Abstract
As the major division of the basal ganglia, neostriatum forms mutual connections with multiple brain areas and is critically involved in motor control and learning/memory. Long-term synaptic plasticity has been widely studied in different species recently. However, there are rare reports about the short-term synaptic plasticity in neostratium. In the present study, using field excitatory postsynaptic potentials recording, we reported one form of short-term synaptic plasticity that is paired pulse depression in juvenile rat dorsal striatum slices induced by stimuli of the white matter. The field excitatory postsynaptic potentials could be abolished by α-amino-3-hydroxy-5-methylizoxazole-4-propionic acid receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione, but not by gamma-aminobutyric acid type A receptor antagonist bicuculline or dopamine D1 receptor antagonist SKF-81297. The paired pulse depression in the corticostratial pathway was different from paired pulse facilitation in the hippocampal CA1 synapse. In addition, the paired pulse depression was not affected by bath application of gamma-aminobutyric acid type A receptor antagonist or dopamine D1 receptor antagonist. However, low calcium and high magnesium could attenuate the paired pulse depression. These findings suggest a more complicated plasticity form in the dorsal striatum of juvenile rats that is different from that in the hippocampus, which is related with extracellular calcium.
Keywords: paired pulses depression, dorsal striatum, calcium, juvenile rats
Abbreviations:
fEPSP, field excitatory postsynaptic potentials; PPD, paired pulse depression; MSN, medium spiny projection neuron
INTRODUCTION
The striatum, as the major division of the basal ganglia, is critically involved in motor control and learning/memory. The most common cell type in the striatum, medium spiny projection neurons (MSNs), receive glutamatergic inputs from the cerebral cortex and thalamus, serotonergic input from the raphe nuclei, dopaminergic input from the substantia nigra as well as cholinergic and gamma-aminobutyric acidergic input from striatal interneuron. In addition, MSNs also receive synapses from axonal collaterals from neighboring MSNs[1], suggesting a more complicated circuit of the striatum. The neuroplasticity in the striatum controls the short-term and long-term selection and the differential amplification of cortical neural signals modulating the processes of motor and behavioral selection within the basal ganglia neural circuit[2,3]. Synaptic plasticity at corticostraital synapses is proposed to contribute to motor learning and habit formation. Unlike hippocampal CA3–CA1 synapses, however, the corticostratial pathway differs regionally in direction of plasticity in spite of the application of identical stimulation protocols: for example high frequency simulation (100 Hz) induces long-term potentiation in the dorsomedial striatum but long-term depression in the dorsolateral striatum[4,5]. In addition, paired pulses induce depression of excitatory postsynaptic potentials (EPSPs)[6,7], and this differs from hippocampus CA3–CA1 synapses which exhibit facilitation in response to paired pulses[8]. These results suggest that the mechanisms of long-term and short-term synaptic plasticity in the striatum may differ from those in the hippocampus.
Short-term synaptic plasticity, including short-term potentiation and depression, lasts from tens of milliseconds to several seconds, and plays an important role in neural encoding[9]. Short-term depression can act as a low-pass filter contributing to spatial-temporal processing[10], serving to control the gain between excitation and inhibition[11], and contributing to the generation of synchronous network activity[12]. Paired pulse depression (PPD), instead of paired pulse facilitation, is a form of short-term synaptic plasticity that was observed in the striatum[6,13]. Although it has been suggested that PPD in the striatum involves presynaptic mechanisms and the concentration of external calcium[6], the detailed mechanism of this form of plasticity is poorly understood.
RESULTS
Dorsal striatum field EPSP (fEPSP) is not affected by D1, gamma-aminobutyric acid type A or muscarinia receptor
Stimulation of the white matter induced two negative extracellular potentials (N1 and N2, Figure 1A) in the neostriatum, as previously described[14,15]. The N2, also referred to population spike or fEPSP, was abolished by bath application of α-amino-3-hydroxy-5-methylizoxazole-4-propionic acid receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione, while the N1 was not affected by 6-cyano-7-nitroquinoxaline-2,3-dione (10 μM) (Figure 1D), suggesting the N2 potential is generated mainly by excitatory postsynaptic currents[14,16]. The population spike (N2) of the striatum was not affected by blockade of gamma-aminobutyric acid type A receptors antagonist bicuculline (20 μM) (Figures 1B, C), while in contrast the fEPSP in hippocampus CA1 synapses was significantly affected by blockade of gamma-aminobutyric acid type A receptor antagonist bicuculline (20 μM) (Figures 2A–C) and paired-pulses induced facilitation, which is significant different from striatum (Figure 2D). In addition, the PS was not affected by D1 receptors antagonist SKF-81297 (20 μM) or muscarinic receptors antagonist succinylcholine chloride (20 μM) (Figures 3, 4), consistent with previous reports[14].
