ABSTRACT
Granulomatous lobular mastitis (GLM) is a rare inflammatory pseudotumor. No therapeutic modality for this disease has been established because of its rarity. The purpose of this study is to evaluate the treatment strategies of GLM. Twelve women who met the histological criteria for GLM were retrospectively studied. The clinical data and the presentation, histopathology, and management of the disease were analyzed by reviewing the patients’ medical records. The diagnosis of GLM was confirmed histologically by core needle biopsy in 9 cases, by vacuum-assisted biopsy in 2 cases, and by excisional biopsy in 1 case. Ten patients received corticosteroid treatment and another two patients were treated with local excision or incision and drainage. The median initial dosage of corticosteroid (Prednisolone) was 30 mg/day (range: 15–60 mg/day), and the dosages were tapered according to improvement. The median duration of corticosteroid treatment was 5 months (range: 1–12 months). The median follow-up period was 22 months (range: 6–104 months), and no patient treated with corticosteroid demonstrated recurrence. However, patients treated with excision or incision and drainage had recurrences. These results suggest that steroid treatment may be the first choice in treatment strategies for GLM.
Key Words: Granulomatous lobular mastitis, corticosteroid treatment
INTRODUCTION
Granulomatous lobular mastitis (GLM) is a rare inflammatory disease of the breast that was first described by Kessler and Wolloch in 1972.1) This disease usually affects women of childbearing age or those with a history of oral contraceptive use.1) The most common clinical finding is a unilateral, discrete breast mass. It is characterized pathologically by chronic granulomatous inflammation of the lobules without necrosis.2) Although GLM is a benign condition, its clinical and radiological features may mimic breast cancer.3-5) The diagnosis is made only after excluding breast cancer and other infective and non-infective causes of granulomatous inflammation, such as tuberculous, parasitic and fungal infections, sarcoidosis, Wegener’s granulomatosis, giant cell arteritis, polyarteritis nodosum, and foreign-body reactions.4, 6, 7)
The treatments for GLM include corticosteroids,8-10) immunosuppressants,11) antibiotics, abscess drainage, and surgical excision.12) Although a preferred therapeutic modality has not been established because of the rarity of this disease, corticosteroid therapy has been shown to be effective for shrinking the granulomatous mass.4,10) Recently, Ogura et al. 13) categorized GLMs into two groups: immunoglobulin G (IgG) 4-related GLMs and non-IgG4-related GLMs. They speculated that patients with IgG4-related GLM might benefit from steroid therapy, as do those with IgG4-related autoimmune disorders. However, the effectiveness of steroid therapy for patients with non-IgG4-related GLM is unknown. We evaluated the effectiveness of corticosteroid treatment for non-IgG4-related GLMs and discussed the strategy of the treatment for GLM.
MATERIALS AND METHODS
We performed a retrospective study of the records from Nagoya University Hospital and KATO Surgery-gynecology-Obstetrics-Breast Clinic for patients treated between October 2005 and October 2012. During this period, 12 women were diagnosed with GLM and treated. All of the women underwent a clinical breast examination to identify palpable lumps, skin thickening, or axillary lymphadenopathy. Mammography (MMG) and ultrasonography (US) were the initial imaging methods used for evaluation, although MMG was not performed in one case due to pain caused by drainage of the sinus tracts. Additional radiological examinations, such as computed tomography (CT) and magnetic resonance imaging (MRI), were performed as needed.
Definitive diagnosis was obtained by core needle biopsy (14-gauge), vacuum-assisted biopsy (11-gauge), or surgical excision. The obtained specimens were fixed overnight in 10% formalin, and the residual tissues were cultured for bacteria. Paraffin-embedded specimens were stained with hematoxylin and eosin (HE), Ziehl-Neelsen, and the periodic acid-Schiff reaction with or without diastase digestion. Other possible causes of granulomatous inflammatory lesions of the breast were excluded by serological tests, histological analysis, and tests of the affected tissue for aerobic and anaerobic bacteria, mycobacteria, and fungi.
