ABSTRACT
Ubiquitous Epstein-Barr virus (EBV) infects not only B cells but also T and NK cells, and is associated with various lymphoid malignancies. The spectrum of EBV-associated lymphoid malignancies is expanding from Burkitt lymphoma to the newly defined systemic EBV+ T cell lymphoproliferative disease of childhood and hydroa vacciniforme-like lymphoma. However, some EBV-associated malignancies are not defined well and overlap other diseases. Furthermore, the role of EBV in tumorigenesis of lymphoid malignancies is still not clear. Further studies are necessary to clarify the pathogenesis of EBV-associated lymphoid malignancies for a better classification of each disease and for the establishment of effective treatment.
Key Words: EBV, latency, systemic EBV+ T cell lymphoproliferative disease of childhood, hydroa vacciniforme-like lymphoma
INTRODUCTION
Epstein-Barr virus (EBV) is a ubiquitous virus which belongs to the γ herpesvirus subfamily.1) γ herpesviruses are well-known as tumor viruses that express virus cancer genes and immortalize infected-lymphocytes. EBV infects not only B cells but also T or natural killer (NK) cells. EBV causes benign lymphoproliferative disease infectious mononucleosis, and is associated with various kinds of lymphoid malignancies.2-4) Since EBV was first isolated from Burkitt lymphoma and its association was reported in 1964, nearly 50 years have passed.5) The spectrum of EBV-associated diseases has been expanding from infectious mononucleosis to overt leukemia/lymphoma. However, the diseases’ definitions are still unclear. Some of the diseases are well defined and known, but others were ill defined and poorly understood.6) In this review, we briefly outline the nature of EBV and summarize the pathogenesis and clinical features of EBV-associated lymphoid malignancies. In particular, we focus on newly defined EBV-associated T cell lymphoproliferative disease or lymphoma.
BIOLOGICAL NATURE OF EBV INFECTION
EBV infects B cells and establishes a life-long infection; the so-called latent infection. In the primary infection, cell-free EBV in the saliva infects naïve B cells in the oropharynx.7) EBV attaches to the cell surface protein CD21, the primary EBV receptor through the viral envelope glycoprotein gp350/220.8) For the penetration of the virus into cell membrane, the viral glycoprotein complex gH-gL-gp42 and co-receptor HLA class II are necessary (Fig. 1).9) EBV initiates a growth-transforming infection, causing naïve B cells to transform into proliferating blasts (Fig. 2). In immunocompetent hosts, both EBV-specific cytotoxic T lymphocytes (CTL) and NK cells control the outgrowth of EBV-transformed cells during primary infection.10) Primary EBV infection is usually asymptomatic, but occasionally progresses to infectious mononucleosis, which resolves spontaneously after the emergence of EBV-specific immunity.10) EBV then establishes a latent infection in memory B cells. After convalescence, EBV persists latently in these memory B cells in an episomal form (Fig. 2). These virus-infected cells persist at a low level for the entire lifetime.7) Occasionally, EBV-infected memory B cells differentiate into plasma cells that undergo lytic infection and produce viruses. Newly infected naïve B cells are controlled by CTL unless immunity is suppressed. In immunocompromised hosts, transformed cells become proliferating blasts that can result in symptomatic disease, such as post-transplant lymphoproliferative disorders.2)
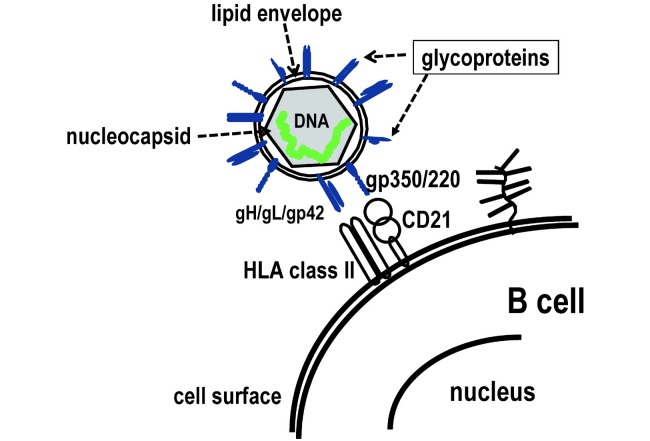
Fig. 1.
Attachment of EBV to B cells through viral glycoproteins and cellular receptors. The schematic diagram of Epstein-Barr virus (EBV) and cell surface of B-cell is shown. EBV infects B cells through the attachments of gp350/220 to CD21 and gH/gL/gp42 to HLA class II.
