ABSTRACT
Infected thoracic aortic aneurysm (ITAA) is a relatively rare disease. The diagnosis of ITAA is generally made comprehensively based on symptoms, laboratory data and CT findings. Several series of blood cultures are mandatory to first detect the infecting organism. ITAA is usually suspected as a result of the CT findings. A short-interval CT re-examination is essential to confirm the correct diagnosis. A CT scan commonly demonstrates a rapid enlargement of the aneurismal lumen and soft tissue mass surrounding the aorta. One of the characteristics of ITAA is the presence of several nodular or saccular aneurysms localized in different aortic portions. Patients with ITAA are associated with high incidences of aneurismal rupture due to the aneurysm’s abrupt growth. Therefore, ITAAs are associated with both high morbidities and mortalities. The major concerns regarding surgical treatment for ITAA are the control of infection, the resection of whole infected tissue, grafting via an aseptic route and the prevention of recrudescent infection. Therefore, effectual antibiotic therapy is mandatory as the first choice of therapy. Ideally surgical intervention is indicated in patients with a controlled infection. It is essential to excise the whole infected aneurysm and to reconstruct in-situ grafting via an aseptic route. However, urgent surgery is often required in patients with an uncontrolled infection because they have an impending aneurismal rupture. In such cases, an extra-anatomical bypass without cardiopulmonary bypass is applicable. Surgical strategies should therefore be determined on a case-by-case basis because these patients present various clinical courses.
Key Words: aneurysm, surgery, infection, artificial graft
INTRODUCTION
An infected thoracic aortic aneurysm (ITAA) is a relatively rare disease, and its incidence has been reported to comprise about 1% of all aneurysms.1,2) Patients with ITAA have a high incidence of aneurismal rupture due to the abrupt growth of the aneurysm. Therefore, ITAA is associated with both high morbidity and mortality, and the mortality has been reported to range from 23 to 37%.1-4) Regrettably, there are no standard guidelines for the medical or surgical treatment of ITAA because it is a rare and frequently lethal disease. The use of antibiotic therapy is essential to control the aortic infection.5)
The major concerns regarding surgical treatment of ITAA are the control of infection, resection of the entire infected tissue, grafting via an aseptic route, and prevention of recurrent infection. Surgical intervention is principally indicated to prevent aneurysm rupture, and is only recommended when the maximum diameter of the aneurysm is over 55 mm or when rapid expansion of the aneurysm is observed. Ideally, surgery is performed only in patients with a controlled infection, and for such patients in-situ reconstruction is also ideal. However, emergency surgery is often required in patients with uncontrolled infection, due to the risk of aneurysmal rupture. In such cases, extra-anatomical bypass is useful for avoiding routing in the infected site and is better than using cardiopulmonary bypass, because cardiopulmonary bypass may reduce the immunologic competence following surgery.
CLASSIFICATION
Infected aortic aneurysms have historically been classified into the following four types; mycotic aneurysms, microbial arteritis with aneurysm, infected preexisting aneurysms, and post-traumatic infected false aneurysms.6) Mycotic aneurysms develop when septic emboli of cardiac origin lodge in the lumen or the vasa vasorum of the aorta. Mycotic aneurysms can occur in both normal and abnormal arteries and develop in virtually any named artery. The predominant organisms are reported to be gram-positive cocci.7) Microbial arteritis with aneurysm is the most common condition that causes ITAA. Diseases of the intima with atherosclerosis allow blood-borne bacteria to inoculate the aortic wall. When an infection is established, suppurations, localized perforation and false aneurysm formation occur. The predominant microorganisms involved in microbial arteritis are Escherichia coli, and Salmonella and Staphylococcus species.8) An infection of preexisting aneurysms is the infection of atheroscleorotic aneurysms. The abdominal aorta is the predominant site, and the most prevalent organisms are Staphylococcus species.9) Post-traumatic infected false aneurysms are the most prevalent type of infected aneurysms that develop in the peripheral arteries. The femoral and carotid arteries are the predominant sites.10) This type of infection may also occur in the thoracic aorta accompanied by endovascular stent grafting.
DIAGNOSIS
The clinical symptoms of ITAA are fever, and chest / abdominal pain or discomfort. Most patients present with a fever of unknown origin and are treated with antibiotics. Infected aneurysms occur in all age groups, but the typical patient is older and has asthelosclerosis.