Figure 1.
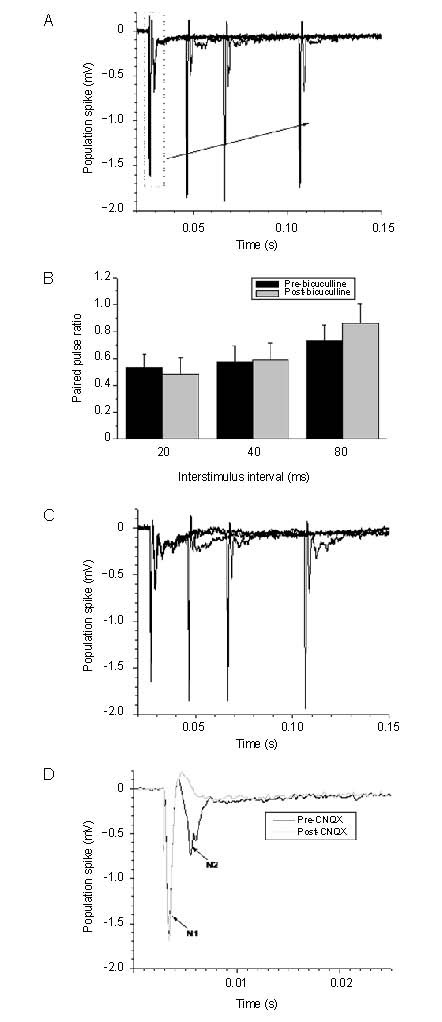
Effect of bicuculline (gamma-aminobutyric acid type A receptor antagonist) and CNQX (α-amino-3-hydroxy-5-methylizoxazole-4-propionic acid receptor antagonist) on striatal population spike. CNQX: 6-cyano-7-nitroquinoxaline-2,3-dione.
(A) Representative trace of paired pulse induced population spike in rat corticostriatal slice, and the insert is the magnified P1.
(B) Averaged paired pulse ratio pre- and post-bicuculline administration; the insert is averaged P1 amplitude before and after bath application of bicuculline. Measurement data are expressed as mean ± SE.
(C) Representative trace of paired pulse induced population spike after bath application of bicuculline.
(D) Representative trace of recording from corticostriatal slice pre- and post-CNQX administration.
Figure 2.
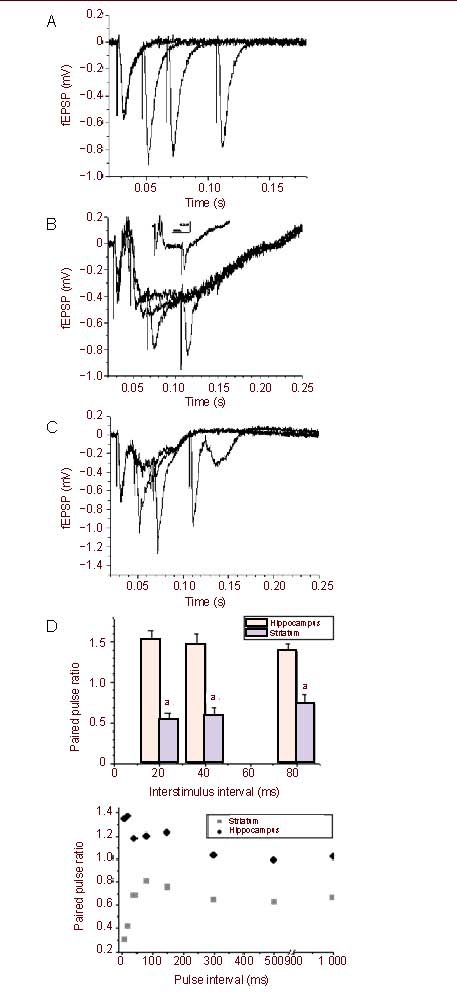
Effect of bicuculline on hippocampal field excitatory postsynaptic potentials (fEPSP) (A–C) and difference of paired pulse ratio between the hippocampus and striatum (D).
