Abstract
Following successful establishment of a rat model of spinal cord hemisection injury by resecting right spinal cord tissues, bone marrow stem cells were transplanted into the spinal cord lesions via the caudal vein while maintaining rectal temperature at 34 ± 0.5°C for 6 hours (mild hypothermia). Hematoxylin-eosin staining showed that astrocytes gathered around the injury site and formed scars at 4 weeks post-transplantation. Compared with rats transplanted with bone marrow stem cells under normal temperature, rats transplanted with bone marrow stem cells under hypothermia showed increased numbers of proliferating cells (bromodeoxyuridine-positive cells), better recovery of somatosensory-evoked and motor-evoked potentials, greater Basso, Beattie, and Bresnahan locomotor rating scores, and an increased degree of angle in the incline plate test. These findings suggested that hypothermia combined with bone marrow mesenchymal stem cells transplantation effectively promoted electrical conduction and nerve functional repair in a rat model of spinal cord hemisection injury.
Keywords: bone marrow mesenchymal stem cells, electrophysiological function, hypothermia, spinal cord hemisection injury, transplantation
Abbreviations:
BMSCs, bone marrow-derived mesenchymal stem cells; SCI, spinal cord injury; SEP, somatosensory-evoked potential; MEP, motor-evoked potential
INTRODUCTION
Bone marrow-derived mesenchymal stem cells (BMSCs) can differentiate into a variety of nervous system cells, thereby providing possible therapeutic strategies for nerve injury repair[1,2,3,4,5,6]. However, BMSC transplantation alone is not sufficient to repair spinal cord injury (SCI), because the majority of BMSCs transplanted into the spinal cord differentiate into glial cells and rarely survive[7]. The microenvironment of the injured spinal cord plays a crucial role in inducing differentiation and survival of transplanted BMSCs[8,9,10,11]. In recent years, hypothermia (33–35°C) has been widely studied as a beneficial approach due to its neuroprotective effects against secondary injury, providing hope for the treatment of brain injury and SCI[12]. The present study hypothesized that hypothermia influences differentiation and survival of transplanted BMSCs, as well as improves the microenvironment of the injured spinal cord. To test this hypothesis, the microenvironment was modified through hypothermia. The aim of the present study was to analyze the effects of BMSC transplantation in combination with hypothermia for the recovery of SCI in a rat model.
Electrophysiological tests, such as somatosensory-evoked potential (SEP) and motor-evoked potential (MEP), provide objective methods to monitor descending, ascending, and segmental pathways in the spinal cord[13,14,15]. These tests have supplemented the behavioral evaluation of neurological functions in humans and animals[16]. The technique of combined MEP and SEP recordings could potentially allow for the assessment of rats with or at risk for SCI, as well as assessment of treatments for experimental cord injury[17]. In the present study, SEP and MEP were utilized to continuously monitor spinal cord hemisection injury and subsequent treatment.
RESULTS
Quantitative analysis of experimental animals
Sixty-eight 3-month-old, Sprague-Dawley rats were included in the study for model establishment through resection of the right hemisphere of the spinal cord. However, eight rats died during SCI modeling. The remaining 60 rats were equally assigned to three groups (n = 20) for analysis: a model group, a BMSC + normal temperature group and a BMSC + mild hypothermia group. In the BMSC + normal temperature group and the model group, rectal temperature was maintained at 37 ± 0.5°C. In the BMSC + mild hypothermia group, rectal temperature was maintained at 34 ± 0.5°C, and BMSCs were transplanted via tail vein injection 6 hours later.
Morphology of transplanted BMSCs
The numbers of BMSCs and colonies significantly increased after 5 days in culture. After 1–3 passages, the cells actively proliferated, the majority of cells adhered to a monolayer with various forms such as spindle-shaped, oval-shaped, flat-shaped, triangular and irregular, and cells exhibited a strong refraction and extended more than two processes, some of which connected to each other. The nucleus and nucleolus remained intact and the cells grew in a confluent layer (Figure 1).
Figure 1.

Morphology of bone marrow-derived mesenchymal stem cells (scale bars: 50 μm).
(A) After 2 days in culture, adherent cells are extended and appear spindle-shaped.
(B) After 7 days in culture, the cells grew around a clone.
