Abstract
The present study observed changes in rat neural cells at various ages (3, 18, 24, and 30 months). With age, neural cells became large and were sparsely arranged, and the number of Nissl bodies decreased. In addition, hypoxia-inducible factor 1α expression increased with increasing age in hippocampal CA1 and CA3 regions, motor cortex, and the first subfolium, especially from 3 to 18 months. In the open-field test, grid crossing decreased with increasing age, especially from 18 months. The number of rearings reached a peak in the 18 months group, and then subsequently decreased. The results suggested that hypoxia-inducible factor 1α played an important role in the nervous system aging process.
Keywords: aging, brain, hypoxia-inducible factor 1α, Nissl body, behavior
Abbreviations:
HIF, hypoxia-inducible factor
INTRODUCTION
Hypoxia-inducible factor 1α (HIF-1α) is a specifically regulated oxygen subunit, which determines activity of HIF-1[1,2,3,4,5,6,7]. By inducing expression of the target gene, HIF-1α regulates anaerobic metabolism and induces anoxic histiocytes to maintain oxygen concentrations, thereby prolonging hypoxia[8,9,10,11]. At present, studies on HIF-1α in the nervous system have primarily focused on regulating the hypoxia response after ischemic brain injury[12,13]. However, a previous study reported the effects of HIF-1α on development and aging in the nervous system[14], but not involved in the aging of the nervous system. The present study was designed to evaluate behavioral changes in rats using the open-field test, as well as to observe expression of Nissl bodies in brain tissue to reflect the function of protein synthesis, and to detect HIF-1α in different brain regions using immunohistochemical techniques to analyze the role of HIF-1α in the aging nervous system.
RESULTS
Quantitative analysis of experimental animals
A total of 40 rats were enrolled in the study. There were 10 rats in each age group (3, 18, 24, and 30 months). All rats were included in the final analysis.
Behavioral changes in rats
Results showed significantly decreased appetite, as well as diminished response and amount of activity in aged rats (24 and 30 months), compared to young rats (3 months) and middle-aged rats (18 months). As shown in the open-field test, the number of grid crossings decreased with age, in particular from 18 to 24 months and from 24 to 30 months (P < 0.05). With increasing age, the number of rearings in each group exhibited a decreasing trend after the initial peak at 18 months. Although there was no statistical difference between 3 to 18 months (P > 0.05), the decrease from 18 to 24 months and from 24 to 30 months was significant (P < 0.05, respectively; Table 1).
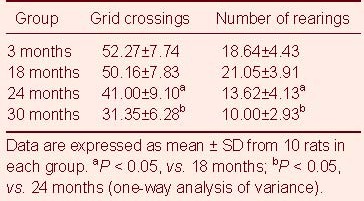
Table 1.
Comparison of grid crossings and the number of rearings (times/3 minutes) in each group in the open-field test
Nissl body content in different brain regions
Nissl staining results showed ordered and concentrated neural cells at 3 months, with abundant Nissl bodies in the cytoplasm. However, neural cells became large and sparsely arranged, and the number of Nissl bodies decreased, in aged rats (24 and 30 months). In particular, neural cells were missing in the hippocampal pyramidal layer at 30 months (Figure 1).
Figure 1.
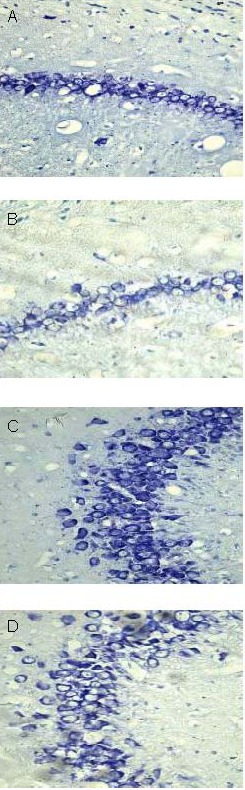
Nissl bodies from neural cells of different brain regions (Nissl staining, optical microscope, × 400).
Hippocampal CA1 (A) and CA3 (C) regions at 3 months: neural cells are ordered and concentrated, with abundant Nissl bodies in the cytoplasm.
Hippocampal CA1 (B) and CA3 (D) regions at 30 months: neural cells are large and sparsely arranged; decreased number of Nissl bodies, and absence of neural cells in the pyramidal layer.