The expression levels of IgG4 and IgG in the GLM lesion were determined immunohistochemically in the paraffin-embedded tissue specimens. Immunohistochemical staining was performed using the avidin-biotin-peroxidase complex technique. The primary antibodies used were an anti-IgG mouse monoclonal antibody (1:2, Histofine, Nichirei Bioscience, Tokyo, Japan) and an anti-IgG4 sheep polyclonal antibody (1:500, The Binding Site, Birmingham, UK). Before incubation with the primary antibodies, antigen retrieval (microwave pretreatment for 10 min at 100°C in pH 6 citrate buffer) was performed separately for IgG4 and IgG. IgG+ or IgG4+ plasma cells were counted in high power fields (hpf, magnification ×300) with intense inflammation, and 4 fields were analyzed per case. The ratio (percentage) of IgG4+ plasma cells/IgG+ plasma cells was calculated in each case. Previous studies had shown that an elevated IgG4+ to IgG+ plasma cell ratio (IgG4/IgG ratio) is helpful in distinguishing IgG4-related from non-IgG4-related inflammatory conditions.14, 15) Those studies revealed that most patients with IgG4-related disease had an IgG4+/IgG+ plasma cell ratio of more than 30%. In contrast, the ratio was less than 10% in cases of non-IgG4-related disease. Therefore, the diagnosis of IgG4-related disease was made in patients having characteristic H&E findings, diffuse IgG4+ plasma cell infiltration, and an IgG4+/IgG+ cell ratio greater than 30%.
RESULTS
Clinical features
The characteristics of the 12 patients are shown in Table 1. The median age was 36 years (range: 25–47). Most of the patients were of reproductive age. None of the patients reported oral contraceptive use. Ten patients had given birth to children. Seven patients were diagnosed within 5 years of their most recent pregnancy, 2 patients had not been pregnant within 5 years prior to the diagnosis, and 1 patient did not list the date of her most recent childbirth. The residual 2 patients who had not experienced childbirth were taking psychotropic medications, such as risperidone or aripiprazole.
Table 1.
Summary of the characteristics of patients with granulomatous lobular mastitis.
| Patient No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 47 | 42 | 33 | 31 | 42 | 38 | 33 | 25 | 37 | 35 | 32 | 44 |
| Parity | NR | 2 | 1 | 0 | 1 | 3 | 1 | 0 | 1 | 1 | 2 | 2 |
| Psychotropic medicine | - | - | - | + | - | - | - | + | - | - | - | - |
| Size of lesion (cm) | 7.5 | 2.5 | 4 | 4 | 6 | 8 | 2.9 | 8 | 10 | 3.3 | 4 | 2.4 |
| Distribution | D/M | L | D/M | D/M | D/M | D/M | S | D/M | D/M | L | L | L |
| Method of diagnosis | V | C | C | C | C | C | C | C | V | C | C | E |
| Ratio of IgG4+ / IgG+ | <5 | <5 | <5 | 5-10 | <5 | <5 | <5 | <5 | <5 | <5 | 5–10 | 5–10 |
| Steroid treatment | + | + | + | + | + | + | + | + | + | + | - | - |
| Local excision | - | - | - | - | - | - | - | - | - | - | - | + |
| Incision and drainage | + | - | + | + | + | + | + | – | + | + | + | - |
| Maximum dose | ||||||||||||
| of steroid (mg) | 50 | 20 | 30 | 30 | 30 | 60 | 15 | 30 | 30 | 20 | - | - |
| Duration of treatment (months) | 1 | 3 | 6 | 5 | 4 | 12 | 4 | 7 | 10 | 5 | 4 | 5 |
| Follow up periods (months) | 6 | 32 | 13 | 21 | 23 | 104 | 20 | 19 | 23 | 10 | 77 | 26 |
| Recurrence | - | - | - | - | - | - | - | - | - | - | + | + |
D/M: Diffuse and/or multiple; L: Localized; S: Subareolar location; V: Vacuum-associated biopsy; C: Core needle biopsy; and E: Excisional biopsy.