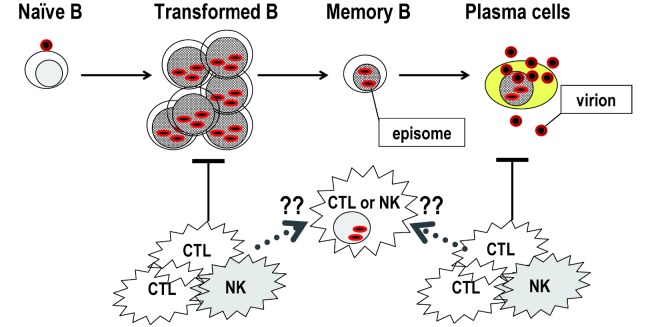
Fig. 2.
EBV infection to lymphocytes. Epstein-Barr virus (EBV)-infected naïve B cells transform and proliferate as activated blasts, but are finally controlled by cytotoxic T lymphocytes (CTL) or natural killer (NK) cells. After convalescence, EBV persists as a latent infection with episomal DNA in memory B cells. Occasionally, memory B cells differentiate into plasma cells that undergo lytic infection and produce virus.
The epithelial cells of Waldeyer’s ring can be infected by EBV and shed viruses during primary infection.11, 12) EBV infects epithelial cells through a CD21-independent mechanism, and the gH mediates EBV attachment to CD21-negative epithelial cells.13) Accumulating evidence suggests that EBV also infects T and NK cells during primary infection. In the tonsils of acute infectious mononucleosis patients, EBV-positive T and NK cells are seen, although they are rare.14, 15) In addition, EBV-infected T and NK cells are detected in the peripheral blood during acute infectious mononucleosis.16) The mechanism underlying EBV-infection of T and NK cells, which do not express CD21, remains unresolved. Interestingly, EBV-infected T or NK cells often express molecules characteristic of cytotoxic cells.17) It has been shown that NK cells activated by EBV-infected B cells acquire CD21 by synaptic transfer, and these ectopic receptors allow EBV binding to NK cell hosts.18) It is plausible that killer cells that closely contact with EBV-infected cells may acquire EBV infection directly and then proliferate with clonality (Fig. 2). Recently, EBV infection to monocytes, which do not express CD21, was reported, although its mechanism is also unclear.19)
EBV-ASSOCIATED LYMPHOID DISEASES
In EBV-infected memory B cells, only transcripts for EBV-encoded small RNAs (EBERs) are expressed (termed latency 0 in Table 1).1, 20) In EBV-associated diseases, viral gene expression is classified into three other latency patterns: type I, type II, and type III. In latency type I, which is found in Burkitt lymphoma,21) EBV nuclear antigen (EBNA)-1 and BamHI A rightward fragments (BARTs) are expressed in addition to EBERs. In latency type II, characteristic to Hodgkin lymphoma,22) latent membrane protein (LMP)-1, LMP-2a, and -2b are expressed in addition to genes expressed in Latency type I. In latency type III, characteristic to post-transplant lymphoproliferative disorders,23) all latency genes, including EBNA-2 and EBNA-3A, -3B, -3C, and -LP, are expressed. As EBNA-3s are dominant CD8+ CTL targets,10) cells in latency type III are usually eliminated by CTL. Thus, latency type III is only maintained in immunosuppressed states, such as those of post-transplant or AIDS patients. On the other hand, in latency types I and II, only a restricted number of less-antigenic EBV latent genes are expressed, allowing EBV-infected cells to evade CTL.10) Although EBV latency patterns can be classified grossly into these four types, this classification is not very strict, and heterogeneous patterns are reported in EBV-associated diseases.24, 25) Patterns of viral gene expression can differ between different cell subsets in the same individual or even the same tissue.
Table 1.
Latency types and patterns of EBV-encoded gene expression
| EBV-encoded gene | Latency type | |||
|---|---|---|---|---|
| 0 | I | II | III | |
| EBNA-1 | – | + | + | + |
| EBNA-2 | – | – | – | + |
| EBNA-3s | – | – | – | + |
| EBNA-LP | – | – | – | + |
| LMP-1 | – | – | + | + |
| LMP-2s | – | – | + | + |
| EBERs | + | + | + | + |
| BARTs | ± | + | + | + |
EBV, Epstein-Barr virus; EBNA, EBV nuclear antigen; LMP, latent membrane protein; EBERs, EBV-encoded small RNAs; BARTs, BamHI A rightward fragments.
Table 2 shows EBV-associated lymphoid malignancies that are described and classified in the 2008 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues.26) Other than the listed diseases, there exist EBV-associated lymphoid malignancies and lymphoproliferative diseases that have not yet been defined; for example, chronic active EBV disease (CAEBV) and EBV-associated hemophagocytic lymphohistiocytosis.
Table 2.
