was considered successful if the rats exhibited all conditions, including limb convulsion, apnea for several seconds after injury, and unconsciousness for 2–10 hours (average 6.5 hours). In the sham-surgery group, only the bone window was exposed.
hUCB-MSC transplantation
Twenty-four hours after modeling, the rats were injected with 3 × 106 hUCB-MSCs suspended in 1 mL PBS via the tail vein. Equal volumes of PBS were administered to the model group, and the sham-surgery group received no treatment. All groups did not receive any immunosuppressive agents.
Neurological evaluation
Neurological function was evaluated using a modified Neurological Severity Score according to Mahmood's method[42] at 3, 7, 14, 21, and 28 days post-TBI by two individuals blinded to the experimental groups. Neurological functions were graded on a scale of 0 to 18. A greater score represented a more severe injury.
Sample preparation of injured brain tissues
Under deep anesthesia with an intraperitoneal injection of 10% chloral hydrate (3 mL/kg), six rats from each group were sacrificed at 3, 7, 14, 21, and 28 days post-transplantation. A synthetic tube was punctured and inserted from the apex of the heart to aorta ascendens. The left auricle was opened, and NaCl solution was transcardially perfused at 37°C for 5-10 minutes, followed by 200 mL 4% paraformaldehyde/0.1 M PBS (pH 7.4). The entire brain tissues were harvested, and the injury focus and surrounding tissues within 3 cm were harvested, further fixed in 4% paraformaldehyde for 24 hours, dehydrated, cleared, paraffin-embedded, and sectioned for hematoxylin-eosin, TUNEL staining, immunohistochemistry, and in situ hybridization.
TUNEL staining for cell apoptosis in brain tissues
TUNEL staining was performed according to kit manufacturer instructions (Tianjin Haoyang Biotechnology, Tianjin, China). TUNEL-positive cells from ten non-overlapping fields of view of areas surrounding the injury were quantified under high-power magnification (× 400; Olympus, Tokyo, Japan), and the average number of cells was calculated.
Immunohistochemical staining for BrdU, BDNF, NGF, VEGF, CD34, and bFGF expression in brain tissues
Immunohistochemistry staining was performed according to kit instructions. To visualize expression of neuron-specific markers, sections were incubated in primary antibodies against human-derived neuronal and angiogenesis-specific trophic factors. Primary antibodies were incubated overnight at 4°C in wet box with the following dilutions: rat anti-human BrdU monoclonal antibody (1:150; Beijing Boaosen Biotechnology, China), rabbit anti-mouse BDNF (1:150; Boster, Wuhan, China), NGF (1:150; Boster), VEGF (1:150; Boster), CD34 (1:100; Boster), bFGF (1:100; Beijing Boaosen Biotechnology) polyclonal antibody. Negative control sections were incubated with no primary antibodies. All sections were subsequently incubated with biotinylated goat anti-rabbit/mouse IgG (1:100; Boster) at 37°C for 20 minutes.
Vascular density and regions were analyzed by CD34 immunohistochemical staining according to Weidner et al[43]. Five random areas from the peripheral TBI region were imaged at a magnification of × 200. Vascular areas were measured using Image-Pro Plus software package (Media Cybernetics, Carlsbad, CA, USA), and vascular density was calculated according to the percentage of vascular areas in randomly selected regions. Cells were quantified by two observers blinded to the experimental treatment. Morphological analysis, as well as expressional analysis of BDNF, NGF, VEGF, and bFGF, in the temporal parietal cortex at different time points in each group was performed. Stained cells in 20 fields of view from each section were quantified at a magnification of × 200, and the results were expressed as absorbance.
In situ hybridization of BDNF and VEGF mRNA expression in brain tissues
In situ hybridization staining was performed according to kit instructions. To visualize expression of neuron-specific markers, sections were processed for immunohistochemistry and in situ hybridization using primary antibodies against human-derived neuronal and angiogenesis-specific trophic factors (BDNF and VEGF in situ hybridization kit; Tianjin Haoyang Biotechnology, China). Negative control sections were incubated without primary antibodies.