The common presenting symptoms were breast pain (11/12, 92%) and breast masses (9/12, 75%). The lesions detected as palpable masses or indurations ranged from 2.4 to 10.0 cm in size (median size of lesion; 6.0 cm). Diffuse and/or multiple lesions were observed in 7 cases (7/12, 58.3%). Four lesions were located at the periphery of the breast (4/12, 33.3%), and a residual lesion was in a subareolar location (1/12, 8.3%). Synchronous or metachronous involvement of the contralateral breast was not found. None of the women had any systemic disorder or history of a specific granulomatous infection.
Diagnostic Pathological Evaluation
Diagnosis of GLM was established histologically in 9 patients by a core needle biopsy, in 2 patients by a vacuum-assisted biopsy, and in one patient by an excisional biopsy. All patients showed non-caseating granulomas involving the mammary lobules, with variable numbers of Langhans-type multinucleated giant cells and inflammatory infiltrates such as neutrophils, polymorphs, lymphocytes, plasma cells, and eosinophils (Fig. 1a and 1b). Microabscess formation was also frequently observed. Specific staining for tuberculosis (Ziehl-Neelsen) and fungal infections (periodic acid-Schiff) were all negative. The inflammation involved the adjacent perilobular and interlobular tissues.
Fig. 1.
Histological findings showing granulomatous inflammation of the lobules without caseation necrosis (1a, Magnification ×40 and the inserted bar 250μm) and the presence of multinucleated giant cells (Langhans’ cells) and plasma cells (1b, Magnification ×400 and the inserted bar 25μm).
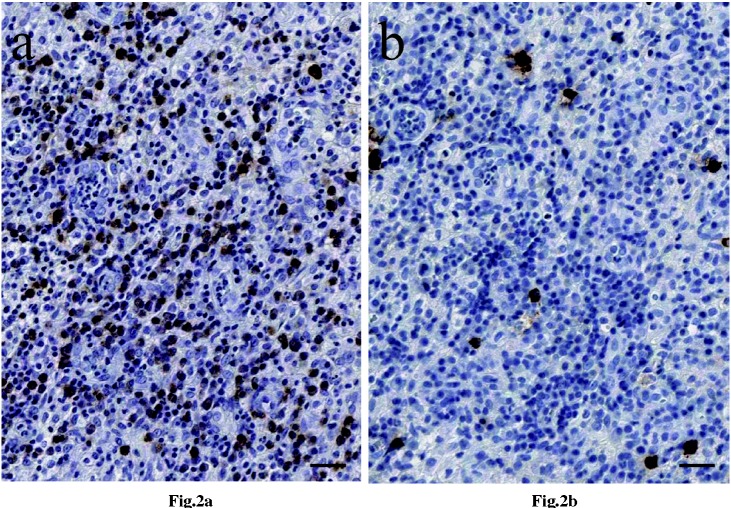
The IgG4+/IgG+ ratios were less than 10% in all patients. The representative images of immunohistochemical staining for IgG and IgG4 are shown in Figure 2a and 2b, respectively. These results indicated that all of our GLMs were the non-IgG4-related type.
Fig. 2.
The following images were from a representative patient No. 8.
a) Immunohistochemical findings for IgG expression in the plasma cells from patients with GLM (Magnification ×300 and the inserted bar 25μm).
b) Immunohistochemical findings for IgG4 expression in the plasma cells from patients with GLM (Magnification ×300 and the inserted bar 25μm).