(A) Representative trace of paired pulse facilitation in rat hippocampal slices before application of gamma-aminobutyric acid type A receptor antagonist bicuculline (20 μM).
(B) Representative trace of paired pulse facilitation in rat hippocampal slices after bath application of gamma-aminobutyric acid type A receptor antagonist bicuculline (20 μM); the insert is averaged P1 amplitude before and after bath application of bicuculline.
(C) Trace of paired pulse facilitation after washout of bicuculline.
(D) Averaged paired pulse ratio from rat hippocampal and corticostriatal slices. Measurement data are expressed as mean ± SE. aP = 0.001, 0.001 2, 0.03, vs. at the hippocampus 20, 40 and 80 interstimulus interval, respectively.
Figure 3.
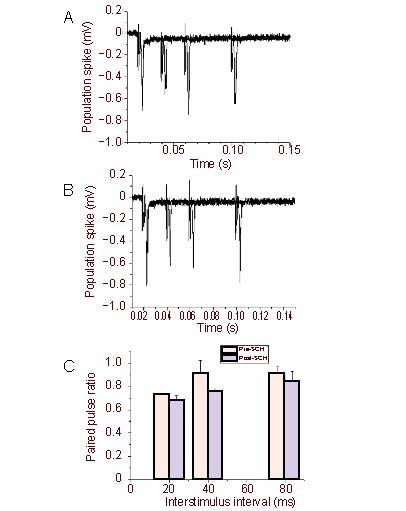
Succinylcholine chloride (muscarinic receptor antagonist) (20 μM) had no effect on the paired pulse depression of population spike/field excitatory postsynaptic potentials.
Representative trace of paired pulse depression in rat hippocampal slices before (A) and after (B) application of succinylcholine chloride. (C) Averaged paired pulse ratio pre- and post-succinylcholine chloride administration. Measurement data are expressed as mean ± SE.
Figure 4.
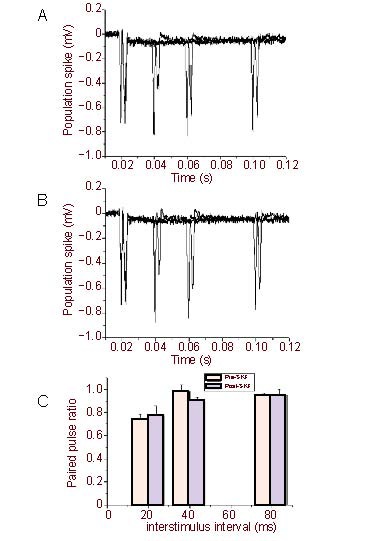
SKF-81297(dopamine D1 receptor antagonist) (20 μM) had no effect on the paired pulse depression of population spike/field excitatory postsynaptic potentials.
Representative trace of paired pulse depression in rat hippocampal slices before (A) and after (B) application of succinylcholine chloride. (C) Averaged paired pulse ratio pre- and post-SKF-81297 administration. Measurement data are expressed as mean ± SE.
The PPD is related with extracellular calcium
It is well known that paired pulse stimulation of the Schaffer collateral pathway induces facilitation of the fEPSP in the hippocampal CA1 area (Figures 2A, D). In contrast, using the identical conditions paired pulse stimuli of the corticostriatl pathway induced depression of the population spike/fEPSP (Figures 1A, B) in the slices of dorsal striatum. In addition, a series of paired pulses, including interstimulus intervals of 20, 40 and 80 ms, all produced depression of population spike/fEPSP (Figure 1B). In addition, we found that even longer interval as 150–1 000 ms also induced depression of population spike/fEPSP in rat dorsal striatum (Figure 2D, lower), which was significantly different from the hippocampus. We next investigated if blockade of gamma-aminobutyric acid, muscarinic or D1 receptors would alter paired pulse induced depression of population spike/fEPSP in striatal slices. Bath application of the gamma-aminobutyric acid type A receptor antagonist bicuculline (20 μM), the muscarinic receptor antagonist succinylcholine chloride (20 μM)[17] or the D2 receptor antagonist SKF-81297 (20 μM) had no effect on the PPD of population spike/fEPSP (Figures 3, 4), neither did any of these drugs alter the amplitudes of population spike/fEPSP. Previous study indicated that the N2 potential was blocked in a perfusion medium containing a lower Ca2+ or a higher Mg2+ concentration than the standard solution[15]. The result indicated that 0.5 mM but not 2.6 mM calcium containing solution can attenuate the PPD (Figure 5).