Blood examination usually reveals leukocytosis and positive levels of blood C reactive protein, but these examinations are nonspecific. Several series of blood cultures are mandatory to confirm the causal bacterial species, however, negative blood cultures are common because that the majority of patients receive antibiotics before confirming the diagnosis of ITAA. The rate of positive cultures is reported to be 50 to 70%.11) The most frequent source of infection has not been identified; however, gram-positive organisms such as Staphylococcus aureus are predominant, in addition to Salmonella species. Gram-negative organisms such as E. coli or Bacteroides species are also frequently observed.2)
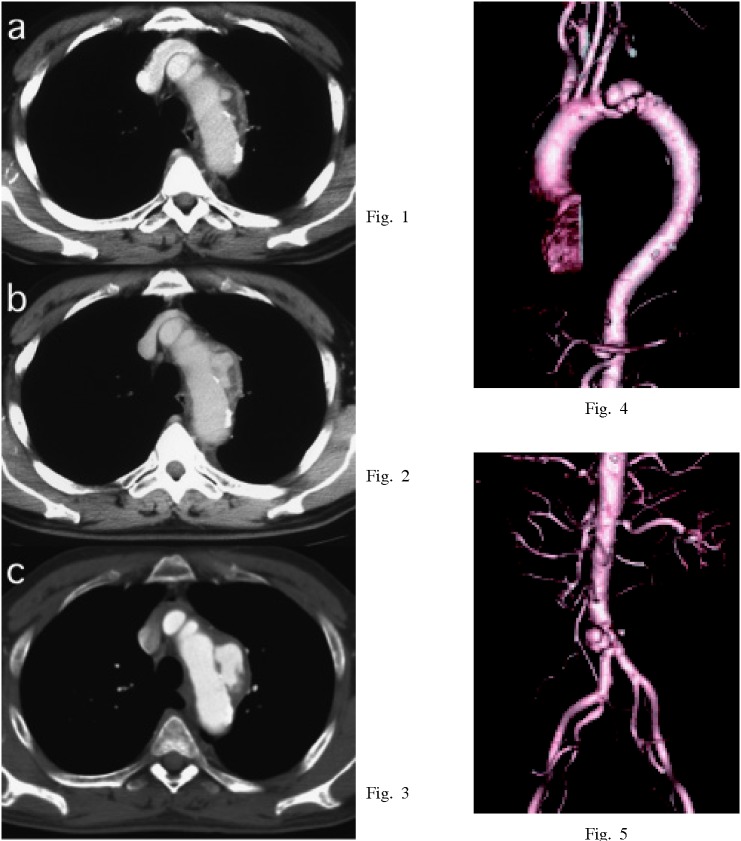
The diagnosis of ITAA is generally made comprehensively based on symptoms, laboratory data and CT findings. ITAA is generally suspected from results of CT findings in patients with symptoms of unknown fever and/or focal chest pain or discomfort. A short-interval CT re-examination is also essential to confirm the correct diagnosis12,13) (Fig.1-3). As with non-infectious aneurysmogenic processes, the CT scan commonly demonstrates rapid enlargement of the aneurysmal lumen and a soft tissue mass surrounding the aorta. CT findings of ITAA often reveal aortic nodularity, an irregular configuration, rupture of calcification, or air in an aortic wall.2) One of the characteristics of ITAA is the presence of several nodular or saccular aneurysms localized in different aortic portions,12) (Fig.4,5). Indeed, infected aneurysms commonly develop as a result of bacteria invation into the aortic vessel wall (microbial arteritis with aneurysm). This type of aneurysms usually reveals several nodular or saccular manifestations. The presence of multiple aneurysms demonstrating either a saccular or nodular type, with a rapid expansion in size, are the most typical CT findings for ITAA.
Fig. 1-5.
Enhanced chest CT findings of a 66-year-old male. Distal arch aorta revealed an ulcer-like protrusion with low density mass under thickened and enhanced adventitia at first (Fig.1). The ulcer-like protrusion expanded abruptly (Fig.2), and the diameter of the aneurysm increased from 40 mm to 70 mm within 10 days (Fig.3). The aneurysm developed as a result of invasion of bacteria into the aortic vessel wall (microbial arteritis with aneurysm). He also had an infrarenal abdominal aortic aneurysm. This aneurysm expanded from 30 mm to 40 mm in diameter within the same period (Fig.5).