Treatment and follow-up
Eleven of the 12 patients had been administered antibiotics, but their GLMs had not improved. After the initial treatment with antibiotics, 10 patients received corticosteroid treatment. The initial dosage of corticosteroid (Prednisolone) was determined by the physician. The median initial dosage was 30 mg/day (range: 15–60 mg/day; mean weight were 63.8 kg), and the initial dosage was tapered weekly or biweekly according to clinical and image improvements. The median duration of corticosteroid treatment was 5 months (range: 1–12 months). Although the corticosteroid dosage was increased in 6 of 10 patients because the focus sizes of their GLMs increased during treatment, all 10 cases eventually improved with the corticosteroid treatment. This treatment was continued until the symptoms were completely resolved. Even if the US findings showed residual GLM lesions, the steroid treatment was terminated if the GLMs became smaller and did not cause pain. Complications due to steroid treatment were observed in only one patient who developed a moon face and acne.
Surgical excision was performed for 1 patient with peripheral, localized disease, and another patient was treated with incision and drainage alone. The therapeutic durations of the two patients were 5 months after local excision and 4 months after incision and drainage, respectively. They required lengthy therapeutic periods due to the recurrence of their GLMs. Because these patients refused steroid treatment, they did not receive corticosteroids after the recurrence. In contrast, no patient receiving the corticosteroid treatment experienced a recurrence during the follow-up period.
DISCUSSION
We investigated the involvement of IgG4 in GLM and found that there was no patient with IgG4-related GLM in our study. Ogura et al. 13) reported two IgG4-related GLM patients whose ages were 62 and 66 year old. These patients were obviously older than women in their reproductive years. Cheuk et al. 16) demonstrated that IgG4 positive cells were scarce in 6 of 7 cases of GLM. These results suggest that IgG4-related GLM may be rare. Although all 12 patients had non-IgG4-related GLM in our study, 10 patients were treated with corticosteroids and all of them improved. Moreover, there was no recurrence in these patients during observation. It should be noted that steroid treatment was effective for non-IgG4-related GLMs.
Kok et al. 7) reported that only 17% of patients (4 of 23 cases) in their study had been diagnosed with GLM using fine needle aspiration cytology (FNAC). However, in our study no case was diagnosed using FNAC alone. Because the definitive diagnosis of GLM requires enough specimens to rule out breast cancer, a core needle biopsy or vacuum-assisted biopsy should be necessary.17, 18)
The initial dosage of corticosteroids was determined by the physician. The optimum initial dosage has not been established; however, in this study, 5 of the 10 patients were administered 30 mg of Prednisolone for several weeks, which was then tapered weekly or biweekly. Although long therapeutic periods were necessary, all patients improved and did not experience recurrence after completion of the steroid treatment. Hugon-Rodin et al. treated 12 GLM patients with prednisone 0.5–1.0 mg/kg/day according to the severity of clinical symptoms during 4.5 ± 1.3 (SD) months and demonstrated a significant decrease in the number of recurrences after prednisone treatment compared with that before treatment.19) In addition, Hovanessian et al. 4) showed that treatment of GLM with corticosteroids before surgical management appears to be beneficial, with 77% of patients showing improvement.
Surgical excision was performed in only one patient with a localized disease. Her therapeutic period was 5 months, including the treatment period for the recurrence. In contrast, no patient who received corticosteroid treatment experienced recurrence during the follow-up period. This result suggests that the effectiveness of the corticosteroid treatment may be greater than that of surgical treatment. Because most cases involved large, diffuse, or multiple lesions, the cosmetic result after the surgical treatment should be considered. Yau et al. 12) recommended a wide surgical excision or mastectomy for GLM with persistent symptoms because of a lower rate of recurrence. In this case, a delay of subsequent plastic surgical procedures for breast asymmetry might be necessary. Therefore, it would be appropriate to treat GLM with a corticosteroid before excision, except in patients whose systemic complications are too severe to administer steroid therapy. In other report, it has been reported that medical treatment with corticosteroids provided significant regression of the inflammatory disease and allowed more conservative surgery. They performed surgical excisions of the remaining lesions after medical treatment.20)
Several presumptive causes of GLM, including alpha-1-antitrypsin deficiency, oral contraceptive usage, pregnancy, lactation, and hyperprolactinemia, have been reported.21, 22) It has been postulated that GLM results from a localized autoimmune response to the retained or extravasated secretions in the breast ducts during the childbearing period due to previous hyperprolactinemia.23) Because the occurrence of GLM without a history of recent pregnancy is uncommon, the serum prolactin level might play an important role in the pathogenesis of GLM in non-pregnant women. Lin et al. 24) reported that GLM was associated with risperidone-induced hyperprolactinemia and discussed the development of GLM associated with high serum prolactin levels in non-pregnant women. In our cases, two non-pregnant women were administered antipsychotic drugs; one woman had been treated with aripiprazole and presented a high level of serum prolactin (179.14 ng/m, normal range: 1.20–29.93) and the other woman was not tested for serum prolactin levels but had received risperidone. These findings support the argument that hyperprolactinemia, whether primary or secondary, might contribute to the pathogenesis of GLM in non-pregnant women. Therefore, the roles of prolactin in the pathogenesis of GLM should be studied in more detail.