Figure 5.
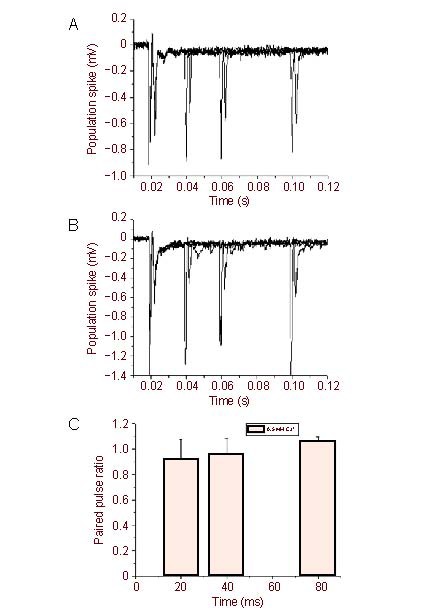
Lowing extracellular calcium partially reduced the striatal paired pulse depression of population spike/field excitatory postsynaptic potential.
Representative trace of paired pulse depression of population spike/field excitatory postsynaptic potential in rat striatal slices by application of 2.6 mM Ca2+, 1.3 mM Mg2+ (A) or 0.5 mM Ca2+, 1.3 mM Mg2+ (B).
(C) Averaged paired pulse ratio via 0.5 mM Ca2+ administration. Measurement data are expressed as mean ± SE.
DISCUSSION
The dorsal striatum is a large forebrain region involved in action initiation, timing, control, learning and memory. Modulation of corticostriatal synaptic transmission plays a large part in controlling the input to as well as the output from MSNs[3]. Both long-term plasticity and short-term plasticity have been identified in the neostriatum using different experimental conditions[18,19,20,21] and the alterations in striatal synaptic plasticity might be implicated in Parkinson's disease and Huntington's disease[16,21]. It was proposed that long-term changes of synaptic transmission were often associated with a modification of short-term synaptic plasticity[22]. Two forms of short-term plasticity at inhibitory synapses, PPD and synaptic augmentation, were studied in adult rat striatal slices using intracellular recordings and were suggested to modify the output of striatum and thus might be an important component of information processing during behaviors involving the striatum[19]. While in the present study, we observed one PPD at the corticostriatal slices mediated by excitatory synapses since the N2 potential (population spike/fEPSP) can be abolished by α-amino-3-hydroxy-5-methylizoxazole-4-propionic acid receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione. Using whole-cell configuration, Mori et al[6] recorded the PPD in MSNs of neonatal Wistar rat corticostriatal slices and suggested the PPD involved presynaptic mechanism. Their findings were not same with the present study in that they found the PPD was not detected when the second pulse was 100 ms after the first pulse while our data showed that even with 500 ms interstimulus interval the PPD was still present. Similar controversy was confirmed by studies from Akopian and Walsh that the PPD existed during the interstimulus interval was 1 second in Fisher rats[23] and from Lighthall indicating that the PPD was gone when interstimulus interval was longer than 30 ms in adult Long-Evans rats[7]. However, one similar PPD was showed up in adult rat striatal inhibitory synapses when interstimulus intervals at several hundred milliseconds when the authors studied other project and they did not give further attention[19], which was similar to the present study.