(C) Cells from the third passage are fused together and arranged in a bunched or radiated shape.
Mild hypothermia and BMSC transplantation significantly improved histology at the injury site
At 4 weeks after injury, spinal cord tissue breakage, scar connections, and structural disorder were visible at the affected site in the model group, as well as a clear cavity formation (Figure 2A). In the normal temperature + BMSC transplantation group, astrocytes aggregated at the edge of the affected site and formed scars at the junction of the intact spinal cord and damaged spinal cord; the tissue cavity was less than in the model group, but larger than in the mild hypothermia + BMSC transplantation group (Figure 2B). In the mild hypothermia + BMSC transplantation group, astrocytes produced reactive hypertrophy, aggregated, and formed scars at the edge of the affected site; some cells were spindle-shaped, with a dense network between processes, and the size of the cavity was reduced (Figure 2C). Immunohistochemical staining showed an increase in the number of bromodeoxyurdine (BrdU)-positive cells at the affected site in the mild hypothermia + BMSC transplantation group and normal temperature + BMSC transplantation group compared with the model group (P < 0.05, P < 0.01; Figure 3) at 4 weeks post-injury.
Figure 2.
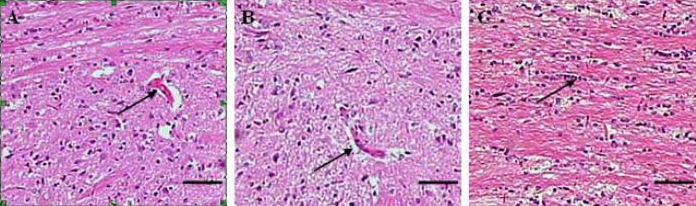
Histological analysis of rats with spinal cord hemisection injury (hematoxylin-eosin staining; scale bars: 100 μm). BMSCs: Bone marrow-derived mesenchymal stem cells.
(A) At 4 weeks after injury in the model group, the damaged spinal cord at the affected site exhibits clear cavity formation (arrow).
(B) In the normal temperature + BMSC transplantation group, astrocytes are aggregated at the edge of the affected site and scars formed at the junction between the intact and damaged spinal cord. The size of the tissue cavity (arrow) is less than in the model group but larger than in the mild hypothermia + BMSC transplantation group.
(C) In the mild hypothermia + BMSC transplantation group, astrocytes produce a reactive hypertrophy, aggregate and form scars at the edge of the affected site. In addition, the cavity is absent (arrow).
Figure 3.
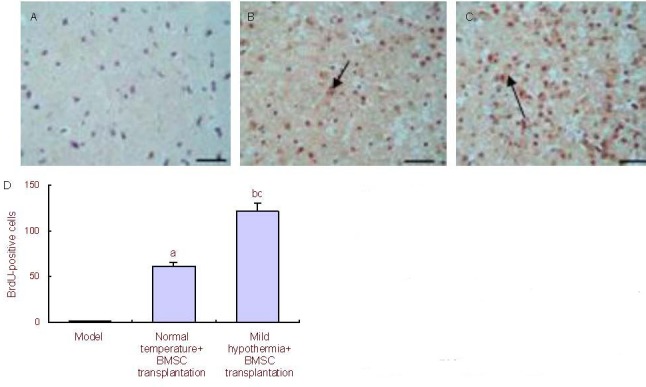
BrdU-positive cells at the affected spinal cord tissues in rats (immunohistochemical staining). BMSC: Bone marrow-derived mesenchymal stem cell; BrdU: bromodeoxyuridine.
(A–C) Model group, normal temperature + BMSC transplantation group, and mild hypothermia + BMSC transplantation group (scale bars: 50 μm). Arrows show BrdU-positive cells.
(D) Quantification of BrdU-positive cells. Following analysis of variance and Dunnett's t-test comparison, aP < 0.05, bP < 0.01, vs. model group; cP < 0.05, vs. normal temperature + BMSC transplantation group.