EBV-associated lymphoid malignancies
| Disease entity | Association to EBV |
Infected cells |
Latency type |
Population at high risk |
|---|---|---|---|---|
| Burkitt lymphoma, endemic | 100% | B | I | Equatorial Africa, New Guinea |
| Burkitt lymphoma, sporadic | 30% | B | I | |
| Hodgkin lymphoma, mixed cellularity | 60–80% | B | II | |
| Hodgkin lymphoma, nodular sclerosis | 20–40% | B | II | |
| Lymphomatoid granulomatosis | 100% | B | II | Western countries |
| EBV+ diffuse large B cell lymphoma of elderly | 100% | B | III? | |
| Post-transplant lymphoproliferative disorders | >90% | B | III | Recipients with heart, lung, or intestine transplantation |
| Lymphoma associated with HIV infection | 40% | B | I-III | |
| Primary effusion lymphoma1) | 70–80% | B | III | HIV-infected individuals |
| Plasmablastic lymphoma | 70% | plasmablasts | I? | HIV-infected individuals |
| Angioimmunoblastic T-cell lymphoma | >90% | B2) | II | |
| Aggressive NK cell leukemia | >90% | NK | II | Asia |
| Extra nodal NK/T cell lymphoma, nasal type | 100% | NK, T | II | East Asia |
| Systemic EBV+ T cell lymphoproliferative disease of childhood | 100% | T | II | East Asia |
| Hydroa vacciniforme-like lymphoma | 100% | γδT, NK | II | Asia, native Americans |
EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; NK, natural killer.
1) Universally associated with human herpesvirus 8.
2) Neoplastic T cells are EBV-negative.
The extent of the association to EBV varies with each disease. Some are 100% associated, but others are associated only in 1/4 to 1/3 of patients. This fact suggests that EBV is not absolutely necessary for the establishment of a disease or for its maintenance. EBV might disappear after the establishment of disease. Moreover, in vitro disappearance of EBV has been reported in EBV-infected B cell lines.27) Although EBV can immortalize B cells in vitro, its in vivo role in tumorigenesis has not been fully clarified. Also, its role in the tumorigenesis of T or NK cells is unclear. Interestingly, a variety of populations are at high risk for EBV-associated malignancies (Table 2). This suggests that genetic backgrounds are related with the pathogenesis of these diseases, although one cannot rule out the possibility that specific EBV strains are prevalent in certain areas. In the following sections, we describe representative EBV-associated lymphoid malignancies. First, we introduce Burkitt lymphoma, whose association to EBV was identified first. Then, newly defined EBV-associated T cell malignancies follow; i.e., systemic EBV+ T cell lymphoproliferative disease of childhood and hydroa vacciniforme-like lymphoma.
BURKITT LYMPHOMA
Burkitt lymphoma is a B-cell lymphoma with an extremely short doubling time that often presents in extranodal sites or as an acute leukemia.28) Translocation involving c-myc is highly characteristic but not specific. Three clinical variants of Burkitt lymphoma are recognized; endemic, sporadic, and immunodeficiency-associated. Endemic Burkitt lymphoma is prevalent in equatorial Africa and New Guinea with an incidence peak at 7 to 8 years28). In this type, the association to EBV is nearly 100%. On the other hand, sporadic Burkitt lymphoma, which is seen in children and young adults throughout the world, only has a 30% association. Immunodeficiency-associated Burkitt lymphoma is primarily seen in association with the human immunodeficiency virus infection. In 25% of cases, neoplastic B cells are positive for EBV.
Endemic Burkitt lymphoma often occurs in the jaws and other facial bones (orbit).29) On the contrary, sporadic Burkitt lymphoma presents with abdominal masses. The ilio-caecal region represents the most frequent site of involvement. In immunodeficiency-associated Burkitt lymphoma, nodal localization is frequent as well as bone marrow involvement. The mass rapidly enlarges due to the short doubling time of the tumor. After starting therapy, a tumor lysis syndrome can occur due to massive tumor cell death.
The tumor cells are medium in size with basophilic cytoplasm and show a diffuse monotonous pattern of growth.28) They have an extremely high proliferation fraction as well as a high fraction of apoptosis. A "starry sky" pattern is usually present, which is imparted by numerous benign macrophages that have ingested apoptotic tumor cells. The tumor cells express surface immunoglobulin M, CD19, CD20, CD22, and CD79a.28) Furthermore, nearly 100% of the cells are positive for Ki67. Rearrangement of immunoglobulin genes is universally seen. Most of the cases have c-myc translocation at band 8q24 to the immunoglobulin heavy chain region (14q32) or to the light chain regions (2p12 or 22q11).4) In EBV-associated cases, EBERs-positive cells are detected by in situ hybridization. The mechanisms whereby EBV contributes to Burkitt lymphoma are still not fully understood. The potent growth transforming ability of the virus suggests that it could act either by initially expanding the population of cells at risk for translocation or by increasing the survival or proliferation of translocation-positive clone.1) Activation-induced cytidine deaminase (AID), which is expressed in B cells within germinal centers, is critically involved in class switch recombination and somatic hypermutation of immunoglobulin loci.30, 31) It is required for the chromosomal breaks in c-myc and its translocations.32) Furthermore, EBV-encoded LMP-1 increases genomic instability through upregulation of AID in B-cell lymphoma.33, 34) These facts indicate that EBV infection plays a pivotal role in the c-myc translocation and tumorigenesis of Burkitt lymphoma. Other cellular genetic changes occur in tumor cells, not to mention that other environmental risk factors that induce genetic mutations must exist for Burkitt lymphoma. Possible agents include plant exposure (Euphorbia tirucalli), co-infections with malaria or arboviruses, or dietary consumption of smoked fish.4, 35)
Treatment of Burkitt lymphoma in most centers is guided by the FAB LMB study or Berlin–Frankfurt–Münster protocols.36) The former consists of initial cytoreduction with cyclophosphamide, prednisolone, and vincristine, followed by more intensive chemotherapy in varying combinations. The risk of pronounced tumor lysis is high in the first few days of therapy, but the use of urate oxidase has reduced this danger substantially. The outcome for sporadic Burkitt lymphoma in high income countries is excellent with an overall cure rate of roughly 90%.