The striatal MSNs receive gluatamtergic input from the cerebral cortex and thalamus, serotonergic input from the raphe, dopaminergic input from the substantia nigra and cholingergic and gamma-aminobutyric acidergic input from striatal interneuron[1]. There are two distinct inhibitory gamma-aminobutyric acidergic circuits in the neostriatum: feedforward circuit and feedback circuit. The feedforward circuit consists of a relatively small population of gamma-aminobutyric acidergic interneurons that receives excitatory input from the neocortex and exerts monosynaptic inhibition onto striatal MSNs. The feedback circuit comprises the numerous spiny projection neurons and their interconnections via local axon collaterals. This network has long been assumed to provide the majority of striatal gamma-aminobutyric acidergic inhibition and to sharpen and shape striatal output through lateral inhibition, producing increased activity in the most strongly excited spiny cells at the expense of their less strongly excited neighbors[24]. However, our data did not support the role of inhibitory gamma-aminobutyric acidergic circuits in the PPD since bicuculline had no effect on the PPD or the population spike. In addition, although MSNs also receive D1 receptor innervations and may be involved in synaptic plasticity in the neostriatum[25], our data did not support their involvements in the PPD.
It was indicated that glutamatergic synapses in the neostriatum were capable of expressing a form of synaptic depression that may involve decreased glutamate release[20] and that depolarization during the first N-methyl-D-aspartate receptor response caused facilitation of the second one by removing voltage-dependent block of N-methyl-D-aspartate receptors by Mg2+ and by activating voltage-dependent Ca2+ channels[26]. However, another study suggested that N-methyl-D-aspartate application depressed the population spike/fEPSP in mouse corticostrital slices in a gamma-aminobutyric acid-independent manner and that the depression was not affected by removal of the cortex[27]. Previous studies have indicated that calcium plays an important role in synaptic transmission at corticostriatal synapses mediated by N-type calcium channels[28]. Although the present study also indicated that lowing the extracellular calcium concentration could reduce the PPD but we still did not see obvious paired pulse facilitation, which was not consistent with Dr Wang's report that lower calcium with the calcium/magnesium ratio at 1:2 could introduce obvious paired pulse facilitation in C57BL/6 mice aged 2–5 months[29] and suggested that the PPD in juvenile rat corticalstriatum was related with calcium. The difference may be related with protocols, species and age, etc., which need to be further studied.
MATERIALS AND METHODS
Design
An in vitro electrophysiological study.
Time and setting
This study was performed at the University of Western Ontario, Canada from November 2010 to March 2011.
Materials
Animals
Wistar rats of either sex, aged 2 weeks, were provided by the Animal Center at University of Western Ontario, Canada.
Reagents
6-cyano-7-nitroquinoxaline-2,3-dione, SKF-81297, bicuculline (Tocris), succinylcholine chloride dehydrate (Sigma) and (+)-α-methyl-4-carboxyphenylglycine (MCPG, Sigma) were applied via the bathing solution.
Methods
Slice preparation
The rats were decapitated under anesthesia with esoflurane and the whole brain was removed, immersed in ice-cold cutting solution containing (in mM): sucrose 194, NaCl 30, KCl 4.5, MgCl2 1, NaHCO3 26, NaH2PO4 1.2, glucose 10, bubbled with 95% oxygen and 5% CO2. The coronary striatum containing cortex and the transverse hippocampus were cut into 350 μm slices using a vibratome (LeicaVT 1200S). The slices were incubated for at least 1 hour at room temperature in artificial cerebrospinal fluid containing (in mM): NaCl 124, KCl 3.0, NaH2PO4 1.25, glucose 10, NaHCO3 26, CaCl2 2.6, MgCl2 1.3, bubbled with 95% oxygen and 5% CO2.
Electrophysiological recording
Recordings were performed at 31°C. A slice was transferred to the recording chamber using a Pasteur pipette and continuously perfused with artificial cerebrospinal fluid saturated by 95% O2 and 5% CO2 at a flow rate of 3 mL/min. A glass microelectrode filled with artificial cerebrospinal fluid (4–6 MΩ) was placed in the dorsal striatum (Figure 6A) or hippocamal CA1 stratum radiatum (Figure 6B). The depth of the electrode was adjusted to obtain a maximal response. Population spike or fEPSP were evoked by constant current stimuli (0.1 ms) via a tungsten concentric bipolar electrode (tip-diameter: 25 mm; MCE-100, David Kopf Instruments, MD, USA) placed in the white matter for striatum recording (Figure 6A) or Schaffer lateral pathway for hippocampus recording (Figure 6B). Variable paired-pulses with different interstimulus intervals (20, 40, 80, or 150, 300, 500, 1 000 ms) were applied to evoke half of the maximal field potential (population spike or fEPSP). The signals were recorded (Digidata 1440A, Molecular Device, USA), amplified (MultiClamp 700B, Molecular Device, USA) and filtered at 5 kHz. Data were analyzed offline using Clampfit 10 (Axon Instruments). The paired pulse ratio refers to the amplitude of the second population or fEPSP relative to that of the first population spike or fEPSP.