The utility of intravenous digital subtraction angiography (DSA) in the aorta and femoral artery has been established. However, multi-detector computed tomography (MDCT) is a more useful method for detecting the shapes and other aspects of aneurysms, (Fig.4,5). It has recently become a routine diagnostic method. Magnetic resonance imaging may also prove helpful when screening certain anatomic sites or when contrast media is contraindicated.14) The diagnosis of the site and severity of inflammation are another concern. Gallium scintigraphy has usually been used, but positron emission tomography (PET) can diagnose the precise site of inflammation. The following SUV (standardized uptake value) is important for deciding positive findings.
SUV = uptake (kBq/mL) / injected dose (kBq) / patient weight (g) 15)
Infected aortic aneurysm occurred in thoracic aorta in 32% of cases, upper abdominal aorta in 26% of cases and lower abdominal aorta in 42% of cases, however, multiple aneurysms were detected in many cases.16)
ANTIBIOTICS THERAPY
Patients with ITAA have a high incidence of aneurysm rupture due to rapid growth of the aneurysm. Therefore, ITAA is associated with both high morbidity and mortality. Antibiotic therapy, using agents which are sensitive and effective against the specific causal bacterial species, is mandatory for treating the infection. However, the actual cause of infection generally cannot be identified. Broad spectrum antibiotic therapy should be initiated in such cases. Ampicillin or Cepharosporin, which combat Salmonella species, should be the first choice antibiotics. Bactericidal antibiotics must be given both before and after surgery, with continuous administration ranging from 4 to 8 weeks.2) Close cooperation with an infection control team is mandatory.
SURGICAL INTERVENTION
Elective surgery is ideal for patients demonstrating a controlled infectious state. However, emergency surgery is often unavoidable due to the risk of aneurysmal rupture. It is still difficult to determine the optimal timing of surgical intervention and the best surgical procedure. Short-interval CT re-examinations are helpful for determining the best timing for surgical intervention. A maximum aneurysm diameter of more than 55 mm is the principle indication for surgical intervention. In addition, a case with an abrupt aneurismal expansion of over 10 mm/week should be indicated for urgent surgery as an impending aneurysmal rupture.
The major concerns about surgical treatment for ITAA are ensuring the resection of the entire infected tissue, reconstruction of the vessels via an aseptic route, and prevention of re-infection. The exclusion or debridement of the infected aneurysm and surrounding tissues is essential, especially during the active infection phase. However, when such patients are complicated with pyothorax, an in-situ reconstruction should be avoided to prevent re-infection, while an extra-anatomical bypass is feasible,17) An in-situ reconstruction can be performed and is ideal, when the entire infected tissue can be successfully excised from the patient, and the infectious state is under sufficient antibiotic therapy control.
The use of a woven Dacron graft soaked with antibiotics has been reported to prevent graft infection even during in-situ replacement.1,18,19) Rifampicin is also commonly used for soaking prosthetic woven Dacron grafts. Experimental studies demonstrated prolonged antistaphylococcal activity of Rifampin-bonded, gelatin-impregnated Dacron grafts after implantation in the arterial circulation.20) A silver-coated polyester prosthesis is also available for management of aortic infections, and favorable outcomes have been reported.21) A cryopreserved homograft is known to better resist bacterial colonization and to present better mechanical properties than prosthestic grafts.22) However, in-situ aortic reconstruction with a cryopreserved homograft in an infected field still carries a high mortality rate.23) In order to reduce the risk of infection after resection of an ITAA, the defect is often filled with the omentum, or grafts are wrapped with the omentum (omentpexy). This is because the omentum has abundant blood circulation and good sterilizing properties.24)
Kam and colleagues reported that endovascular aortic repair (TEVAR) could be successfully carried out in an HIV positive immunocompromised host with ITAA.25) However, TEVAR for ITAA is considered to be associated with a high risk of recurrence of infection, especially when aneurysms form a fistula with the gastroinstestinal tract or respiratory tract.26) Stellemes and collegues performed TEVAR as emergency therapy despite suspected aortic infection in 6 cases and reported 1 hospital mortality. They concluded that TEVAR as emergency therapy is feasible, but life-long clinical and morphological surveillance remains mandatory as recurring infection cannot be entirely excluded.27) Endovascular stenting may be indicated for high co-morbidity patients under a controlled infection state. The bridge use of TEVAR can be considered to prevent an aneurismal rupture, but open surgery should be scheduled under cautious follow-up. The surgical strategies for ITAA should be determined on a case-by-case basis and include a careful CT follow-up.