SYSTEMIC EBV+ T CELL LYMPHOPROLIFERATIVE DISEASE OF CHILDHOOD
Systemic EBV+ T-cell lymphoproliferative disease of childhood is a life-threatening illness of children and young adults characterized by a clonal proliferation of EBV-infected T-cells with an activated cytotoxic phenotype.37) This entity has some overlapping clinicopathologic features with CAEBV and EBV-associated hemophagocytic lymphohistiocytosis, both of which are EBV-associated T or NK cell lymphoproliferative diseases.6, 38, 39) The disease is most prevalent in East Asia,37) but has also been reported in Mexico, and rarely in Western countries.40) It occurs most often in children and young adults.41)
Systemic EBV+ T-cell lymphoproliferative disease of childhood can occur shortly after primary acute EBV infection or in the setting of CAEBV. Previously healthy patients present with acute onset of fever and general malaise suggestive of an acute viral respiratory illness. Within a period of weeks to months, patients develop hepatosplenomegaly and liver failure, sometimes accompanied by lymphadenopathy. Other symptoms and signs are thrombocytopenia, anemia, skin rash, diarrhea, and uveitis. Laboratory tests show abnormal liver function tests and often an abnormal EBV serology with high anti-viral capsid antigen IgG antibodies. The disease is sometimes complicated by hemophagocytic syndrome, coagulopathy, digestive tract ulcer/perforation, central nervous system involvement, myocarditis, interstitial pneumonia, multiorgan failure and sepsis.42, 43) Interstitial pneumoniae, calcifications in basal ganglia, and coronary aneurysms are occasionally seen without any symptoms.
EBV+ T cells often infiltrate liver, spleen, lymph nodes and bone marrow, and less frequently myocardium, gastrointestinal tracts, and muscles.37, 44) The infiltrating lymphocytes are usually small to medium in size and lack significant atypia. The typical phenotypes are CD4+ or CD8+ T cells with cytotoxic makers (perforin+, TIA+ and granzyme B+).39, 45) The majority of the cells are alpha-beta T cells, but a few cases with gamma-delta T cells have also been reported. EBERs are positive, but positive cells are often sporadic. The cells have monoclonally rearranged TCR genes, and all cases harbor EBV in a clonal episomal form.
For making a diagnosis, biopsy and histopathology are useful. However, biopsies cannot always be performed due to the lack of nodal sites or the difficulty of access. Because EBV-infected lymphocytes exist in the peripheral blood, peripheral blood lymphocytes can be examined instead of tissue specimens. Patients have much higher viral loads in their peripheral blood than latently infected individuals. By real-time PCR, a huge amount of EBV-DNA is detectable in peripheral blood mononuclear cells of patients with systemic EBV+ T-cell lymphoproliferative disease of childhood.39, 42, 46) To diagnose the disease, not only must the EBV load be measured, but EBV-infected cells must also be identified. Immunobead sorting followed by quantitative PCR has been used to determine which cells harbored EBV.47) This technique is convenient and sensitive, but sometimes produces ambiguous results due to its indirectness. Recently, we established a novel assay to identify and quantify EBV-infected cells using flowcytometric in situ hybridizasion.48) Using this assay, simultaneous staining with antibodies to both surface antigens and EBER can be performed. With the new method, the absolute number of EBV-infected cells and more precise phenotypes of them can be determined.49)