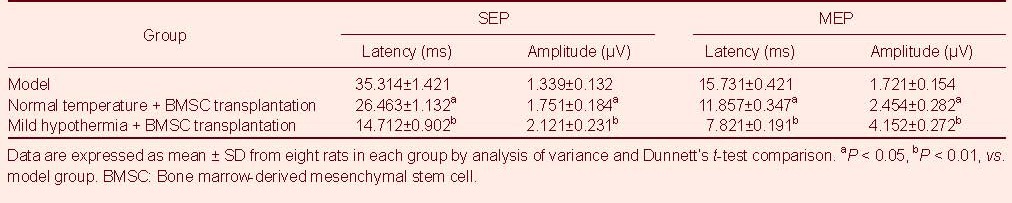
Mild hypothermia + BMSC transplantation improved electrophysiological function in rats
SEP and MEP testing results showed that both waveforms disappeared after the establishment of spinal cord hemisection injury. At 8 weeks post-injury, SEP and MEP measurements were slightly restored in the normal temperature + BMSC transplantation group. In addition, SEP and MEP levels were completely restored in the mild hypothermia + BMSC transplantation group, as well as increased volatility. SEP and MEP latency and amplitude are shown in Table 1.
Table 1.
Somatosensory-evoked potential (SEP) and motor-evoked potential (MEP) in each group at 8 weeks post- transplantation
Mild hypothermia + BMSC transplantation improved behaviors
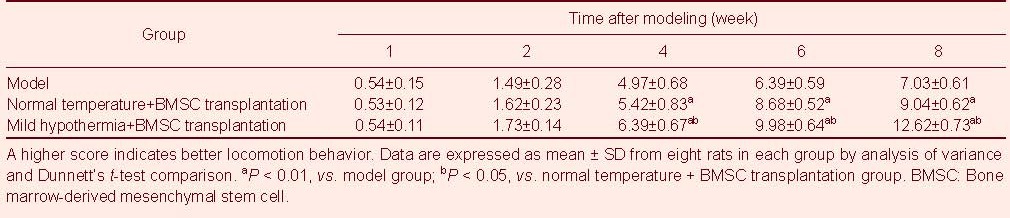
Basso, Beattie, and Bresnahan (BBB) scores
Following injury, the rats exhibited full paraplegia, with no activity in the hind limb or tail, as well as urination dysfunctions without defecation dysfunction. Retraction to the puncture began to emerge at 1 week post-injury, hind limb movement occurred at 2 weeks post-injury and became clearer at 4 weeks. Hind limbs exhibited coordinated activities at 6 weeks, and urinary function was partially restored, although residual urine remained in the bladder. The three groups exhibited similar changes after injury. BBB scores in the normal temperature + BMSC transplantation and mild hypothermia + BMSC transplantation groups were greater than in the model group at 4 weeks post-injury (P < 0.01). BBB scores in the mild hypothermia + BMSC transplantation group were greater than in the normal atmospheric temperature + BMSC transplantation group (P < 0.05; Table 2).
Table 2.
Basso, Beattie, and Bresnahan scores from rats in each group at different time points after spinal cord hemisection
Incline plate test
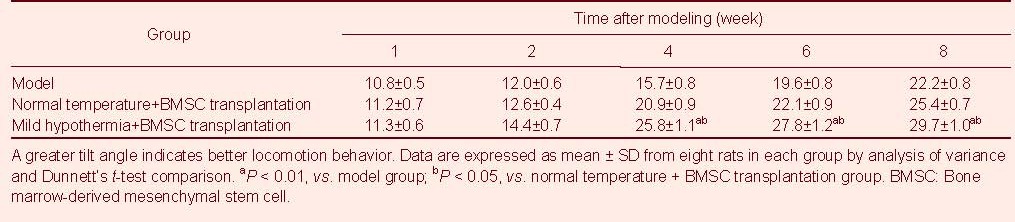
At 4–8 weeks post-injury, the tilt angle in the incline plate test was greater in the mild hypothermia + BMSC transplantation group than in the model group or normal temperature + BMSC transplantation group (P < 0.05, P < 0.01, respectively; Table 3).
Table 3.
Tilt angles of rats in each group at different time points after spinal cord hemisection (°)
DISCUSSION
In recent years, mild hypothermia (33-35°C) has received increased attention for the treatment of central nervous system injury. Clinical studies have shown that mild hypothermia effectively reduces secondary nerve injury and protects the brain and spinal cord against severe injury. The protective effects depend on the following mechanisms: reduced release of excitatory amino acids, inhibition of calcium influx, regulation of calmodulin kinase II and protein kinase C activity, inhibition of inflammatory response following cerebral ischemia, suppression of edema formation, decreased oxygen metabolic rate, decreased production of free radicals, and inhibition of necrosis and mitochondrial release of cytochrome C-induced neuronal apoptosis[18,19,20].