HIF-1α expression in different brain regions
Immunohistochemistry results revealed HIF-1α expression primarily in the cytoplasm and nucleus. In the hippocampus and motor cortex, pyramidal cells expressed HIF-1α, and in the first subfolium, Purkinje cells were positive (Figure 2). Significant differences existed (P < 0.05) in the increased number of HIF-1α-positive cells with increasing age in the CA1 and CA3 regions of the hippocampus, motor cortex, and the first subfolium, and this increase was most significant from 3 to 18 months (Table 2).
Figure 2.
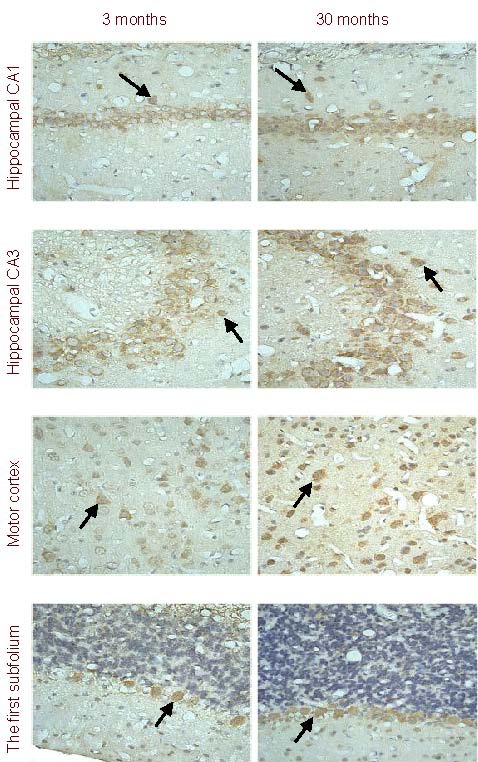
Cells expressing hypoxia-inducible factor 1α (HIF-1α) in various brain regions (immunohistochemistry, × 400).
HIF-1α expression (arrows) is visible in pyramidal cells of the hippocampus and motor cortex, as well as Purkinje's cells in the first subfolium. In addition, there is a difference in the number of cells expressing HIF-1α within a region at different ages. This figure shows increased numbers of cells expressing HIF-1α in each brain region at 30 months compared to 3 months.
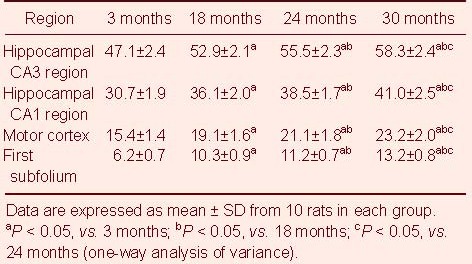
Table 2.
Quantification of cells expressing hypoxia-inducible factor 1α (HIF-1α) (/400-fold visual field) in different brain regions of each group
DISCUSSION
The process of neural aging is complex[15,16,17,18]. Therefore, it is necessary to investigate all changes related to aging in the nervous system, in particular within the brain. In the present study, rats aged 3, 18, 24, and 30 months were selected to represent adolescence, middle age, early old age, and middle-late old age, respectively. Results showed that each observed index significantly changed with age.
The classic open-field experiment is used to study exploratory behavior and emotion in rats[19]. Results from the present study showed a decreased number of grid crossings with age, and this trend increased noticeably into old age, which was consistent with previous results[20]. These behavioral changes could be due to decreased excitability in the central nervous and motor systems during the aging process. The number of rearings increased to a peak at 18 months, but decreased in the number of rearings significantly decreased thereafter. These physiological changes were most severe after 18 months.
The Nissl body has been used as an index for functional status in neural cells. The present results showed a significant decrease in the number and size of Nissl bodies in neural cells of different brain regions, which suggested that protein synthetic function decreased with age. This change was most obvious in the hippocampal pyramidal cell layer at 30 months. These results suggested that decreased numbers of Nissl bodies could be related to hypomnesis with aging[21].
HIF-1 is the first identified factor to mediate hypoxia tolerance in mammals. The molecule is a heterodimer, composed of the HIF-1α subunit and HIF-1β subunit. HIF-1α is specific to HIF-1 and determines its activity. Previous studies have showed that HIF-1 mediates hypoxia tolerance[22,23]. Decreased intracellular oxygen concentration results in increased HIF-1α activity[24], and active HIF-1α directly or indirectly combines with the transcription initiation complex to regulate downstream genes via an effective transcriptional activation domain at the carboxyl terminus. These downstream genes contain coding genes for erythropoietin, vascular endothelial growth factor, glucose transporter protein, and nitric oxide synthase[25,26]. In addition, results have shown that increased gene transcription improves survival in hypoxic cells[27].