Most cases have a fulminant clinical course resulting in death, usually lasting from days to weeks. However, some cases show a subacute course of several months to a year. No standard treatment has been established. Systemic EBV+ T-cell lymphoproliferative disease of childhood is usually resistant to conventional chemotherapies. Hematopoietic stem cell transplantation has been introduced as a curative therapy, although its indication and standard regimens have not been established.50-52)
HYDROA VACCINIFORME-LIKE LYMPHOMA
Hydroa vacciniforme-like lymphoma is an EBV+ cutaneous malignancy associated with photosensitivity. Although this condition is rare, it affects children and adolescents from Asia, Native Americans from Central and South America, and from Mexico.37, 53-56)
This disease is characterized by a papulovesicular eruption that generally proceeds to ulceration and scarring. Eruptions usually occur on the sun-exposed areas, particularly on the cheeks, nose, ears, lower lip and dorsal surfaces of hands.56, 57) In some cases, systemic symptoms, including fever, wasting, lymphadenopathy, and hepatosplenomegaly, may be present.37) With systemic spread, the clinical course is much more aggressive and the prognosis is poor. Some patients develop hypersensitivity to mosquito bites.58)
In the skin, the infiltrates show extension from epidermis to the subcutis, showing necrosis, angiocentricity and angioinvasion. The periappendageal infiltration is also present, and the epidermis is usually ulcerated. The infiltrating lymphocytes are usually small to medium in size and lack significant atypia.37) In hydroa vacciniforme-like eruptions, T cells infiltrate the superficial dermis and the subcutaneous tissue. Using the flowcytometric in situ hybridizasion assay, we recently showed that in five out of seven cases of hydroa vacciniforme-like lymphoma, EBV-infected cells were CD3+CD4– CD8– TCRγδ+ T cells.49) This observation accords with other recent reports.58, 59) Gamma-delta T cells make up the major T cell population in the skin and mucosal epithelium. They secrete various cytokines and have cytolytic properties.60) These results indicate that gamma-delta T cells play a central role in the formation of hydroa vacciniforme eruptions.49)
The prognosis of hydroa vacciniforme-like lymphoma varies. Some cases have eventual resolution of their disease in adult life, but others develop progressive disease with worsening of cutaneous symptoms and systemic dissemination.37, 57) No standard treatment has been established. Advanced hydroa vacciniforme-like lymphomas are usually resistant to conventional chemotherapies. Hematopoietic stem cell transplantation has been introduced as a curative therapy, although its indication and standard regimens have not been established.
CONCLUSIONS
The spectrum of EBV-associated lymphoid malignancies is expanding. However, some EBV-associated malignancies are not defined well and overlap other diseases. Further studies are necessary to clarify the pathogenesis of EBV-associated lymphoid malignancies for making better classifications of each disease and for the establishment of effective treatment.
ACKNOWLEDGEMENTS
We thank the following for their invaluable suggestions and encouragement: Dr. Koichi Ohshima (Kurume University), Drs. Keiji Iwatsuki and Tsuneo Morishima (Okayama University), and Dr. Shigeo Nakamura (Nagoya University).
REFERENCES
- 1).Rickinson AB, Kieff E. Epstein-Barr Virus and Its Replication. In: Knipe DM, Howly PM. Virology, 5th ed., vol. 2. pp. 2603–2654, 2006, Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia.
- 2).Cohen JI. Epstein-Barr virus infection. N Engl J Med, 2000; 343: 481–492. [DOI] [PubMed]
- 3).Williams H, Crawford DH. Epstein-Barr virus: the impact of scientific advances on clinical practice. Blood, 2006; 107: 862–869. [DOI] [PubMed]
- 4).Rickinson AB, Kieff E. Epstein-Barr Virus. In: Knipe DM, Howly PM. Virology, 5th ed., vol. 2. pp.2655–2700, 2006, Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia.