PATHOLOGICAL FINDINGS
Specimens obtained from the ITAA usually reveal the presence of lymphocytes and neutrophils, while plasma cells invade the aortic tissue with signs of an infected aneurysm. These findings are identical even in patients treated surgically during the controlled infection phase. Pathological findings are helpful for confirm infectious aneurysms.
CLINICAL OUTCOMES
Although ITAA is a relatively rare disease, it has a high morbidity and mortality rate. Intensive antibiotic therapy is an integral part in the effective management of this disease.28) The type, duration and dose of antibiotics should be determined according to the guidelines for infected endocarditis.5) The mortality of ITAA is much higher than for non-infected aneurysms because the prognosis of ITAA is greatly influenced by the management of infection. Therefore, antibiotic therapy is the most essential part of the treatment for ITAA.
Clinical outcomes for ITAA vary according to the surgical strategy employed. There was no large volume clinical study of extra-anatomic reconstruction for ITAA. A clinical study of axillobifemoral bypass for infected infra-renal abdominal aortic aneurysm has been reported.29) It showed that early and overall mortality were 24% and 42%, respectively. A higher rate of late complications was reported, including aortic stump rupture or graft infection. Several series have reported satisfactory outcomes with in-situ reconstruction, but results varied according to the graft materials. Reconstruction with in-situ cryopreserved allograft showed superior disease-related survival-free reoperation than that treated with prostatic graft.22) The U.S. cryopreserved aortic allograft registry reported a 30-day mortality rate of 13% and an overall mortality rate of 25% during a mean follow-up period of 5.3 months. It was associated with a high complication rate, including limb amputation, hemorrhage, graft occlusion and reinfection (3 to 14%).23)
Reconstruction with an antibiotic-soaked Dacron graft has also been reported with better clinical outcomes. Oderich and coworkers reported an operative mortality of 21% in 43 cases and Muller and coworkers reported an overall hospital mortality of 36% in 33 cases with infected aortic aneurysm.30) However, there are few reports about surgical outcomes of ITAA. Hsu and coworkers reported a hospital mortality of 10% in 10 patients who underwent in-situ aortic arch replacement for infected aortic arch aneurysm. They also reported that major complication including hypoxic encephalopathy occurred in 70% patients and late prosthetic graft infection occurred in 10% of patients.31) We experienced 8 cases of ITAA.12) Extra-anatomic reconstruction was performed in 1 patient and in-situ reconstruction was performed in 7 other patients. There were no hospital mortalities but a patient who underwent extra-anatomic reconstruction died suddenly 2 years after surgery.
Even when patients can survive the perioperative period, long-term reinfection can occur clinically. It may present as a pseudoaneurysm or hemorrhage. Indications for life-long antibiotic therapy are not well documented, however, cautious follow-up is essential for such patients. The survival rate has been reported to be 67–76% at 2 years and 47% at 5 years due to late aortic events.32,33)
CONCLUSION
Patients with ITAA are associated with a high incidence of aneurismal rupture due to the abrupt growth of the aneurysms. Therefore, ITAA is associated with both high morbidity and mortality. The major concerns regarding surgical treatment for ITAA are control of infection, resection of whole infected tissue, grafting via an aseptic route and prevention of recrudescent infection. Therefore, effectual antibiotic therapy is mandatory as the first choice of therapy. Surgical intervention is indicated ideally in patients with a controlled infection. It is essential to excise the whole infected aneurysm and to reconstruct in-situ grafting via an aseptic route. However, urgent surgery is often required in patients with an uncontrolled infection due to impending aneurismal rupture. In such cases, an extra-anatomical bypass without cardiopulmonary bypass is applicable, because cardiopulmonary bypass may reduce the immunologic competence and thereby enhance septicemia. Surgical strategies should therefore be determined on a case-by-case basis because patients may present various clinical courses.
