Abstract
The present study analyzed the anatomical association between intracranial subarachnoid space and the cervical lymphatic system. X-ray contrast medium and Microfil® (Microfil compounds fill and opacify microvascular and other spaces of non-surviving animals and post-mortem tissue under physiological injection pressure) were injected into the cisterna magna of the rabbit, and perineural routes of cerebrospinal fluid outflow into the lymphatic system were visualized. Under a surgical operating microscope, Microfil was found within the subarachnoid space and along the olfactory nerves. At the nasal mucosa, a lymphatic network was identified near the olfactory nerves, which crossed the nasopharyngeal region and finally emptied into the superficial and deep cervical lymph nodes. Under a light microscope, Microfil was visible around the olfactory nerves and within lymphatic vessels. These results suggested that cerebrospinal fluid drained from the subarachnoid space along the olfactory nerves to nasal lymphatic vessels, which in turn, emptied into the cervical lymph nodes. This anatomical route, therefore, allowed connection between the central nervous system and the lymphatic system.
Keywords: central nervous system, cerebrospinal fluid, lymph, subarachnoid space
Abbreviations:
CSF, cerebrospinal fluid; SAS, subarachnoid space
INTRODUCTION
Under normal conditions, the cerebrospinal fluid (CSF) is produced mainly via a process of ultrafiltration at the non-tight junction level of capillary endothelial walls in the choroid plexus. However, very little evidence is available that supports a role for arachnoid villi and granulations in this process. Recent studies have suggested that CSF movement directly into the cranial venous system might only occur under high pressure, which suggests that arachnoid projections might exhibit accessory functions, rather than representing primary locations where CSF is absorbed[1,2].
According to the traditional theory, the central nervous system is regarded as an immune-exempt organ. Lymphatic vessels do not exist within the parenchyma of the central nervous system[3]. CSF is typically thought to be absorbed through arachnoid villi. However, a relatively large volume of literature supports an anatomical association between CSF and the lymph system[4,5,6]. Alternative routes for CSF drainage into the peripheral lymphatic system exist. Among them, the route along the fila olfactoria and olfactory nerves has been suggested to be a main route of CSF drainage from the subarachnoid space (SAS) into nasal lymphatics[7,8,9].
The present study analyzed the anatomical and histological characteristics of the olfactory route for CSF drainage into peripheral lymphatic vessels. The olfactory route was initially revealed by X-ray cisternography and subarachnoid injection of Microfil®. Subsequently, the ultrastructure of olfactory nerves and nasal mucosa, in relation to cerebrospinal fluid drainage, was demonstrated.
RESULTS
Quantitative analysis of experimental animals
A total of ten male and ten female New Zealand white rabbits were included in the present study. Five male and five female rabbits, respectively were infused with medical contrast medium for cisternography, and the remaining ten rabbits were injected at the subarachnoid space for Microfil examinations. One rabbit injected with Microfil into the cisterna magna was excluded, because Microfil did not properly distribute. Microfil was observed throughout the subarachnoid compartment at the base of the brain and in the orbit.
Cisternography
Conventional radiographs, taken 30 minutes after cisternal infusion of 3 mL contrast medium, revealed perineural routes of CSF outflow into the lymphatic system. Contrast medium was observed in the orbit, nasal cavities, and subsequently in the hard and soft palate (Figure 1). Details of CSF outflow were detected by direct magnification of the X-ray technique: lateral projections during the first minute of infusion revealed contrast medium sinking downwards into the spinal compartment. The medium filled the basal cisterns first and then spread in the direction of the cribriform plate. Reaching the cribriform plate at 10 minutes after start of infusion, the contrast medium leaked immediately into the nasal cavities.
Figure 1.
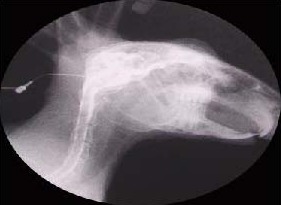
Cisternography of X-ray.
In conventional radiographs, the contrast medium (Omnipaque) is injected into the cisterna magna, and the perineural routes of contrast medium outflow into the lymphatic system are visualized.
The picture is taken 30 minutes after cisternal infusion of 3-mL contrast medium. The contrast medium is visible in the orbit, nasal cavities, and subsequently in the hard and soft palate.