- 5).Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet, 1964; 1: 702–703. [DOI] [PubMed]
- 6).Cohen JI, Kimura H, Nakamura S, Ko YH, Jaffe ES. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8–9 September 2008. Ann Oncol, 2009; 20: 1472–1482. [DOI] [PMC free article] [PubMed]
- 7).Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med, 2004; 350: 1328–1337. [DOI] [PubMed]
- 8).Fingeroth JD, Weis JJ, Tedder TF, Strominger JL, Biro PA, Fearon DT. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci, U S A 1984; 81: 4510–4514. [DOI] [PMC free article] [PubMed]
- 9).Spriggs MK, Armitage RJ, Comeau MR, Strockbine L, Farrah T, Macduff B, Ulrich D, Alderson MR, Mullberg J, Cohen JI. The extracellular domain of the Epstein-Barr virus BZLF2 protein binds the HLA-DR beta chain and inhibits antigen presentation. J Virol, 1996; 70: 5557–5563. [DOI] [PMC free article] [PubMed]
- 10).Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA, Brenner MK, Rooney CM. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med, 1996; 2: 551–555. [DOI] [PubMed]
- 11).Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat Med, 2002; 8: 594–599. [DOI] [PubMed]
- 12).Tugizov SM, Berline JW, Palefsky JM. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat Med, 2003; 9: 307–314. [DOI] [PubMed]
- 13).Molesworth SJ, Lake CM, Borza CM, Turk SM, Hutt-Fletcher LM. Epstein-Barr virus gH is essential for penetration of B cells but also plays a role in attachment of virus to epithelial cells. J Virol, 2000; 74: 6324–6332. [DOI] [PMC free article] [PubMed]
- 14).Anagnostopoulos I, Hummel M, Kreschel C, Stein H. Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood, 1995; 85: 744–750. [PubMed]
- 15).Hudnall SD, Ge Y, Wei L, Yang NP, Wang HQ, Chen T. Distribution and phenotype of Epstein–Barr virus-infected cells in human pharyngeal tonsils. Mod Pathol, 2005; 18: 519–527. [DOI] [PubMed]
- 16).Kasahara Y, Yachie A, Takei K, Kanegane C, Okada K, Ohta K, Seki H, Igarashi N, Maruhashi K, Katayama K, Katoh E, Terao G, Sakiyama Y, Koizumi S. Differential cellular targets of Epstein-Barr virus (EBV) infection between acute EBV-associated hemophagocytic lymphohistiocytosis and chronic active EBV infection. Blood, 2001; 98: 1882–1888. [DOI] [PubMed]
- 17).Takahashi E, Asano N, Li C, Tanaka T, Shimada K, Shimada S, Yoshino T, Kojima M, Hara K, Eimoto T, Nakamura S. Nodal T/NK-cell lymphoma of nasal type: a clinicopathological study of six cases. Histopathology, 2008; 52: 585–596. [DOI] [PubMed]
- 18).Tabiasco J, Vercellone A, Meggetto F, Hudrisier D, Brousset P, Fournie JJ. Acquisition of viral receptor by NK cells through immunological synapse. J Immunol, 2003; 170: 5993–5998. [DOI] [PubMed]
- 19).Calattini S, Sereti I, Scheinberg P, Kimura H, Childs RW, Cohen JI. Detection of EBV genomes in plasmablasts/plasma cells and non-B cells in the blood of most patients with EBV lymphoproliferative disorders using Immuno-FISH. Blood, 2010; 116: 4546–4549. [DOI] [PMC free article] [PubMed]
- 20).Kis LL, Takahara M, Nagy N, Klein G, Klein E. Cytokine mediated induction of the major Epstein-Barr virus (EBV)-encoded transforming protein, LMP-1. Immunol Lett, 2006; 104: 83–88. [DOI] [PubMed]
- 21).Tao Q, Robertson KD, Manns A, Hildesheim A, Ambinder RF. Epstein-Barr virus (EBV) in endemic Burkitt’s lymphoma: molecular analysis of primary tumor tissue. Blood, 1998; 91: 1373–1381. [PubMed]
- 22).Deacon EM, Pallesen G, Niedobitek G, Crocker J, Brooks L, Rickinson AB, Young LS. Epstein-Barr virus and Hodgkin’s disease: transcriptional analysis of virus latency in the malignant cells. J Exp Med, 1993; 177: 339–349. [DOI] [PMC free article] [PubMed]
- 23).Young L, Alfieri C, Hennessy K, Evans H, O’Hara C, Anderson KC, Ritz J, Shapiro RS, Rickinson A, Kieff E, Cohen JI. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med, 1989; 321: 1080–1085. [DOI] [PubMed]
- 24).Yoshioka M, Ishiguro N, Ishiko H, Ma X, Kikuta H, Kobayashi K. Heterogeneous, restricted patterns of Epstein-Barr virus (EBV) latent gene expression in patients with chronic active EBV infection. J Gen Virol, 2001; 82: 2385–2392. [DOI] [PubMed]
- 25).Xue SA, Labrecque LG, Lu QL, Ong SK, Lampert IA, Kazembe P, Molyneux E, Broadhead RL, Borgstein E, Griffin BE. Promiscuous expression of Epstein-Barr virus genes in Burkitt’s lymphoma from the central African country Malawi. Int J Cancer, 2002; 99: 635–643. [DOI] [PubMed]
- 26).Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed., 2008, WHO Press, Lyon.
- 27).Shimizu N, Tanabe-Tochikura A, Kuroiwa Y, Takada K. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt’s lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J Virol, 1994; 68: 6069–6073. [DOI] [PMC free article] [PubMed]
- 28).Leoncini L, Raphael M, Stein H, Harris NL, Jaffe ES, Kluin PM. Burkitt lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed., pp.262–266, 2008, IARC Press, Lyon.