In summary, our retrospective analysis demonstrated that treatment with a corticosteroid was effective for GLMs. Although corticosteroid treatment requires a lengthy therapeutic period, there was no recurrence in patients during observation. Steroid treatment may be the first choice for the treatment of GLM. However, if GLM resists steroid treatment, other treatments, such as surgical excision or immunosuppressants, should be considered.
ACKNOWLEDGEMENTS
The authors thank Dr. Koji Oda, Department of Breast Oncology, Aichi Cancer Center Aichi Hospital, and Dr. Yukihiro Yokoyama, Division of Surgical Oncology, Department of Surgery, Nagoya Graduate School of Medicine, for advice on our investigation. There were no supporting grants or institutional/corporate affiliations associated with this study.
REFERENCES
- 1).Kessler E, Wolloch Y: Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol 1972; 58: 642–646. [DOI] [PubMed]
- 2).Going JJ, Anderson TJ, Wilkinson S, Chetty U: Granulomatous lobular mastitis. J Clin Pathol 1987; 40:535–540. [DOI] [PMC free article] [PubMed]
- 3).Han BK, Choe YH, Park JM, Moon WK, Ko YH, Yang JH, Nam SJ: Granulomatous mastitis: mammographic and sonographic appearances. AJR Am J Roentgenol. 1999; 173: 317–20. [DOI] [PubMed]
- 4).Hovanessian Larsen LJ, Peyvandi B, Klipfel N, Grant E, Iyengar G: Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2009; 193: 574–81. [DOI] [PubMed]
- 5).Naraynsingh V, Hariharan S, Dan D, Harnarayan P, Teelucksingh S: Conservative management for idiopathic granulomatous mastitis mimicking carcinoma: case reports and literature review. Breast Dis. 2010; 31: 57–60. [DOI] [PubMed]
- 6).Ocal K, Dag A, Turkmenoglu O, Kara T, Seyit H, Konca K: Granulomatous mastitis: clinical, pathological features, and management. Breast J. 2010;16:176–82. [DOI] [PubMed]
- 7).Kok KY, Telisinghe PU: Granulomatous mastitis: presentation, treatment and outcome in 43 patients. Surgeon. 2010; 8: 197–201. [DOI] [PubMed]
- 8).DeHertogh DA, Rossof AH, Harris AA, Economou SG: Prednisone management of granulomatous mastitis. N Engl J Med. 1980; 303: 799–800. [DOI] [PubMed]
- 9).Erozgen F, Ersoy YE, Akaydin M, Memmi N, Celik AS, Celebi F, Guzey D, Kaplan R: Corticosteroid treatment and timing of surgery in idiopathic granulomatous mastitis confusing with breast carcinoma. Breast Cancer Res Treat. 2010; 123: 447–52. [DOI] [PubMed]
- 10).Sakurai K, Fujisaki S, Enomoto K, Amano S, Sugitani M: Evaluation of follow-up strategies for corticosteroid therapy of idiopathic granulomatous mastitis. Surg Today. 2011; 41: 333–7. [DOI] [PubMed]
- 11).Akbulut S, Arikanoglu Z, Senol A, Sogutcu N, Basbug M, Yeniaras E, Yagmur Y: Is methotrexate an acceptable treatment in the management of idiopathic granulomatous mastitis? Arch Gynecol Obstet. 2011; 284: 1189–95. [DOI] [PubMed]