Macroscopic distribution of Microfil
Under macroscopic examination, some contrast agent filled and opacified the head and cervical superficial lymphatic vessels, and was also located in subcutaneous tissues of both ears, as well as periorbital and nasal regions. Simultaneously, lymphatic vessels located beneath the submaxillary gland and cervical skin were slightly opacified by Microfil. However, the majority of Microfil was found in deep cervical lymphatic vessels along the posterior pharyngeal wall to the cervical lymphatic trunks and thoracic duct (Figure 2).
Figure 2.
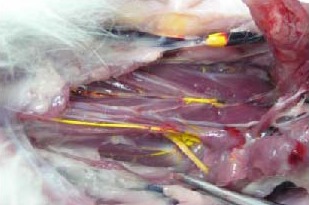
Macroscopic view of Microfil distribution in cervical lymphatic vessels.
some Microfil fills and opacifies the head and cervical superficial lymphatic vessels. However, the majority of Microfil is observed in deep cervical lymphatic vessels along the posterior pharyngeal wall to the cervical lymphatic trunks and thoracic duct.
After the animals were decapitated, Microfil was observed clustered along the sagittal sinus, cisterna magna, and olfactory bulb under a surgical operating microscope. In addition, Microfil was distributed in a patchy pattern along the fila olfactoria external to the cranium, as well as in extensive lymphatic networks associated with the submucosa of the olfactory epithelium, ethmoid turbinates, and adjacent nasal septum (Figure 3).
Figure 3.
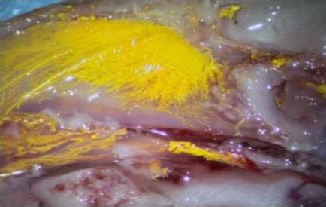
Microscopic view of Microfil distribution in nasal and anterior skull base region.
Microfil is visible in a patchy distribution along the fila olfactoria external to the cranium, as well as in extensive lymphatic networks associated with the submucosa of the olfactory epithelium, ethmoid turbinates, and adjacent nasal septum.
Microfil was detected within the SAS of the optic nerve sheath and accumulated in the conjunction optic nerve of the retina. In addition, lymphatics containing Microfil were observed in mucosa of the lateral wall of the dorsum nasi, close to the nostrils. These vessels penetrated through cartilage of the lateral wall and joined larger superficial lymphatic ducts in subcutaneous adipose tissue. The arterial vessels of these preparations were filled with blue Microfil via injection into both carotid arteries, and the CSF compartment was infused with a yellow agent. Results demonstrated yellow lymphatic vessels that were clearly discernable from the blue blood vessels (Figure 4).
Figure 4.

Varied colored Microfil display in nasal septum lymphatic vessel and artery.
When arterial vessels are filled with blue Microfil via injection into both carotid arteries and the cerebrospinal fluid compartment is infused with yellow agent, the yellow lymphatic vessels are clearly discernable from the blue-colored blood vessels.
Microscopic distribution of Microfil
Under microscopic histological examination, Microfil was detected in a patchy distribution along the fila olfactoria as it exited the cribriform plate. The yellow Microfil appeared dark-brown after fixation. Microfil was observed in perineurial spaces and was particularly concentrated around emerging olfactory nerve roots. Microfil was associated closely with fila olfactoria located free in perineurial spaces or contained within endothelial lined vessels (Figure 5). Most lymphatic vessels within the olfactory submucosa appeared to comprise a single layer of endothelial cells, which resembled lymphatic vessels (Figure 6). Microfil-filled lymphatic vessels were particularly conspicuous around the fila olfactoria, close to the point of exit from the cribriform plate. However, lymphatics filled with contrast agent were observed scattered throughout the olfactory submucosa. There was no Microfil in arteries or veins of the olfactory submucosa.
Figure 5.
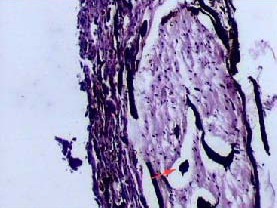
Histological observation of Microfil in olfactory nerve (hematoxylin-eosin staining, × 100).
The yellow Microfil (red arrow) appears dark-brown after fixation. Microfil is observed in perineurial spaces.
Figure 6.