REFERENCES
- 1).Aleksic L, Leyh R, Schorn B. Extra-anatomic management of homograft reinfection after thoracic aortic rupture. Thorac Cardiovasc Surg 2006; 54: 428–430. [DOI] [PubMed]
- 2).Muller BT, Wegener OR, Grabitz K, Pillny M, Thomas L & Sandmann W. Mycotic aneurysm of the thoracic and abdominal aorta and iliac arteries:Experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg 2001; 33: 106–13. [DOI] [PubMed]
- 3).Oz MC, Brener BJ, Buda JA, Todd G, Brenner RW, Goldenkranz RJ, McNicholas KW, Lemole GM & Lozner JS. A ten-year experiense with bacterial aortits. J Vasc surg 1989; 10: 439–49. [DOI] [PubMed]
- 4).Fichelle JM, Tabet G, Cormier P. Infected infrarenal aortic aneurysms: when is in situ reconstruction safe? J Vasc Surg 1993; 17: 635–645. [DOI] [PubMed]
- 5).Miyatake K, Akaishi M & Kawazoe K. Guideline for the prevention and treatment of Infective Endocarditis. Circulation Jounal 2003; 67: 1039–1076.
- 6).Weaver MR, Reddy DJ. Infected aneurysms, In Rutherford’s Vascular Surgery 7th edition, edited by Cronenwett and Johnston, pp 2156, 2010, Saunders, Elsevier, Philadelphia.
- 7).Magilligan DJ & Quinn EL. Active infective endocarditis. In Endocarditis: Medical and surgical management. Edited by Magilligan DJ, Quinn EL, pp187–203, 1986, Marcel Dekker, New York.
- 8).Wilson SE, Van Wagenen P & Passaro E Jr. Arterial infection. Curr Probl Surg 1978; 15: 1–96. [DOI] [PubMed]
- 9).Jarrett F, Darling RC & Mundth ED, Austen WG. (1975). Experience with infected aneurysms of the abdominal aorta. Arch Surg 1975; 110: 1281–1286. [DOI] [PubMed]
- 10).Johnson JR, Ledgewood AM, Lucas CE. (1983). Mycotic aneurysm: New concepts in surgery. Arch Surg 1983; 118: 577–582. [DOI] [PubMed]
- 11).Reddy DJ, Shepard AD, Evans JR, Wright DJ, Smith RF, Ernst CB. Management of infected aortoiliac-aneurysms. Arch Surg. 1991; 126: 873–878. [DOI] [PubMed]
- 12).Nakashima M, Usui A, Oshima H & Ueda Y. The treatment of infectious aneurysms in the thoracic aorta; our experience in treating five consecutive patients. Interact Cardiovasc Thorac Surg. 2010; 10: 334–337. [DOI] [PubMed]
- 13).Walsh DW, Hb VB, Haggerty MF. Mycotic aneurysm of the aorta: MRI and MRA features. J Magn Reson Imaging 1997; 7: 312–315. [DOI] [PubMed]
- 14).Yasuhara H & Muto T. Infected abdominal aortic aneurysm presenting with sudden appearance: diagnostic importance of serial computed tomography. Ann Vasc Surg. 2001; 15: 582–585. [DOI] [PubMed]
- 15).Tokuda Y, et al. Diagnostics of thoracic aortic vascular prosthesis infection using FDG-PET/CT, Eur Cardio Thorac Surg in press.