The present study analyzed the effects of mild hypothermia combined with BMSC transplantation in a rat model of SCI. Histological and hematoxylin-eosin staining results exhibited typical neuronal-like morphological changes with a reduced cavity in the mild hypothermia + BMSC transplantation group at 4 weeks post-injury. Tissue repair was better than in the model group or normal temperature + BMSC transplantation group. Immunohistochemical staining was also utilized to determine the number of BrdU-positive neurons at the affected site in rats: mild hypothermia + BMSC transplantation group > normal temperature + BMSC transplantation group > model group. BBB scores and inclined plane test results were statistically significant at 4 weeks post-injury: mild hypothermia + BMSC transplantation group > normal temperature + BMSC transplantation group > model group. The preliminary study addressed the effects of temperature manipulation on SEP/MEP signals, as well as the effects of transplanting BMSC-derived immature oligodendrocytes to aid recovery of spinal cord structure and function. These results provided a better understanding for the development of diagnostic and therapeutic methods to evaluate and treat spinal cord hemisection[21,22,23]. In the present study, amplitude, latency, and frequency following effects were observed in rats of the model group, normal temperature + BMSC transplantation group and mild hypothermia + BMSC transplantation group. SEP and MEP amplitude and latency improved in rats following BMSC transplantation. These results suggested that mild hypothermia + BMSC transplantation helped to restore conduction in demyelinated axons. However, the diversity and prolonged time course of somatic and autonomic effects suggested the influence of an additional mechanism, such as enhanced synaptic transmission[24]. In summary, BMSC transplantation in combination with mild hypothermia promoted survival and proliferation of transplanted cells at the injury site, as well as promoted restoration of electrophysiological functions in a rat model of SCI.
MATERIALS AND METHODS
Design
A randomized, controlled, animal study.
Time and setting
The present study was performed between March 2009 and May 2011 at the Laboratory Animal Center of Tianjin Medical University Hospital in China.
Materials
One 1-month-old, Sprague-Dawley rat was used for BMSC extraction, and 68 healthy, female, 3-month-old, Sprague-Dawley rats (weighing 200–250 g) were purchased from the Animal Laboratory, Chinese Academy of Medical Sciences, China (license No. SCXK (Jin) 20070001). Rats were housed at 18–26°C in 40–70% relative humidity. The temperature within the cage was 1–2°C greater than the general environment, and humidity was 5–10% greater. Ventilation of the cages was performed 8–12 times per hour. The experimental disposal of animals complied with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[25].
Methods
Rat BMSC cultivation
The 1-month-old Sprague-Dawley rat was anesthetized with an intraperitoneal injection of 2.5% ketamine 20 mg/kg and then sacrificed. The rats were then disinfected in 75% alcohol for approximately 10 minutes. Bilateral tibia and femur were removed under sterile conditions; the bone ends were resected, and 1 mL of Dulbecco's modified Eagle's medium (DMEM) complete medium (Gibco BRL Life Technologies, Gaithersburg, ML, USA) was collected using a No. 10 syringe. The cells were washed and prepared into a single-cell suspension at the density of 3 × 104/mL. In brief, the flask was fully washed and 15 mL of DMEM containing 0.05% bovine serum albumin was added; 24 hours later, the medium was collected and cells were centrifuged using 3-kDa centrifugal ultrafiltration tubes at 3 800 r/min for 30 minutes. The cells were then inoculated into 100-mL culture flasks and incubated at 37°C in 5% CO2 saturated humidity. Culture medium was replenished 24 hours later and then replaced every 3 days. Non-adherent cells were removed and adherent cells were expanded until the cultures were subconfluent. The cells were then processed through sequential passages. The majority of contaminating hematopoietic stem cells was progressively lost, and after the second passage, cultures contained a morphologically homogenous cell population that was designated BMSCs. This cell population was confirmed by fluorescence-activated cell-sorting analysis, showing a lack of typical hematopoietic cell surface marker expression, including CD45, CD34, and CD14, but positive expression of CD71, CD105, and CD44[26]. Cells between passage 3 and passage 6 were used for the experiments. Proliferating cells were labeled by BrdU diluted in culture medium (15 μM BrdU) (Takara Biotechnology (Dalian), Dalian, China)[27,28].