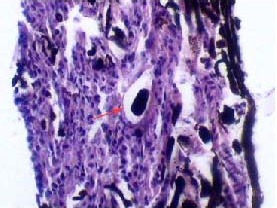
Histological observation of Microfil in nasal septum lymphatic vessels (hematoxylin-eosin staining, × 400).
Most lymphatic vessels within the olfactory submucosa comprise a single layer of endothelial cells, which is characteristic of lymphatic vessels. Dark-brown Microfil (red arrow) is visible in lymphatic vessels.
DISCUSSION
Results from the present study suggested that a significant portion of CSF was removed from the subarachnoid space by peripheral lymphatic vessels, particularly in nasal lymphatic vessels. These conclusions were based on experiments and intensive analysis to explore CSF transport routing, which spanned over 100 years and was conducted in various experimental animals, such as dogs, rabbits, rats, and sheep[10,11,12,13]. The methods for determining passage between SAS and lymphatic vessels included subarachnoid injection of ink, X-ray contrast, and Microfil. All experimental results demonstrated that the olfactory route was a major passage for CSF absorption through the lymphatic system.
Microfil compounds, which are based on silicone rubber, can fill and opacify microvasculars and other spaces of non-surviving animals and post-mortem tissue under physiological injection pressure. The continuous, closed, vascular system lends itself to flow through injection or perfusion techniques. Following injection, Microfil compounds form a three-dimensional cast of the vasculature. Johnston et al[6] showed that this was possible in sheep, pigs, rabbits, rats, mice, monkeys, and humans; immediately upon sacrifice (or up to 7 hours after death in humans), yellow Microfil was injected into the CSF compartment. Microfil was observed primarily in the subarachnoid space around the olfactory bulbs and cribriform plate. Along the olfactory nerves, the contrast agent entered extensive lymphatic networks in the submucosa, which were associated with olfactory and respiratory epithelium. Results suggested that CSF absorption into the nasal lymphatic vessels is a characteristic feature of all mammals, including humans. In the present study, Microfil injections into the cistern magna of rabbits revealed a similar phenomenon in all animals but one.
Under macroscopic examination, Microfil in the SAS was primarily distributed along the cistern magna, skull base, sagittal sinus, optic nerve, olfactory bulb, and extracranial, mostly along the fila olfactoria. Some Microfil filled and opacified the head and cervical superficial lymphatic vessels. However, the majority of Microfil was observed in deep cervical lymphatic vessels along the posterior pharyngeal wall to the cervical lymphatic trunks and thoracic duct. Morphological analysis revealed that the olfactory route was the main route for CSF drainage into lymphatic vessels, particularly the primary route went into the deep lymphatic vessels system.
Under light microscopic histological analysis, Microfil was observed in perineurial spaces and was particularly concentrated around emerging olfactory nerve roots. Microfil-filled lymphatics were particularly conspicuous around the fila olfactoria, close to the point of exit from the cribriform plate. However, lymphatics filled with the contrast agent were observed scattered throughout the olfactory submucosa, which suggested that the high-injection pressure destroyed the lymphatic vessels. There was no Microfil in the arteries or veins of olfactory submucosa.
The mechanism of CSF drainage into lymphatic vessels via the olfactory route remains controversial[5,7,14,15].
Different models have been proposed by various scholars: (1) the SAS is present along olfactory nerves in the cribriform plate and continues with lymphatic vessels of the nasal mucosa; (2) the SAS extends along the nerve to the nasal mucosa. From this space, cerebrospinal fluid leaks out into nasal mucosa and enters lymphatic vessels; (3) the SAS is continuous with the perineurial space of the olfactory nerves. The CSF reaches nasal mucosa through this space and disperses through connective tissue and enters lymphatic vessels[7,14]; and (4) the SAS is continuous with the perineurial space and with nasal lymphatic vessels[14,15,16].
Microfil is a relatively viscous silastic material, which would not be expected to be readily absorbed from an interstitial compartment. Therefore, results from the present study suggested that the hypothesis proposed by Johnston et al[6] was most likely; either a direct connection or labyrinth structure must exist between the CSF and lymph compartments to facilitate uptake of intracisternally administered Microfil into lymphatic vessels.