- 12).Yau FM, Macadam SA, Kuusk U, Nimmo M, Van Laeken N: The surgical management of granulomatous mastitis. Ann Plast Surg. 2010; 64: 9–16. [DOI] [PubMed]
- 13).Ogura K, Matsumoto T, Aoki Y, Kitabatake T, Fujisawa M, Kojima K: IgG4-related tumour-forming mastitis with histological appearances of granulomatous lobular mastitis: comparison with other types of tumour-forming mastitis. Histopathology. 2010; 57: 39–45. [DOI] [PubMed]
- 14).Zen Y, Nakanuma Y: IgG4-related disease: a cross-sectional study of 114 cases. Am J Surg Pathol. 2010; 34: 1812–9. [DOI] [PubMed]
- 15).Kasashima S, Zen Y, Kawashima A, Konishi K, Sasaki H, Endo M, Matsumoto Y, Kawakami K, Kasashima F, Moriya M, Kimura K, Ohtake H, Nakanuma Y: Inflammatory abdominal aneurysm: close relationship to IgG4-related periaortitis. Am J Surg Pathol. 2008; 32: 197–204. [DOI] [PubMed]
- 16).Cheuk W, Chan AC, Lam WL, Chow SM, Crowley P, Lloydd R, Campbell I, Thorburn M, Chan JK: IgG4-related sclerosing mastitis: description of a new member of the IgG4-related sclerosing diseases. Am J Surg Pathol. 2009; 33: 1058–64. [DOI] [PubMed]
- 17).Hirata S, Saito T, Kiyanagi K, Kitada M, Yamazaki K, Sasajima T, Ohsaki Y, Miyokawa N: Granulomatous mastitis diagnosed by core-needle biopsy and successfully treated with corticosteroid therapy: a case report. Breast Cancer. 2003; 10: 378–81. [DOI] [PubMed]
- 18).Kuba S, Yamaguchi J, Ohtani H, Shimokawa I, Maeda S, Kanematsu T: Vacuum-assisted biopsy and steroid therapy for granulomatous lobular mastitis: report of three cases. Surg Today. 2009; 39: 695–9. [DOI] [PubMed]
- 19).Hugon-Rodin J, Plu-Bureau G, Hugol D, Gompel A: Management of granulomatous mastitis: a series of 14 patients. Gynecol Endocrinol. 2012; 28: 921–4. [DOI] [PubMed]
- 20).Gurleyik G, Aktekin A, Aker F, Karagulle H, Saglamc A: Medical and surgical treatment of idiopathic granulomatous lobular mastitis: a benign inflammatory disease mimicking invasive carcinoma. J Breast Cancer. 2012; 15: 119–23. [DOI] [PMC free article] [PubMed]
- 21).Bani-Hani KE, Yaghan RJ, Matalka II, Shatnawi NJ: Idiopathic granulomatous mastitis: time to avoid unnecessary mastectomies. Breast J. 2004; 10: 318–22. [DOI] [PubMed]
- 22).Azlina AF, Ariza Z, Arni T, Hisham AN: Chronic granulomatous mastitis: diagnostic and therapeutic considerations. World J Surg. 2003; 27: 515–8. [DOI] [PubMed]
- 23).Cserni G, Szajki K: Granulomatous Lobular Mastitis Following Drug-Induced Galactorrhea and Blunt Trauma. Breast J. 1999; 5: 398–403. [DOI] [PubMed]
- 24).Lin CH, Hsu CW, Tsao TY, Chou J: Idiopathic granulomatous mastitis associated with risperidone-induced hyperprolactinemia. Diagn Pathol. 2012: 5; 7:2. [DOI] [PMC free article] [PubMed]