- 29).Burkitt D. A sarcoma involving the jaws in African children. Br J Surg, 1958; 46: 218–223. [DOI] [PubMed]
- 30).Honjo T, Muramatsu M, Fagarasan S. AID: how does it aid antibody diversity? Immunity, 2004; 20: 659–668. [DOI] [PubMed]
- 31).Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat Rev Immunol, 2012; 12: 517–531. [DOI] [PMC free article] [PubMed]
- 32).Robbiani DF, Bothmer A, Callen E, Reina-San-Martin B, Dorsett Y, Difilippantonio S, Bolland DJ, Chen HT, Corcoran AE, Nussenzweig A, Nussenzweig MC. AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell, 2008; 135: 1028–1038. [DOI] [PMC free article] [PubMed]
- 33).He B, Raab-Traub N, Casali P, Cerutti A. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J Immunol, 2003; 171: 5215–5224. [DOI] [PMC free article] [PubMed]
- 34).Kim JH, Kim WS, Park C. Epstein-Barr virus latent membrane protein 1 increases genomic instability through Egr-1-mediated up-regulation of activation-induced cytidine deaminase in B-cell lymphoma. Leuk Lymphoma, in press. [DOI] [PubMed]
- 35).Osato T, Mizuno F, Imai S, Aya T, Koizumi S, Kinoshita T, Tokuda H, Ito Y, Hirai N, Hirota M, Koshimizu K, Kofi-Tsekpo WM, Were JBO, Mugamibi M. African Burkitt’s lymphoma and an Epstein-Barr virus-enhancing plant Euphorbia tirucalli. Lancet, 1987; 1: 1257–1258. [DOI] [PubMed]
- 36).Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, Harrison CJ, Israels T, Bailey S. Burkitt’s lymphoma. Lancet, 2012; 379: 1234–1244. [DOI] [PubMed]
- 37).Quintanilla-Martinez L, Kimura H, Jaffe ES. EBV+ T-cell lymphoma of childhood. In: Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed., pp.278–280, 2008,WHO Press, Lyon.
- 38).Ohshima K, Kimura H, Yoshino T, Kim CW, Ko YH, Lee SS, Peh SC, Chan JK. Proposed categorization of pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol Int, 2008; 58: 209–217. [DOI] [PubMed]
- 39).Kimura H, Ito Y, Kawabe S, Gotoh K, Takahashi Y, Kojima S, Naoe T, Esaki S, Kikuta A, Sawada A, Kawa K, Ohshima K, Nakamura S. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood, 2012; 119: 673–686. [DOI] [PubMed]
- 40).Quintanilla-Martinez L, Kumar S, Fend F, Reyes E, Teruya-Feldstein J, Kingma DW, Sorbara L, Raffeld M, Straus SE, Jaffe ES. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood, 2000; 96: 443–451. [PubMed]
- 41).Isobe Y, Aritaka N, Setoguchi Y, Ito Y, Kimura H, Hamano Y, Sugimoto K, Komatsu N. T/NK cell type chronic active Epstein-Barr virus disease in adults: an underlying condition for Epstein-Barr virus-associated T/NK-cell lymphoma. J Clin Pathol, 2012; 65: 278–282. [DOI] [PubMed]
- 42).Kimura H, Hoshino Y, Kanegane H, Tsuge I, Okamura T, Kawa K, Morishima T. Clinical and virologic characteristics of chronic active Epstein-Barr virus infection. Blood, 2001; 98: 280–286. [DOI] [PubMed]
- 43).Kimura H, Morishima T, Kanegane H, Ohga S, Hoshino Y, Maeda A, Imai S, Okano M, Morio T, Yokota S, Tsuchiya S, Yachie A, Imashuku S, Kawa K, Wakiguchi H. Prognostic factors for chronic active Epstein-Barr virus infection. J Infect Dis, 2003; 187: 527–533. [DOI] [PubMed]
- 44).Okano M, Kawa K, Kimura H, Yachie A, Wakiguchi H, Maeda A, Imai S, Ohga S, Kanegane H, Tsuchiya S, Morio T, Mori M, Yokota S, Imashuku S. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol, 2005; 80: 64–69. [DOI] [PubMed]
- 45).Ohshima K, Suzumiya J, Sugihara M, Nagafuchi S, Ohga S, Kikuchi M. CD95 (Fas) ligand expression of Epstein-Barr virus (EBV)-infected lymphocytes: a possible mechanism of immune evasion in chronic active EBV infection. Pathol Int, 1999; 49: 9–13. [DOI] [PubMed]
- 46).Ohga S, Nomura A, Takada H, Ihara K, Kawakami K, Yanai F, Takahata Y, Tanaka T, Kasuga N, Hara T. Epstein-Barr virus (EBV) load and cytokine gene expression in activated T cells of chronic active EBV infection. J Infect Dis, 2001; 183: 1–7. [DOI] [PubMed]