BDNF mRNA probes for in situ hybridization:
5-CTT ACT ATG GTT ATT TCA TAC TTT GGT TGC
VEGF mRNA probes for in situ hybridization:
5-AGG AAC ATT TAC ACG TCT GCG GAT CTT GAT GTT CCT
Diethyl pyrocarbonate (1:1 000) was added to all solutions in the experiment to terminate RNA activity. Procedures of brain tissue section preparation were identical to immunohistochemistry methods. Tissues were incubated with hybridization fluid (25 μL/section) (probe density of 8 μg/mL hybridization solution) at 37°C in a humidity chamber for 2–4 hours. Negative controls were incubated in 0.01 M PBS. Sections were incubated with biotinylated rat anti-digoxin (25 μL/section) at 37°C for 45 minutes, followed by horseradish peroxidase-avidin (25 μL/section) at 37°C for 45 minutes. Five random areas from the peripheral TBI region were imaged with a magnification of × 200. Positive cells were quantified using Image-Pro Plus software package (Media Cybernetics, Carlsbad, CA, USA) by two observers blinded to the experimental treatment. Expression of BDNF and VEGF mRNA in the temporal parietal cortex of each group was analyzed. Positive cells from 20 fields of view from each section were quantified at a magnification of × 200, and the results were expressed as absorbance.
Statistical analysis
All data were expressed as mean ± SD. Significance was analyzed using independent samples t-test. Statistical significance was set to P < 0.05.
Acknowledgments:
Acknowledgments: We thank Xianhua Wang, Department of Pathology, Hebei United University and Xiuhua Han, Animal Laboratory of the Affiliated Hospital, Hebei united University for technical support. The authors also thank Aiguo Meng, Yurong Chang, and Aiying Dong at the Hematology Laboratory of the Affiliated Hospital of Hebei United University for their help.
Footnotes
Funding: The study was supported by Medical Scientific Research Program of Hebei Province in 2010, Hebei Provincial Health Department, No. 20100131.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Care and Research Committee of Hebei United University, Tanshan, Heibei Province, China
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org, and entering Vol. 7, No. 10, 2012 after selecting the “NRR Current Issue” button on the page.
(Edited by Yang XF, Bao JF/Su LL/Song LP)
REFERENCES
- [1].Moppett IK. Traumatic brain injury: assessment, resuscitation and early management. Br J Anaesth. 2007;99(1):18–31. doi: 10.1093/bja/aem128. [DOI] [PubMed] [Google Scholar]
- [2].Zadeh G, Guha A. Angiogenesis in nervous system disorders. Neurosurgery. 2003;53(6):1362–1376. doi: 10.1227/01.neu.0000093425.98136.31. [DOI] [PubMed] [Google Scholar]
- [3].Cheng B, Matton MP. NT-3 and BDNF protect CNS neurons against metabolic/excitotoxic insults. Brain Res. 1994;640(1-2):56–67. doi: 10.1016/0006-8993(94)91857-0. [DOI] [PubMed] [Google Scholar]
- [4].Ferrer I, Ballabriga J, Marti E, et al. BDNF up-regulates TrkB protein and prevents the death of CA1 neurons following transient forebrain ischemia. J Brain Pathol. 1998;8(2):253–261. doi: 10.1111/j.1750-3639.1998.tb00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kawamata T, Dietrich WD, Schallert T, et al. Intracisternal basic fibroblast growth factor enhances functional recovery and up-regulates the expression of a molecular marker of neuronal sprouting following focal cerebral infarction. Proc Natl Acad Sci U S A. 1997;94(15):8179–8184. doi: 10.1073/pnas.94.15.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jin K, Mao XO, Greenberg DA, et al. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66(3):236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- [7].Marti HJ, Bernaudin M, Bellail A, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156(3):965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Park HJ, Lee PH, Bang OY, et al. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson's disease. J Neurochem. 2008;107(1):141–151. doi: 10.1111/j.1471-4159.2008.05589.x. [DOI] [PubMed] [Google Scholar]
- [9].Mazzini L, Mareschi K, Ferrero I, et al. Stem cell treatment in Amyotrophic Lateral Sclerosis. J Neurol Sci. 2008;265(1-2):78–83. doi: 10.1016/j.jns.2007.05.016. [DOI] [PubMed] [Google Scholar]
- [10].Tang J, Xie Q, Pan G, et al. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg. 2006;30(2):353–361. doi: 10.1016/j.ejcts.2006.02.070. [DOI] [PubMed] [Google Scholar]
- [11].Wu KH, Zhou B, Yu CT, et al. Therapeutic potential of human umbilical cord derived stem cells in a rat myocardial infarction model. Ann Thorac Surg. 2007;83(4):1491–1498. doi: 10.1016/j.athoracsur.2006.10.066. [DOI] [PubMed] [Google Scholar]
- [12].Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92(6):692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- [13].Mahmood A, Lu D, Qu C, et al. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J Neurosurg. 2006;104(2):272–277. doi: 10.3171/jns.2006.104.2.272. [DOI] [PubMed] [Google Scholar]