HIF-1α expression increased in the rat brain with increasing age, suggesting that aging promoted expression of HIF-1α. It is possible that this was related to cerebral blood flow changes during the aging process. Results showed significantly increased HIF-1α expression from 3 to 18 months, as well as from 24 to 30 months. It is possible that these changes could be due to an increased oxygen demand or decreased cerebral blood flow. In addition, HIF-1α expression varied in different brain regions; expression was greatest in the hippocampus and weakest in the subfolium. These differences could be due to several processes. First, ischemia sensitivity varies in different brain regions; the hippocampus is most sensitive to cerebral ischemia and hypoxia. Second, different brain regions contain different types of cells. According to results from previous studies, as well as the present study, HIF-1α was primarily expressed in large neural cells, including pyramidal cells and Purkinje cells[22,23]. The present study suggested that HIF-1α had multiple effects: activating expression of the erythropoietin gene to increase erythropoiesis and elevate oxygen concentration, up-regulation of vascular endothelial growth factor to promote angiogenesis, increased nitric oxide synthase expression to dilate blood vessels, induced expression of the glycolysis gene to facilitate anaerobic metabolism, and increased apoptosis[28,29,30].
In addition, behavioral results showed that horizontal crossing numbers in the open-field test decreased with age, although HIF-1α expression increased with age. These results suggested that increased HIF-1α expression in neural cells was a compensatory mechanism in response to declining rat behaviors. Increased HIF-1α expression and improved anti-anoxia in the nervous system could delay the age-related changes in rats.
In summary, results suggested that HIF-1α was associated with brain aging and played an important role in this process.
MATERIALS AND METHODS
Design
Aging, controlled, animal experiment.
Time and setting
The study was performed at the Medical College of Xi’an Jiaotong University, China from December 2008 to May 2010.
Materials
A total of 40 male, Sprague-Dawley rats at different ages were obtained from Chengdu Dashuo Animal Reproduction Base (lisence No. SCXK (Chuan) 2008-24). The rats were assigned to various groups, according to age: 3 months (250 ± 20 g), 18 months (500 ± 20 g), 24 months (600 ± 20 g), and 30 months (650 ± 20 g), with 10 rats in each group, respectively. The rats were housed at 22 ± 2°C and controlled humidity (55 ± 5%), with a 12-hour dark/light cycle for 1 week. Animal procedures were in strict accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[31].
Methods
Behavioral changes in rats of different ages, as determined by the open-field test
Rats were housed in a standard environment. After a 2-hour fast, behaviors were assessed at 8 a.m. and 4 p.m. using the open-field test[32]. The rats were placed in an open field (Xinruan, Shanghai, China), which was 100 cm × 100 cm × 50 cm, with a black wall and bottom composed of 25 equally sized sections. The number of times the rats crossed from the grid from the center was quantified during a 3-minute period, as well as the frequency of erect forelimb, including the number of times the forelimb touched the wall and was elevated 1 cm from the ground. Testing was performed for 5 days.
Tissue preparation
After 1 week, the rats were anesthetized with 10% chloral hydrate (3 mL/kg). A thoracotomy was performed, and a cannula was inserted into the ascending aorta via the left ventricle. The right atrium was incised, and 250 mL room temperature heparinized saline was infused, followed by 4% paraformaldehyde/0.01 mM phosphate-buffered saline (250 mL). The brains were then quickly removed. The cerebrum, behind the anterior margin of the optic chiasma and cerebellum, was separated and placed in 4% paraformaldehyde for 14 hours. The specimens were dehydrated, permeabilized, and embedded in wax. The sections were then serially cut into 6-μm thick coronal slices.