- 16).Miller DV, et al. Surgical pathology of infected aneurysms of the descending thoracic and abdominal aorta: clinicopathologic correlations in 29 cases (1976 to 1999). Hum Pathol 2004; 35: 1112. [DOI] [PubMed]
- 17).Yokote J, Usui A, Oshima H, Usami N, Yokoi K & Ueda Y. Total thoracic aorta reconstruction against graft infection in a patient with chronic empyema developed after lung cancer surgery. J Thorac Cardiovasc Surg. 2009; 138: 779–81. [DOI] [PubMed]
- 18).Vogt PR, Turina MI. Management of infected aortic grafts:Development of less invasive surgery using cryopreserved hommmografts. Ann Thorac Surg 1999; 67: 1986–9. [DOI] [PubMed]
- 19).Tylor LM Jr, Deitz DM, McConnell DB & Porter JM. Treatment of infected abdominal aneurysms by extraanatomic bypass, aneuryusm excision, and drainage. Am J Surg 1988; 155: 655–8. [DOI] [PubMed]
- 20).Gupta AK, Bandyk DF, Johnson RL. In situ repair of mycotic abdominal aortic aneurysms with rifanpin-bonded gelatin-impregnated Dacron grafts: a preliminary case report. J Vasc Surg 1996; 24: 472–476. [DOI] [PubMed]
- 21).Batt M, Magne JL, Alric P, Muzi A, Ruotolo C, Ljungstrom KG, Garcia-Casas R, Simms M. In situ revascularization with silver-coated polyester grafts to trat aortic infection: early and midterm results. J Vasc Surg. 2003; 38: 983–989. [DOI] [PubMed]
- 22).Vogt PR, Brunner-LaRocca HP, Lachart M, Ruef C, Turina MI. Technical details with the use of cryopreserved arterial allografts for aortic infection: Influence on early and midterm mortality. J Vasc Surg. 2002; 35: 80–85. [DOI] [PubMed]
- 23).Noel AA, Gloviczki P, Cherry KJ Jr., Safi H, Goldstone J, Morasch MD, Johansen KH. Abdominal aortic reconstruction in infected fields: early results of the United States cryopreserved aortic allograft registry. J Vasc Surg. 2002; 35: 847–852. [DOI] [PubMed]
- 24).Douglas EP, Blair A. Management of aortobronchial fistula with graft replacement and omentopexy. Ann Thorac Surg 1990; 50: 972–974. [DOI] [PubMed]
- 25).Kam MH, Ttoh LK, S.G. Tan, Wong D, Chia KH. A case report of endovascular stenting in Salmonella Mycotic aneurysm: A successful procedure in an immynocompromeised patient. Ann Acad Med Singapore. 2007; 36: 1028–1031. [PubMed]
- 26).González-Fajardo JA, Gutiérrez V, Martín-Pedrosa M, Del Rio L, Carrera S, Vaquero C. Endovascular repair in the presence of aortic infection. Ann Vasc Surg. 2005; 19: 94–98. [DOI] [PubMed]
- 27).Stellmes A, Von Allmen R, Derungs U, Dick F, Makaloski V, Do DD, Schmidli J, Czerny M. Thoracic endovascular aortic repair as emergency therapy despite suspected aortic infection. Interact Cardiovasc Thorac Surg. 2013; 16: 459–64. [DOI] [PMC free article] [PubMed]
- 28).Hollier LH, Money SR, Creely B, Bower TC & Kazmier FJ. Direct replacement of mycotic thoracoabdominal aneurysms. J Vasc surg Vol. 1993; 18: 477–85. [DOI] [PubMed]
- 29).Bacourt F & Koskas F. Axillobifemoral bypass and aortic exclusion for vascular septic lesions: A multicenter retrospective study of 98 cases. Ann Vasc Surg 1992; 6: 119–126. [DOI] [PubMed]
- 30).Oderich GS, Panneton JM, Bower TC, Cherry KJ Jr, Rowland CM, Noel AA, Hallett JW Jr, Gloviczki P. Infected aortic aneurysms: aggressive presentation, complicated early outcome but durable results. J Vasc Surg 2001; 34: 900–908. [DOI] [PubMed]
- 31).Hsu RB & Lin FY. Surgery for infected aneurysm of the aortic arch. J Thorac Cardiovasc Surg 2007; 134: 1157–1162. [DOI] [PubMed]
- 32).Yeager RA, taylor LMJ, Monca GL, Edwards JM, Nicoloff AD, McConnell DB, Porter JM. Improved results with conventional management of infrarenal aortic infection. J Vasc Surg 1999; 30: 76–83. [DOI] [PubMed]
- 33).Young RM, Cherry KJJ, Davis PM, Glovicski P, Bower TC, Panneton JM, Hallett JW Jr. The results of in situ prosthetic replacement for infected aortic grafts. Ann J Surg 1999; 178: 136–140. [DOI] [PubMed]