CSF drainage into lymphatic vessels is significant for the physiology and pathophysiology of the central nervous system and related disease. Lymphatic CSF transport influences intracranial pressure and CSF circulation: Mollanji et al[17] concluded that sealing the cribriform plate extracranially impairs the ability of CSF pressure-regulating systems to compensate for volume infusions in a sheep model. The time spent for India ink to move from the CSF into the cervical lymph nodes increase was shown to be dependent on the model of transforming growth factor-β1 induced hydrocephalus in a mouse model[18]. This suggested that the cribriform-lymphatic connection is disrupted in these animals.
The blockage of lymphatic CSF transport impacts functions of the central nervous system. Sun et al[19] observed the influence of cerebral lymphatic drainage blockade on regional cerebral blood flow and brain edema in a rat model of experimental subarachnoid hemorrhage, concluding that a blocked cerebral lymphatic drainage pathway further deteriorates secondary cerebral ischemia and brain edema following subarachnoid hemorrhage.
A lymphatic-CSF relationship also implies an immunological relationship. Induction of experimental autoimmune encephalomyelitis in a rat model results in a severe immune response, followed by cerebral lesions. Removal of deep and superficial cervical lymph nodes following induction of autoimmune encephalomyelitis significantly reduces pathological severity[20,21]. Therefore, the cervical lymph nodes could provide the prime immune cells needed for targeting the brain. Previous results have suggested that lymphatic drainage of brain antigens contributes to the pathogenesis of Alzheimer's disease and multiple sclerosis[22].
In conclusion, results from the present study demonstrated the presence of an olfactory route for cerebrospinal fluid drainage into the nasal lymphatics. According to radiographs and Microfil data, these pathways could be used for high-flow CSF absorption. In rabbits, the lymphatic pathways were as important as the absorption route, which was considered to be important for cerebrospinal fluid absorption and antigen movement from the subarachnoid space to the cervical lymph nodes, where the immune response occurred.
MATERIALS AND METHODS
Design
A contrasting, observational, animal experiment.
Time and setting
This study was performed at the Laboratory of Yuquan Hospital, China from March to October 2009.
Materials
To avoid the influence of animal gender, ten male and ten female New Zealand white rabbits, weighing 2-3 kg and aged 4-6 months, were used for the experiments. The rabbits were purchased from the Fangyuanyuan Breeding Farm of Beijing (license No. SCXK (Jing) 2009-0014). All experimental procedures were conducted in accordance with the Institutional Guidelines for the Care and Use of Laboratory Animals in Yuquan Hospital, Beijing, China, and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Methods
Cisterna magna puncture
The rabbits were placed into a stereotactic frame in a prone position, and the atlanto-occipital membrane was exposed by a midsagittal incision of the scalp. A 22-gauge venous indwelling trocar was introduced into the cisterna magna at an angle of 30° between the trocar and horizontal plane. Clear CSF discharge from the trocar demonstrated that the puncture was successful (Figure 7). The troca was fixed along the skull bone and the skin with chemical glue. X-ray contrast and Microfil were injected into the cisterna magna via the troca.
Figure 7.
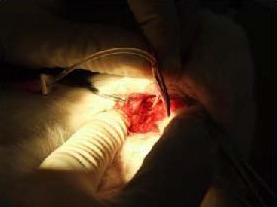
Cisterna magna puncture in a rabbit model.
The rabbits were fixed in a prone position and the atlanto-occipital membrane was exposed.
A 22-gauge venous indwelling trocar was introduced into the cisterna magna at an angle of 30° between the trocar and horizontal plane. Clear cerebrospinal fluid discharge from the trocar was considered a successful puncture.
Cisternography
At the start of examination, the rabbits were anesthetized with 1% pentobarbital (1 mL/kg) intravenous injection, and the injection was repeated every 30 minutes with half of the initial dosage. During this process, a self-made microcatheter was connected to the troca, and a pressure transducer was connected to the cisternal catheter via a three-way stopcock. Using an adjustable syringe pump, 0.1 mL/min of X-ray contrast medium, Omnipaque (Shanghai GE Healthcare, Shanghai, China), was infused over 60 minutes. The infusion was interrupted when intracranial pressure reached > 2.67 kPa. X-ray (DigiArc-100, Beijing East Whale Imaging Technology, Beijing, China) studies were performed at 1, 10, 20, 30, and 60 minutes, respectively, after infusion. The contrast medium reached the cribriform plate at 10 minutes and the distal optic nerve at 20 minutes, respectively. The medium then leaked into the nasal cavities and opacified the cervical lymphatic vessels at 30 minutes after start of infusion.