Nissl staining
The 6-μm paraffin sections were rinsed twice in xylene for 15 minutes each, followed by 95% ethanol and 80% ethanol, for 5 minutes each, followed by rinsing for 5 minutes in running water. After dewaxing, the sections were incubated in 1% toluidin blue (Tianyuan Biotechnology, Shanghai, China) at 55°C for 30 minutes, and subsequently rinsed for 5 minutes in distilled water. The sections were then incubated in 80% ethanol, 90% ethanol, and 95% ethanol for 5 minutes each, followed by three rinses in xylene for 15 minutes each[33]. Finally, the sections were mounted in neutral gum, and observed under an optical microscope (B-type biomicroscope, Global Motic Group, Xiamen, China).
HIF-1α expression, as determined by immunohistochemistry
The sections were deparaffinized, hydrated, and subjected to antigen retrieval for 10 minutes, according to manufacturer instructions. The sections were incubated in rabbit anti-HIF-1α polyclonal antibody (1:200; Boster, Wuhan, China)[13,34] at 4°C overnight, followed by biotinylated sheep anti-rabbit antibody (1:200) for 40 minutes at room temperature. The sections were then incubated for 30 minutes in streptavidin with horseradish peroxidase (1:200) at room temperature, followed by diaminobenzidine chromogen for 5 minutes. The sections were then counterstained with hematoxylin and subsequently dehydrated and mounted. Images from the hippocampal CA1 and CA3 regions, the motor cortex, and the first subfolium were collected and analyzed (Q550CW; Leica, Solms, Germany). Under a light microscope (× 400; B-type biomicroscope, Global Motic Group), five different visual fields were randomly selected from each section for quantification. The mean number represented the number of positive cells in that section.
Statistical analysis
All data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Measurement data were expressed as mean ± SD, and comparisons were made using one-way analysis of variance. P < 0.05 was considered statistically significant.
Footnotes
Funding: This work was supported by grants from Foundation of Second Hospital of Medical College of Xi’an Jiaotong University, No. 2005-yl-01; the National Natural Science Foundation of China (The mechanism of erythropoietin-TAT regulating Keap l-Nrf2 pathway on anti-aging process in nerve, 2011), No. 81170330.
Conflicts of interest: None declared.
Ethical approval: This study received approval from the Animal Ethics Committee of the Medical College of Xi’an Jiaotong University, China.
(Edited by Liu YL, Li HF/Qiu Y/Song LP)
REFERENCES
- [1].Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12(12):5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sendoel A, Kohler I, Fellmann C, et al. HIF-1 antagonizes p53-mediated apoptosis through a secreted neuronal tyrosinase. Nature. 2010;465(7298):577–583. doi: 10.1038/nature09141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20(1):51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Corzo CA, Condamine T, Lu L, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207(11):2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Takubo K, Goda N, Yamada W, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7(3):391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- [6].Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15(20):2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Semenza GL. Physiology meets biophysics: visualizing the interaction of hypoxia-inducible factor 1 alpha with p300 and CBP. Proc Natl Acad Sci U S A. 2002;99(18):11570–11572. doi: 10.1073/pnas.192442299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- [9].Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- [10].Greijer AE, van der Groep P, Kemming D, et al. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) J Pathol. 2005;206(3):291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- [11].Leiser SF, Begun A, Kaeberlein M. HIF-1 modulates longevity and healthspan in a temperature-dependent manner. Aging Cell. 2011;10(2):318–326. doi: 10.1111/j.1474-9726.2011.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Matsuda T, Abe T, Wu JL, et al. Hypoxia-inducible factor-1alpha DNA induced angiogenesis in a rat cerebral ischemia model. Neurol Res. 2005;27(5):503–508. doi: 10.1179/016164105X25144. [DOI] [PubMed] [Google Scholar]