Establishment of SCI models
A total of 68 female, Sprague-Dawley rats were maintained in the laboratory for 2 weeks. At the beginning of the experiment, the rats were anesthetized with an intraperitoneal injection of 2.5% ketamine (20 mg/kg). The rats were fixed in a prone position on the surgery table to prepare the skin specimens, which were then disinfected. T9 spinous processes were selected, and a 2–3 cm length of skin and subcutaneous tissue were incised along the posterior median line. The paraspinal muscles were stripped, exposing T8-9 spinous processes and lamina. Using forceps, the T8-9 spinous processes and lamina were resected, exposing dura mater. The right hemisphere of the spinal cord was then resected. The wound was rinsed with a penicillin-saline solution and sutured. The rat bladders were squeezed twice daily, morning and evening, to express urine, until resumption of micturition reflex. Paralysis of the injured right hind limb was considered successful model establishment[29,30].
Mild hypothermia and normal temperature BMSC transplantation
A HP-V26 temperature meter (Beijing Shun Jie Xinlong Technology, Beijing, China) was used for continuous monitoring of rectal temperature. Following SCI, rats in normal temperature + BMSC transplantation group were placed on the surgical table at room temperature and rectal temperature was maintained at 37 ± 0.5°C. After 6 hours, a 1-mL BMSC (1 × 1010/L) suspension was injected through the tail vein using a 1-mL syringe. In the mild hypothermia + BMSC transplantation group, the rats were placed on an ice blanket, maintaining rectal temperature at 34 ± 0.5°C; after 6 hours, a 1-mL BMSC (1 × 1010/L) suspension was injected into the tail vein using a 1-mL syringe. Then animals were then housed in individual cages.
Evaluation of functional recovery
Eight rats from each group were selected to assess functional recovery. Each behavioral test was observed by two independent investigators.
BBB score: The open-field locomotion scale was used to assess movement, weight support, and coordination. Scores were achieved using a standardized BBB locomotor scoring system. The BBB scores range from 0 (flaccid paralysis) to 21 (normal gait). Rats were acclimated to the testing environment (90 cm diameter plastic wading pool; 4 cm height) prior to testing[31,32], and testing was performed at 1, 2, 4, 6, and 8 weeks post-surgery. BBB scores were averaged for each group. Inclined plate test: An inclined plate surface was covered with a 6-mm-thick rubber pad, and the rats were placed on an axis perpendicular to the longitudinal axis of the inclined plate. The incline angle gradually increased, and the rats were forced to remain on the inclined plate for at least 5 seconds to record maximum angle. The inclined plate angle was measured three times in each rat to obtain an average value. Three groups were measured at 1, 2, 4, 6, and 8 weeks post-surgery[33,34,35].
Histological analysis after spinal cord hemisection
At 4 weeks post-surgery, two rats were randomly selected from each group and were anesthetized intraperitoneally with chloral hydrate (35 mg/100 g). The dissected spinal cord tissues (T9) were fixed for 3 hours in 4% paraformaldehyde, soaked overnight in 10% paraformaldehyde, followed by 30% sucrose, and cut into 15-mm thick sagittal and parasagittal sections using a cryostat (Oxford Instruments, Oxford, UK). Hematoxylin-eosin staining and 1% cresyl violet staining were performed for general histological examination under microscope observation (Olympus, Tokyo, Japan).
Immunohistochemical analysis of BrdU-positive cells at the affected site
At 4 weeks post-surgery, two rats were randomly selected from each group for BrdU detection. Immunohistochemistry for BrdU detection required 2 N HCl-pretreatment of spinal cord tissue sections (T9) to open histones and make DNA accessible. All stainings were performed on free-floating, 40-μm thick sections. The sections were incubated in monoclonal mouse anti-rat BrdU antibody (10 μM; Boehringer Mannheim, Santa Cruz, CA, USA) at 4°C overnight in a humidified chamber, followed by biotinylated horse anti-mouse IgG antibody (1:167; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C for 45 minutes. A total of 10 visual fields were randomly selected from each section under a high-power microscope (Olympus) to quantify BrdU-positive cells from each field (200 ×) of vision. The mean served as the number of BrdU-positive cells per group.