- [14].Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- [15].Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev. 2001;122(7):713–734. doi: 10.1016/s0047-6374(01)00224-x. [DOI] [PubMed] [Google Scholar]
- [16].Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91(8):1017–1026. [PubMed] [Google Scholar]
- [17].Secco M, Zucconi E, Vieira NM, et al. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008;26(1):146–150. doi: 10.1634/stemcells.2007-0381. [DOI] [PubMed] [Google Scholar]
- [18].Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25(6):1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- [19].Lu D, Mahmood A, Chopp M. Biologic transplantation and neurotrophin-induced neuroplasticity after traumatic brain injury. J Head Trauma Rehabil. 2003;18(4):357–376. doi: 10.1097/00001199-200307000-00006. [DOI] [PubMed] [Google Scholar]
- [20].Li N, Lu X, Zhao X, et al. Endothelial nitric oxide synthase promotes bone marrow stromal cell migration to the ischemic myocardium via upregulation of stromal cell-derived factor-1alpha. Stem Cells. 2009;27(4):961–970. doi: 10.1002/stem.6. [DOI] [PubMed] [Google Scholar]
- [21].Zhou B, Han ZC, Poon MC, et al. Mesenchymal stem/stromal cells (MSC) transfected with stromal derived factor 1 (SDF-1) for therapeutic neovascularization: enhancement of cell recruitment and entrapment. Med Hypotheses. 2007;68(6):1268–1271. doi: 10.1016/j.mehy.2006.09.066. [DOI] [PubMed] [Google Scholar]
- [22].Rink A, Fung KM, Trojanowski JQ, et al. Evidence of apoptotic cell death after experimental traumatic brain injury in the rat. Am J Pathol. 1995;147(6):1575–1583. [PMC free article] [PubMed] [Google Scholar]
- [23].Mahmood A, Lu D, Lu M, et al. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53(3):697–703. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- [24].Mahmood A, Lu D, Qu C, et al. Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurgery. 2005;57(5):1026–1031. doi: 10.1227/01.neu.0000181369.76323.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen NY, Zhang J, Wang DL, et al. Experiment on directional differentiation of human umbilical cord blood mesenchymal stem cells into neural-like cells induced in Vitro by retinoic acid combined with brain-derived neurotrophic factors. Zhonghua Yixue Quanke Zazhi. 2010;13(9):947–949. [Google Scholar]
- [26].Liao W, Xie J, Zhong J, et al. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation. 2009;87(3):350–359. doi: 10.1097/TP.0b013e318195742e. [DOI] [PubMed] [Google Scholar]
- [27].Fatar M, Stroick M, Griebe M, et al. Lipoaspirate-derived adult mesenchymal stem cells improve functional outcome during intracerebral hemorrhage by proliferation of endogenous progenitor cells stem cells in intracerebral hemorrhages. Neurosci Lett. 2008;443(3):174–178. doi: 10.1016/j.neulet.2008.07.077. [DOI] [PubMed] [Google Scholar]
- [28].Lu D, Li Y, Wang L, et al. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001;18(8):813–819. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- [29].Chen X, Katakowski M, Li Y, et al. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J Neurosci Res. 2002;69(5):687–691. doi: 10.1002/jnr.10334. [DOI] [PubMed] [Google Scholar]
- [30].Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- [31].Adams JH, Doyle D, Ford I, et al. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15(1):49–49. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- [32].Povlishock JT, Erb DE, Astruc JC. Axonal response to traumatic brain injury: reactive axonal change, differentation, and neuroplasticity. J Neurotrauma. 1992;9(suppl 1):S189–200. [PubMed] [Google Scholar]
- [33].Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59(4):514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- [34].Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004;21(1):33–39. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- [35].Newman MP, Féron F, Mackay-Sim A. Growth factor regulation of neurogenesis in adult olfactory epithelium. Neuroscience. 2000;99(2):343–350. doi: 10.1016/s0306-4522(00)00194-9. [DOI] [PubMed] [Google Scholar]
- [36].Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2(1):24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- [37].Vicario-Abejón C, Collin C, McKay RD, et al. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci. 1998;18(18):7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Qu R, Li Y, Gao Q, et al. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007;27(4):355–363. doi: 10.1111/j.1440-1789.2007.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- [40].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [41].Feeney DM, Boyeson MG, Linn RT, et al. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981;211(1):67–77. doi: 10.1016/0006-8993(81)90067-6. [DOI] [PubMed] [Google Scholar]
- [42].Mahmood A, Lu D, Wang L, et al. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49(5):1196–1204. [PubMed] [Google Scholar]
- [43].Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]