Figure 6.
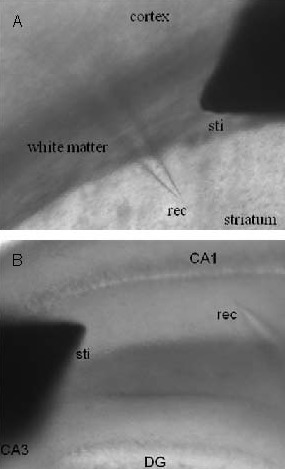
Schematic diagram of the field potential recording from the dorsal striatum (A) and hippocampus (B).
Sti: Stimulator; rec, recording electrode; DG: dentate gyrus.
Statistical analysis
Data were expressed as mean ± SE. Statistical analyses were performed using one-way analysis of variance followed by the Bonferroni test or two-way repeated analysis of variance, using software of Origin7.
Footnotes
Funding: The study was supported by a grant from the Canadian Institutes of Health Research, No. 15514 & 44008.
Conflicts of interest: None declared.
Ethical approval: The experiment was approved by the Animal Ethics Committee of the University of Western Ontario, Canada.
(Edited by Zhu LQ, Zhao MG/Wang L)
REFERENCES
- [1].Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13(7):259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- [2].Di Filippo M, Picconi B, Tantucci M, et al. Short-term and long-term plasticity at corticostriatal synapses: implications for learning and memory. Behav Brain Res. 2009;199(1):108–118. doi: 10.1016/j.bbr.2008.09.025. [DOI] [PubMed] [Google Scholar]
- [3].Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58(7):951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dang MT, Yokoi F, Yin HH, et al. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci U S A. 2006;103(41):15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol. 2000;84(3):1422–1429. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]
- [6].Mori A, Takahashi T, Miyashita Y, et al. Two distinct glutamatergic synaptic inputs to striatal medium spiny neurones of neonatal rats and paired-pulse depression. J Physiol. 1994;476(2):217–228. doi: 10.1113/jphysiol.1994.sp020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lighthall JW, Park MR, Kitai ST. Inhibition in slices of rat neostriatum. Brain Res. 1981;212(1):182–187. doi: 10.1016/0006-8993(81)90048-2. [DOI] [PubMed] [Google Scholar]
- [8].O’Mara SM, Commins S, Anderson M. Synaptic plasticity in the hippocampal area CA1-subiculum projection: implications for theories of memory. Hippocampus. 2000;10(4):447–456. doi: 10.1002/1098-1063(2000)10:4<447::AID-HIPO11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- [9].O’Donovan MJ, Rinzel J. Synaptic depression: a dynamic regulator of synaptic communication with varied functional roles. Trends Neurosci. 1997;20(10):431–433. doi: 10.1016/s0166-2236(97)01124-7. [DOI] [PubMed] [Google Scholar]
- [10].Fortune ES, Rose GJ. Short-term synaptic plasticity as a temporal filter. Trends Neurosci. 2001;24(7):381–385. doi: 10.1016/s0166-2236(00)01835-x. [DOI] [PubMed] [Google Scholar]
- [11].Varela JA, Song S, Turrigiano GG, et al. Differential depression at excitatory and inhibitory synapses in visual cortex. J Neurosci. 1999;19(11):4293–4304. doi: 10.1523/JNEUROSCI.19-11-04293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tsodyks M, Uziel A, Markram H. Synchrony generation in recurrent networks with frequency-dependent synapses. J Neurosci. 2000;20(1):RC50. doi: 10.1523/JNEUROSCI.20-01-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nisenbaum ES, Berger TW, Grace AA. Presynaptic modulation by GABAB receptors of glutamatergic excitation and GABAergic inhibition of neostriatal neurons. J Neurophysiol. 1992;67(2):477–481. doi: 10.1152/jn.1992.67.2.477. [DOI] [PubMed] [Google Scholar]