SEP and MEP measurements in rats following spinal cord hemisection
At 8 weeks post-model establishment, SEPs and MEPs were measured in eight rats per group using Keypoint myoelectricity-evoked potential equipment (Medtronic, Minneapolis, MN, USA). Rats were anesthetized intraperitoneally with 10% chloral hydrate, and a stimulus electrode was subsequently fixed to the hind legs. Recording electrodes were placed on the hind-limb cortical sensory area between the coronal suture and sagittal suture lines[36] using a brain localization testing instrument (Wuhan Vaidya Medical Technology, Wuhan, China). Reference electrodes were placed 0.5 cm posterior to the recording electrodes, eliciting direct-current square wave electrical stimulation with an intensity of 5–15 mA, pulse width of 0.2 ms, and frequency of 3 Hz, which was superimposed for 50-60 times. SEP latency and amplitude were recorded, and nerve electrophysiological recovery was observed. Stimulating electrodes were placed in the cerebral cortex motor area, with a stimulus intensity of 40 mA, a pulse width of 0.1 ms, and a frequency of 1 Hz, which was superimposed 300–500 times. The scanning speed was 5 ms/D and sensitivity was 5 μV/D. MEP latency and amplitude changes were recorded[35,37].
Statistical analysis
Data are expressed as mean ± SD, and a completely randomized design analysis of variance was performed using SPSS 15.0 statistical software. Two-sample comparison was performed with the Dunnett's t-test, and a level of P < 0.05 was considered significant. All analyses were performed using SPSS statistical software (Version 15.0; SPSS, Chicago, IL, USA).
Acknowledgments:
We express our sincere gratitude to Dr. Jingjian Ma from the Department of Neurosurgery, General Hospital of Tianjin Medical University, China for revising this manuscript.
Footnotes
Funding: This study was sponsored by the Science and Technology Foundation of Tianjin Health Bureau, No. 2010ky04, and Application Basic and Front Technology Projects of Tianjin (Science and Technology Foundation of Tianjin), No.12JCYBJC18000.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Scientific Review Committee and the Institutional Review Board of Tianjin Medical University in China.
(Edited by Bu XY, Yang XY/Yang Y/Song LP)
REFERENCES
- [1].Gu Y, Wang J, Ding F, et al. Neurotrophic actions of bone marrow stromal cells on primary culture of dorsal root ganglion tissues and neurons. J Mol Neurosci. 2010;40(3):332–341. doi: 10.1007/s12031-009-9304-6. [DOI] [PubMed] [Google Scholar]
- [2].Ban DX, Ning GZ, Feng SQ, et al. Combination of activated Schwann cells with bone mesenchymal stem cells: the best cell strategy for repair after spinal cord injury in rats. Regen Med. 2011;6(6):707–720. doi: 10.2217/rme.11.32. [DOI] [PubMed] [Google Scholar]
- [3].Schira J, Gasis M, Estrada V, et al. Significant clinical, neuropathological and behavioural recovery from acute spinal cord trauma by transplantation of a well-defined somatic stem cell from human umbilical cord blood. Brain. doi: 10.1093/brain/awr222. in press. [DOI] [PubMed] [Google Scholar]
- [4].Alexanian AR, Fehlings MG, Zhang Z, et al. Transplanted neurally modified bone marrow-derived mesenchymal stem cells promote tissue protection and locomotor recovery in spinal cord injured rats. Neurorehabil Neural Repair. 2011;25(9):873–880. doi: 10.1177/1545968311416823. [DOI] [PubMed] [Google Scholar]
- [5].Wright KT, El Masri W, Osman A, et al. Concise review: Bone marrow for the treatment of spinal cord injury: mechanisms and clinical applications. Stem Cells. 2011;29(2):169–178. doi: 10.1002/stem.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mothe AJ, Bozkurt G, Catapano J, et al. Intrathecal transplantation of stem cells by lumbar puncture for thoracic spinal cord injury in the rat. Spinal Cord. 2011;49(9):967–973. doi: 10.1038/sc.2011.46. [DOI] [PubMed] [Google Scholar]