- [13].Wu HQ, Zhang B, Zhan SQ, et al. The expression of HIF-1α and EPO after forebrain ischemia in rats. Zhongfeng yu Shenjing Jibing Zazhi. 2006;13(1):34–36. [Google Scholar]
- [14].Tomita S, Ueno M, Sakamoto M, et al. Defective brain development in mice lacking the Hif-1alpha gene in neural cells. Mol Cell Biol. 2003;23(19):6739–6749. doi: 10.1128/MCB.23.19.6739-6749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237(4811):143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- [16].Fonseca DB, Sheehy MR, Blackman N, et al. Reversal of a hallmark of brain ageing: liofuscin accumulation. Neurobiol Aging. 2005;26(1):69–76. doi: 10.1016/j.neurobiolaging.2004.02.013. [DOI] [PubMed] [Google Scholar]
- [17].Rutten BP, Schmitz C, Gerlach OH, et al. The aging brain: accumulation of DNA damage or neuron loss? Neurobiol Aging. 2007;28(1):91–98. doi: 10.1016/j.neurobiolaging.2005.10.019. [DOI] [PubMed] [Google Scholar]
- [18].Escames G, López A, García JA, et al. The role of mitochondria in brain aging and the effects of melatonin. Curr Neuropharmacol. 2010;8(3):182–193. doi: 10.2174/157015910792246245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].de Cabo de la Vega C, Pujol A, Paz Viveros M. Neonatally administered naltrexone affects several behavioral responses in adult rats of both genders. Pharmacol Biochem Behav. 1995;50(2):277–286. doi: 10.1016/0091-3057(94)00314-9. [DOI] [PubMed] [Google Scholar]
- [20].Ji Y, Li Y, Jiang H, et al. Effect of chronic stress on the behavior and estradiol level in different age rats. Shandong Daxue Xuebao: Yixue Ban. 2007;45(1):14–17. [Google Scholar]
- [21].Ris L, Godaux E. Synapse specificity of long-term potentiation breaks down with aging. Learn Mem. 2007;14(3):185–189. doi: 10.1101/lm.451507. [DOI] [PubMed] [Google Scholar]
- [22].Bernaudin M, Nedelec AS, Divoux D, et al. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22(4):393–403. doi: 10.1097/00004647-200204000-00003. [DOI] [PubMed] [Google Scholar]
- [23].Jones NM, Lee EM, Brown TG, et al. Hypoxic preconditioning produces differential expression of hypoxia-inducible factor-1alpha (HIF-1alpha) and its regulatory enzyme HIF prolyl hydroxylase 2 in neonatal rat brain. Neurosci Lett. 2006;404(1-2):72–77. doi: 10.1016/j.neulet.2006.05.049. [DOI] [PubMed] [Google Scholar]
- [24].Papandreou I, Cairns RA, Fontana L, et al. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3(3):187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- [25].Fedele AO, Whitelaw ML, Peet DJ. Regulation of gene expression by the hypoxia-inducible factors. Mol Interv. 2002;2(4):229–243. doi: 10.1124/mi.2.4.229. [DOI] [PubMed] [Google Scholar]
- [26].Semenza GL. O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1. J Appl Physiol. 2004;96(3):1170–1177. doi: 10.1152/japplphysiol.00770.2003. [DOI] [PubMed] [Google Scholar]
- [27].Park HK, Seol IJ, Kim KS. Protective effect of hypoxic preconditioning on hypoxic-ischemic injured newborn rats. J Korean Med Sci. 2011;26(11):1495–1500. doi: 10.3346/jkms.2011.26.11.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shao G, Gao CY, Lu GW. Alterations of hypoxia-inducible factor-1 alpha in the hippocampus of mice acutely and repeatedly exposed to hypoxia. Neurosignals. 2005;14(6):255–261. doi: 10.1159/000088641. [DOI] [PubMed] [Google Scholar]
- [29].Helton R, Cui Jk, Scheel JR, et al. Brain-specific knock-out of hypoxia inducible factor-lα reduces rather than increases hypoxic ischemic-damage. J Neurosci. 2005;25(16):4099–4107. doi: 10.1523/JNEUROSCI.4555-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Manotham K, Tanaka T, Ohse T, et al. A biologic role of HIF-1 in the renal medulla. Kidney Int. 2005;67(2):1428–1439. doi: 10.1111/j.1523-1755.2005.00220.x. [DOI] [PubMed] [Google Scholar]
- [31].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [32].Rafay J, Flak P. Crossbreeding parameters of rabbit motion under open field test conditions. World Rabbit Science. 1998;7(1):9–14. [Google Scholar]
- [33].Yu SS, Zhao J, Zheng WP, et al. Neuroprotective effect of 4-hydroxybenzyl alcohol against transient focal cerebral ischemia via anti-apoptosis in rats. Brain Res. 2010;1308:167–175. doi: 10.1016/j.brainres.2009.10.037. [DOI] [PubMed] [Google Scholar]
- [34].Hughes JM, Groot AJ, van der Groep P, et al. Active HIF-1 in the normal human retina. J Histochem Cytochem. 2010;58(3):247–254. doi: 10.1369/jhc.2009.953786. [DOI] [PMC free article] [PubMed] [Google Scholar]