- 47).Kimura H, Hoshino Y, Hara S, Sugaya N, Kawada J, Shibata Y, Kojima S, Nagasaka T, Kuzushima K, Morishima T. Differences between T cell-type and natural killer cell-type chronic active Epstein-Barr virus infection. J Infect Dis, 2005; 191: 531–539. [DOI] [PubMed]
- 48).Kimura H, Miyake K, Yamauchi Y, Nishiyama K, Iwata S, Iwatsuki K, Gotoh K, Kojima S, Ito Y, Nishiyama Y. Identification of Epstein-Barr virus (EBV)-infected lymphocyte subtypes by flow cytometric in situ hybridization in EBV-associated lymphoproliferative diseases. J Infect Dis, 2009; 200: 1078–1087. [DOI] [PubMed]
- 49).Kawabe S, Ito Y, Gotoh K, Kojima S, Matsumoto K, Kinoshita T, Iwata S, Nishiyama Y, Kimura H. Application of flow cytometric in situ hybridization assay to Epstein-Barr virus (EBV)-associated T/NK lymphoproliferative diseases. Cancer Sci, 2012; 103: 1481–1488. [DOI] [PMC free article] [PubMed]
- 50).Gotoh K, Ito Y, Shibata-Watanabe Y, Kawada J, Takahashi Y, Yagasaki H, Kojima S, Nishiyama Y, Kimura H. Clinical and virological characteristics of 15 patients with chronic active Epstein-Barr virus infection treated with hematopoietic stem cell transplantation. Clin Infect Dis, 2008; 46: 1525–1534. [DOI] [PubMed]
- 51).Sato E, Ohga S, Kuroda H, Yoshiba F, Nishimura M, Nagasawa M, Inoue M, Kawa K. Allogeneic hematopoietic stem cell transplantation for Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disease in Japan. Am J Hematol, 2008; 83: 721–727. [DOI] [PubMed]
- 52).Kawa K, Sawada A, Sato M, Okamura T, Sakata N, Kondo O, Kimoto T, Yamada K, Tokimasa S, Yasui M, Inoue M. Excellent outcome of allogeneic hematopoietic SCT with reduced-intensity conditioning for the treatment of chronic active EBV infection. Bone Marrow Transplant, 2011; 46: 77–83. [DOI] [PubMed]
- 53).Iwatsuki K, Xu Z, Takata M, Iguchi M, Ohtsuka M, Akiba H, Mitsuhashi Y, Takenoshita H, Sugiuchi R, Tagami H, Kaneko F. The association of latent Epstein-Barr virus infection with hydroa vacciniforme. Br J Dermatol, 1999; 140: 715–721. [DOI] [PubMed]
- 54).Barrionuevo C, Anderson VM, Zevallos-Giampietri E, Zaharia M, Misad O, Bravo F, Caceres H, Taxa L, Martinez MT, Wachtel A, Piris MA. Hydroa-like cutaneous T-cell lymphoma: a clinicopathologic and molecular genetic study of 16 pediatric cases from Peru. Appl Immunohistochem Mol Morphol, 2002; 10: 7–14. [DOI] [PubMed]
- 55).Chen HH, Hsiao CH, Chiu HC. Hydroa vacciniforme-like primary cutaneous CD8-positive T-cell lymphoma. Br J Dermatol, 2002; 147: 587–591. [DOI] [PubMed]
- 56).Cho KH, Lee SH, Kim CW, Jeon YK, Kwon IH, Cho YJ, Lee SK, Suh DH, Chung JH, Yoon TY, Lee SJ. Epstein-Barr virus-associated lymphoproliferative lesions presenting as a hydroa vacciniforme-like eruption: an analysis of six cases. Br J Dermatol, 2004; 151: 372–380. [DOI] [PubMed]
- 57).Iwatsuki K, Satoh M, Yamamoto T, Oono T, Morizane S, Ohtsuka M, Xu ZG, Suzuki D, Tsuji K. Pathogenic link between hydroa vacciniforme and Epstein-Barr virus-associated hematologic disorders. Arch Dermatol, 2006; 142: 587–595. [DOI] [PubMed]
- 58).Hirai Y, Yamamoto T, Kimura H, Ito Y, Tsuji K, Miyake T, Morizane S, Suzuki D, Fujii K, Iwatsuki K. Hydroa Vacciniforme Is Associated with Increased Numbers of Epstein-Barr Virus-Infected gammadeltaT Cells. J Invest Dermatol, 2012; 132: 1401–1408. [DOI] [PubMed]
- 59).Tanaka C, Hasegawa M, Fujimoto M, Iwatsuki K, Yamamoto T, Yamada K, Kawa K, Saikawa Y, Toga A, Mase S, Wada T, Takehara K, Yachie A. Phenotypic analysis in a case of hydroa vacciniforme-like eruptions associated with chronic active Epstein-Barr virus disease of gammadelta T cells. Br J Dermatol, 2012; 166: 216–218. [DOI] [PubMed]
- 60).Kaufmann SH. gamma/delta and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci U S A, 1996; 93: 2272–2279. [DOI] [PMC free article] [PubMed]