- [7].Chen JR, Cheng GY, Sheu CC, et al. Transplanted bone marrow stromal cells migrate, differentiate and improve motor function in rats with experimentally induced cerebral stroke. J Anat. 2008;213(3):249–258. doi: 10.1111/j.1469-7580.2008.00948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huang T, Solano J, He D, et al. Traumatic injury activates MAP kinases in astrocytes: mechanisms of hypothermia and hyperthermia. J Neurotrauma. 2009;26(9):1535–1545. doi: 10.1089/neu.2008.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Uccelli A, Benvenuto F, Laroni A, et al. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011;24(1):59–64. doi: 10.1016/j.beha.2011.01.004. [DOI] [PubMed] [Google Scholar]
- [10].Hilfiker A, Kasper C, Hass R, et al. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg. 2011;396(4):489–497. doi: 10.1007/s00423-011-0762-2. [DOI] [PubMed] [Google Scholar]
- [11].Alexanian AR, Kwok WM, Pravdic D, et al. Survival of neurally induced mesenchymal cells may determine degree of motor recovery in injured spinal cord rats. Restor Neurol Neurosci. 2010;28(6):761–767. doi: 10.3233/RNN-2010-0547. [DOI] [PubMed] [Google Scholar]
- [12].Braisted JE, Catalano SM, Stimac R, et al. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. J Neurosci. 2000;20(15):5792–5801. doi: 10.1523/JNEUROSCI.20-15-05792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Agrawal G, Sherman D, Maybhate A, et al. Slope analysis of somatosensory evoked potentials in spinal cord injury for detecting contusion injury and focal demyelination. J Clin Neurosci. 2010;17(9):1159–1164. doi: 10.1016/j.jocn.2010.02.005. [DOI] [PubMed] [Google Scholar]
- [14].Hu Y, Wen CY, Li TH, et al. Somatosensory-evoked potentials as an indicator for the extent of ultrastructural damage of the spinal cord after chronic compressive injuries in a rat model. Clin Neurophysiol. 2011;122(7):1440–1447. doi: 10.1016/j.clinph.2010.12.051. [DOI] [PubMed] [Google Scholar]
- [15].Clarke CJ, Galen S, Allan DB, et al. Correlations between recovery of ambulatory capacity and lower-limb somatosensory evoked potentials in incomplete spinal cord injury. Arch Phys Med Rehabil. 2010;91(10):e19. [Google Scholar]
- [16].Hu L, Zhang ZG, Hung YS, et al. Single-trial detection of somatosensory evoked potentials by probabilistic independent component analysis and wavelet filtering. Clin Neurophysiol. 2011;122(7):1429–1439. doi: 10.1016/j.clinph.2010.12.052. [DOI] [PubMed] [Google Scholar]
- [17].Garcia RM, Qureshi SA, Cassinelli EH, et al. Detection of postoperative neurologic deficits using somatosensory-evoked potentials alone during posterior cervical laminoplasty. Spine J. 2010;10(10):890–895. doi: 10.1016/j.spinee.2010.08.018. [DOI] [PubMed] [Google Scholar]
- [18].Jori FP, Napolitano MA, Melone MA, et al. Molecular pathways involved in neural in vitro differentiation of marrow stromal stem cells. J Cell Biochem. 2005;94(4):645–655. doi: 10.1002/jcb.20315. [DOI] [PubMed] [Google Scholar]
- [19].Mazzini L, Mareschi K, Ferrero I, et al. Stem cell treatment in Amyotrophic Lateral Sclerosis. J Neurol Sci. 2008;265(1-2):78–83. doi: 10.1016/j.jns.2007.05.016. [DOI] [PubMed] [Google Scholar]
- [20].Lyden PD, Krieger D, Yenari M, et al. Therapeutic hypothermia for acute stroke. Int J Stroke. 2006;1(1):9–19. doi: 10.1111/j.1747-4949.2005.00011.x. [DOI] [PubMed] [Google Scholar]
- [21].Ma Y, Hu Y, Valentin N, et al. Time jitter of somatosensory evoked potentials in recovery from hypoxic-ischemic brain injury. J Neurosci Methods. 2011;201(2):355–360. doi: 10.1016/j.jneumeth.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Agrawal G, Thakor NV, All AH. Evoked potential versus behavior to detect minor insult to the spinal cord in a rat model. J Clin Neurosci. 2009;16(8):1052–1055. doi: 10.1016/j.jocn.2008.08.009. [DOI] [PubMed] [Google Scholar]