Subarachnoid injection of Microfil
At the start of preparation, the rabbits were intraperitoneally anaesthetized with 1 mL/kg 1% pentobarbital (Beijing Double-Crane Pharmaceutical, Beijing, China). Subsequently, the rabbits were euthanized with an intraperitoneal injection of 1% pentobarbital (3 mL/kg). Microfil was used to fill vascular and other spaces of non-surviving animals (http://www.flowtech-inc.com, Flow Tech, Carver, MA, USA). It was available in a variety of colors and was designed to facilitate visual and microscopic analysis of microcirculation. A volume of 10 mL yellow Microfil was manually infused into the cisterna magna over 5-10 minutes; 3 mL of diluent was used for each 1 mL of yellow Microfil, and the material catalyzed with 10% (of total volume) of the curing agent. In two male and three female rabbits, respectively, blue Microfil was injected into the blood vasculature, and yellow Microfil was simultaneously injected into the CSF compartment. The carotid arteries were catheterized and 10 mL blue Microfil (MV-120) was simultaneously infused into both arteries. An injection was considered successful if the Microfil fully filled and stained the nasal lymphatic vessel.
Histological assessments
After the infusions were complete, the Microfil was allowed to set for 2 hours. The anterior cervical skin was incised midsaggitally from the incisor to suprasternal fossa. The superficial and deep lymphatic vessel was observed step by step. After cervical anatomical dissection was complete, the animals were sacrificed. The skin was removed and the heads were cut along a parasagittal plane. Partial dissection was performed under a surgical operating microscope (Leica MS-II Leica, Nussloch, Germany). Subsequently, the olfactory bulbs and olfactory tracts were removed from the cribriform plate and the subjacent parts of the nasal mucosa. Tissues were harvested and fixed in 10% formalin for 48 hours. Prior to sectioning for histology, the samples were processed through 70%, 95%, and 100% ethanol, as well as methylbenzene and wax. Paraffin-embedded samples were cut into 4-μm sections using a microtome (Leica, Nussloch, Germany), and were stained with hematoxylin-eosin. Histological assessments were performed using an Olympus light microscope (Olympus, Tokyo, Japan).
Footnotes
Funding: The study was supported by the National Natural Science Foundation of China, No. 30700858; the Research Fund of Capital Medical Development, No. 2009-3047; and Wushunde Medical Research Fund of Tsinghua University in 2011.
Conflicts of interest: None declared.
Ethical approval: The experiments were performed with the Institutional Guidelines for the Care and Use of Laboratory Animals Yuquan Hospital, Tsinghua University, China.
(Edited by Zhao XX, Xiao N/Su LL/Song LP)
REFERENCES
- [1].Johanson CE. Ventricles and Cerebrospinal fluid. In: Conn PM, editor. Neuroscience in Medicine. Philadelphia: J.B. Lippincott Company; 1995. [Google Scholar]
- [2].Papaiconomou C, Zakharov A, Azizi N, et al. Reassessment of the pathways responsible for cerebrospinal fluid absorption in the neonate. Childs Nerv Syst. 2004;20(1):29–36. doi: 10.1007/s00381-003-0840-z. [DOI] [PubMed] [Google Scholar]
- [3].Brinker T, Lüdemann W, Berens von Rautenfeld D, et al. Dynamic properties of lymphatic pathways for the absorption of cerebrospinal fluid. Acta Neuropathol. 1997;94(5):493–498. doi: 10.1007/s004010050738. [DOI] [PubMed] [Google Scholar]
- [4].Mollanji R, Papaiconomou C, Boulton M, et al. Comparison of cerebrospinal fluid transport in fetal and adult sheep. Am J Physiol Regul Integr Comp Physiol. 2001;281(4):1215–1223. doi: 10.1152/ajpregu.2001.281.4.R1215. [DOI] [PubMed] [Google Scholar]
- [5].Weller RO, Kida S, Zhang ET. Pathways of fluid drainage from the brain-morphological aspects and immunological significance in rat and man. Brain Pathol. 1992;2(4):277–284. doi: 10.1111/j.1750-3639.1992.tb00704.x. [DOI] [PubMed] [Google Scholar]