- [14].Cordingley GE, Weight FF. Non-cholinergic synaptic excitation in neostriatum: pharmacological evidence for mediation by a glutamate-like transmitter. Br J Pharmacol. 1986;88(4):847–856. doi: 10.1111/j.1476-5381.1986.tb16258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Misgeld U, Okada Y, Hassler R. Locally evoked potentials in slices of rat neostriatum: a tool for the investigation of intrinsic excitatory processes. Exp Brain Res. 1979;34(3):575–590. doi: 10.1007/BF00239150. [DOI] [PubMed] [Google Scholar]
- [16].Calabresi P, Pisani A, Mercuri NB, et al. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci. 1996;19(1):19–24. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- [17].Jonsson M, Dabrowski M, Gurley DA, et al. Activation and inhibition of human muscular and neuronal nicotinic acetylcholine receptors by succinylcholine. Anesthesiology. 2006;104(4):724–733. doi: 10.1097/00000542-200604000-00017. [DOI] [PubMed] [Google Scholar]
- [18].Akopian G, Musleh W, Smith R, et al. Functional state of corticostriatal synapses determines their expression of short- and long-term plasticity. Synapse. 2000;38(3):271–280. doi: 10.1002/1098-2396(20001201)38:3<271::AID-SYN6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- [19].Fitzpatrick JS, Akopian G, Walsh JP. Short-term plasticity at inhibitory synapses in rat striatum and its effects on striatal output. J Neurophysiol. 2001;85(5):2088–2099. doi: 10.1152/jn.2001.85.5.2088. [DOI] [PubMed] [Google Scholar]
- [20].Lovinger DM, Tyler EC, Merritt A. Short- and long-term synaptic depression in rat neostriatum. J Neurophysiol. 1993;70(5):1937–1949. doi: 10.1152/jn.1993.70.5.1937. [DOI] [PubMed] [Google Scholar]
- [21].Gustafson N, Gireesh-Dharmaraj E, Czubayko U, et al. A comparative voltage and current-clamp analysis of feedback and feedforward synaptic transmission in the striatal microcircuit in vitro. J Neurophysiol. 2006;95(2):737–752. doi: 10.1152/jn.00802.2005. [DOI] [PubMed] [Google Scholar]
- [22].Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3(Suppl):1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- [23].Akopian G, Walsh JP. Reliable long-lasting depression interacts with variable short-term facilitation to determine corticostriatal paired-pulse plasticity in young rats. J Physiol. 2007;580(Pt 1):225–240. doi: 10.1113/jphysiol.2006.115790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tepper JM, Wilson CJ, Koos T. Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain Res Rev. 2008;58(2):272–281. doi: 10.1016/j.brainresrev.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Martella G, Tassone A, Sciamanna G, et al. Impairment of bidirectional synaptic plasticity in the striatum of a mouse model of DYT1 dystonia: role of endogenous acetylcholine. Brain. 2009;132(Pt 9):2336–2349. doi: 10.1093/brain/awp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Akopian G, Walsh JP. Corticostriatal paired-pulse potentiation produced by voltage-dependent activation of NMDA receptors and L-type Ca(2+) channels. J Neurophysiol. 2002;87(1):157–165. doi: 10.1152/jn.00115.2001. [DOI] [PubMed] [Google Scholar]
- [27].Schotanus SM, Chergui K. NR2A-containing NMDA receptors depress glutamatergic synaptic transmission and evoked-dopamine release in the mouse striatum. J Neurochem. 2008;106(4):1758–1765. doi: 10.1111/j.1471-4159.2008.05512.x. [DOI] [PubMed] [Google Scholar]
- [28].Lovinger DM, Merritt A, Reyes D. Involvement of N-and non-N-type calcium channels in synaptic transmission at corticostriatal synapses. Neuroscience. 1994;62(1):31–40. doi: 10.1016/0306-4522(94)90312-3. [DOI] [PubMed] [Google Scholar]
- [29].Wang Y. Differential effect of aging on synaptic plasticity in the ventral and dorsal striatum. Neurobiol Learn Mem. 2008;89(1):70–75. doi: 10.1016/j.nlm.2007.08.015. [DOI] [PubMed] [Google Scholar]