- [23].Salama H, Hatem B, Ashraf B, et al. PO31-FR-15 Spinal cord motor evoked potential monitoring during scoliosis surgery and perioperative somatosensory evoked potentials outcomes. J Neurol Sci. 2009;285(Sup 1):S324. [Google Scholar]
- [24].Gressens P, Dingley J, Plaisant F, et al. Analysis of neuronal, glial, endothelial, axonal and apoptotic markers following moderate therapeutic hypothermia and anesthesia in the developing piglet brain. Brain Pathol. 2008;18(1):10–20. doi: 10.1111/j.1750-3639.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [26].Sarnowska A, Braun H, Sauerzweig S, et al. The neuroprotective effect of bone marrow stem cells is not dependent on direct cell contact with hypoxic injured tissue. Exp Neurol. 2009;215(2):317–327. doi: 10.1016/j.expneurol.2008.10.023. [DOI] [PubMed] [Google Scholar]
- [27].Beggs KJ, Lyubimov A, Borneman JN, et al. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15(8-9):711–721. doi: 10.3727/000000006783981503. [DOI] [PubMed] [Google Scholar]
- [28].Bae JS, Han HS, Youn DH, et al. Bone marrow-derived mesenchymal stem cells promote neuronal networks with functional synaptic transmission after transplantation into mice with neurodegeneration. Stem Cells. 2007;25(5):1307–1316. doi: 10.1634/stemcells.2006-0561. [DOI] [PubMed] [Google Scholar]
- [29].Furuya T, Hashimoto M, Koda M, et al. Treatment of rat spinal cord injury with a Rho-kinase inhibitor and bone marrow stromal cell transplantation. Brain Res. 2009;1295:192–202. doi: 10.1016/j.brainres.2009.07.087. [DOI] [PubMed] [Google Scholar]
- [30].Ohta M, Suzuki Y, Noda T, et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 2004;187(2):266–278. doi: 10.1016/j.expneurol.2004.01.021. [DOI] [PubMed] [Google Scholar]
- [31].Liu WG, Wang ZY, Huang ZS. Bone marrow-derived mesenchymal stem cells expressing the bFGF transgene promote axon regeneration and functional recovery after spinal cord injury in rats. Neurol Res. 2011;33(7):686–693. doi: 10.1179/1743132810Y.0000000031. [DOI] [PubMed] [Google Scholar]
- [32].Wang YT, Lu XM, Zhu F, et al. The use of a gold nanoparticle-based adjuvant to improve the therapeutic efficacy of hNgR-Fc protein immunization in spinal cord-injured rats. Biomaterials. 2011;32(31):7988–7998. doi: 10.1016/j.biomaterials.2011.07.009. [DOI] [PubMed] [Google Scholar]
- [33].Fischer FR, Peduzzi JD. Functional recovery in rats with chronic spinal cord injuries after exposure to an enriched environment. J Spinal Cord Med. 2007;30(2):147–155. doi: 10.1080/10790268.2007.11753926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wan H, Li DZ, Yang F, et al. Research about schwann cells and PLGA implanted to rat transected spinal cord. Zhonghua Wai Ke Za Zhi. 2007;45(12):843–846. [PubMed] [Google Scholar]
- [35].Shao Y, Ma H, Wu Y, et al. Effect of nerve growth factor on changes of myelin basic protein and functional repair of peripheral nerve following sciatic nerve injury in rats. Chin J Traumatol. 2002;5(4):237–240. [PubMed] [Google Scholar]
- [36].Tharion G, Indirani K, Durai M, et al. Motor recovery following olfactory ensheathing cell transplantation in rats with spinal cord injury. Neurol India. 2011;59(4):566–572. doi: 10.4103/0028-3886.84339. [DOI] [PubMed] [Google Scholar]
- [37].Chen Y, Wang S, Geng B, et al. Experimental study on adenosine triphosphate combining bone marrow mesenchymal stem cells transplantation in treatment of spinal cord injury in rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24(10):1233–1238. [PubMed] [Google Scholar]