- [6].Johnston M, Zakharov A, Koh L, et al. Subarachnoid injection of Microfil reveals connections between cerebrospinal fluid and nasal lymphatics in the non-human primate. Neuropathol Appl Neurobiol. 2005;31(6):632–640. doi: 10.1111/j.1365-2990.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- [7].Johnston M, Zakharov A, Papaiconomou C, et al. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;10(1):2. doi: 10.1186/1743-8454-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zakharov A, Papaiconomou C, Djenic J, et al. Lymphatic cerebrospinal fluid absorption pathways in neonatal sheep revealed by subarachnoid injection of Microfil. Neuropathol Appl Neurobiol. 2003;29(6):563–573. doi: 10.1046/j.0305-1846.2003.00508.x. [DOI] [PubMed] [Google Scholar]
- [9].Kapoor KG, Katz SE, Grzybowski DM, et al. Cerebrospinal fluid outflow: an evolving perspective. Brain Res Bull. 2008;77(6):327–334. doi: 10.1016/j.brainresbull.2008.08.009. [DOI] [PubMed] [Google Scholar]
- [10].Greitz D, Greitz T, Hindmarsh T. A new view on the CSF-circulation with the 7. potential for pharmacological treatment of childhood hydrocephalus. Acta Paediatr. 1997;86(2):125–132. doi: 10.1111/j.1651-2227.1997.tb08850.x. [DOI] [PubMed] [Google Scholar]
- [11].Zakharov A, Papaiconomou C, Koh L, et al. Integrating the roles of extracranial lymphatics and intracranial veins in cerebrospinal fluid absorption in sheep. Microvasc Res. 2004;67(1):96–104. doi: 10.1016/j.mvr.2003.08.004. [DOI] [PubMed] [Google Scholar]
- [12].Johnston M, Papaiconomou C. Cerebrospinal fluid transport: a lymphatic perspective. News Physiol Sci. 2002;17(6):227–230. doi: 10.1152/nips.01400.2002. [DOI] [PubMed] [Google Scholar]
- [13].Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2005;20(2):6. doi: 10.1186/1743-8454-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19(6):480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- [15].Bradbury MW, Westrop RJ. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J Physiol. 1983;339(1):19–34. doi: 10.1113/jphysiol.1983.sp014731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gomez DG, Fenstermacher JD, Manzo RP, et al. Cerebrospinal fluid absorption in the rabbit: olfactory pathways. Acta Otolaryngol. 1985;100(1):29–36. doi: 10.3109/00016488509126567. [DOI] [PubMed] [Google Scholar]
- [17].Mollanji R, Bozanovic-Sosic R, Zakharov A, et al. Blocking cerebrospinal fluid absorption through the cribriform plate increases resting intracranial pressure. Am J Physiol Regul Integr Comp Physiol. 2002;282(6):R1593–1599. doi: 10.1152/ajpregu.00695.2001. [DOI] [PubMed] [Google Scholar]
- [18].Moinuddin SM, Tada T. Study of cerebrospinal fluid flow dynamics in TGF-beta 1 induced chronic hydrocephalic mice. Neurol Res. 2000;22(2):215–222. doi: 10.1080/01616412.2000.11741064. [DOI] [PubMed] [Google Scholar]
- [19].Sun BL, Xia ZL, Wang JR, et al. Effects of blockade of cerebral lymphatic drainage on regional cerebral blood flow and brain edema after subarachnoid hemorrhage. Clin Hemorheol Microcirc. 2006;34(1-2):227–232. [PubMed] [Google Scholar]
- [20].Harling-Berg C, Knopf PM, Merriam J, et al. Role of cervical lymph nodes in the systemic humoral immune response to human serum albumin microinfused into rat cerebrospinal fluid. J Neuroimmunol. 1989;25(2-3):185–193. doi: 10.1016/0165-5728(89)90136-7. [DOI] [PubMed] [Google Scholar]
- [21].Weller RO, Galea I, Carare RO, et al. Pathophysiology of the lymphatic drainage of the central nervous system: Implications for pathogenesis and therapy of multiple sclerosis. Pathophysiology. 2010;17(4):295–306. doi: 10.1016/j.pathophys.2009.10.007. [DOI] [PubMed] [Google Scholar]
- [22].Weller RO, Djuanda E, Yow HY, et al. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117(1):1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]


