ABSTRACT
The central nervous system, in particular the spinal cord, is a rare site for primary lymphoma occurrence, with very few published cases. We report an extremely rare primary lymphoma in the cauda equina in a single case with literature review. An immunocompetent 59-year-old male, who complained of progressive low back and bilateral leg pain for 7 months, was studied. Magnetic resonance imaging (MRI) revealed an intradural space-occupying lesion from T12 to S1, poorly demarcated to the normal cauda equina. The intradural lesion showed T1 low intensity, T2 low isointensity, and marked homogeneous enhancement with gadolinium-diethylenetriaminepentaacetic acid on MRI. We performed spinal tap to obtain additional information about the intradural lesion. Large-sized atypical lymphoid cells were found during pathological examination. Fluorodeoxyglucose accumulation was found only in the lumbar area, which corresponded with the MRI findings, and the primary lymphoma site was defined as the cauda equina area. For further detailed pathological diagnosis, we performed surgical biopsy of the cauda equina. Morphological and immunohistochemical assessment made a diagnosis of diffuse large B-cell lymphoma of the cauda equina. The patient received radiotherapy to the lumbosacral area (50 Gy) and methotrexate (MTX) therapy after surgery. The patient was able to walk without help after the therapies. Follow-up MRI performed 1 year after biopsy showed remission of the lesion. MRI and spinal tap were effective tools for the early definitive diagnosis of cauda equina lymphoma. Combined treatment with radiotherapy and MTX should be performed as early as possible.
Key Words: primary lymphoma, cauda equina, B-cell lymphoma
INTRODUCTION
Primary central nervous system (CNS) lymphoma (PCNSL) is a relatively rare tumor, accounting for 1%–7% of primary brain tumors and 1%–2% of all cases of non-Hodgkin lymphoma1). Primary lymphoma existing in the spinal cord is also rare, representing <1% of PCNSLs in adults2). In addition, primary lymphoma in the cauda equina is even more rare. We identified an extremely rare case of primary lymphoma in the whole cauda equina area, characterized by disappearance of the cauda equina nerves, using magnetic resonance imaging (MRI). The purpose of this report was to describe the clinical features and pathological findings of the case of cauda equina lymphoma, with a review of the literature.
CASE REPORT
A 59-year-old immunocompetent man, with a 7-month history of severe low back and bilateral leg pain, was studied. Symptoms had gradually progressed after onset, and the patient could not walk because of severe leg pain. The patient’s Eastern Cooperative Oncology Group performance status3) was 3 and previous history included hypertension, diabetes mellitus, and cerebral subarachnoid hemorrhage, for which surgery had been performed. Physical examination revealed bilateral absence of lower limb reflexes, numbness of both lower extremities, and mild motor weakness in bilateral iliopsoas and hamstring muscles. Routine hematological and biochemical tests, as well as human immunodeficiency virus serology, were normal. Serum interleukin-2 and serum lactate dehydrogenase (LDH) levels were normal at 458U/ml and 149 IU/l, respectively.
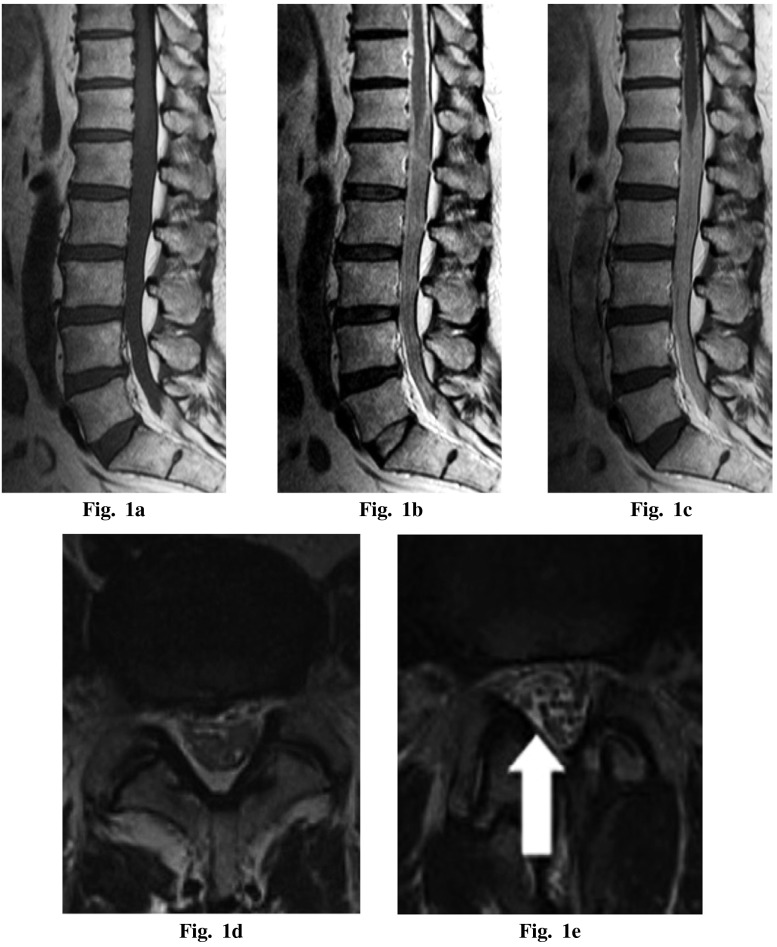
MRI of the lumbar spine revealed an intradural space-occupying lesion from T12 to S1, which was poorly demarcated to the normal cauda equina. The intradural lesion showed T1 low intensity, T2 low isointensity, and marked homogeneous enhancement with gadolinium (Gd)-diethylenetriaminepentaacetic acid on MRI (Figures 1a–d). No other region of the patient’s brain or spine was found to be involved. Malignant lymphoma and metastatic lesion were the differential diagnosis of this lesion.
Fig. 1.
Magnetic resonance imaging — (a) preoperative T1-weighted sagittal image, (b) preoperative T2-weighted sagittal image, (c) preoperative T1-weighted sagittal image with gadolinium enhancement showing an intrathecal mass at the lesion and marked enhancement with gadolinium, (d) preoperative T2-weighted axial image at L4/5 level (e) postoperative T2-weighted axial image at L4/5 level. Swelling of the cauda equina was diminished (white arrow).
We attempted to collect a cerebrospinal fluid sample by spinal tap in order to obtain additional information about the intradural lesion. We performed spinal tap under fluoroscopy at the L3–4 level. Initially, no sample was obtained because of a lack of cerebrospinal fluid. We then administered 3 ml of normal saline into the spinal canal and collected the injected sample. Large-sized atypical lymphoid cells were identified by pathological examination, and the lumbar spinal lesion was diagnosed as malignant lymphoma. The cerebrospinal fluid protein level was found to be elevated at 0.48 g/dl.
We performed fludeoxyglucose (FDG)-positron emission tomography. FDG accumulation was found in the spinal intradural area (T12–S1), which corresponded with the MRI findings. FDG accumulation was not found in any other location. Moreover, whole-body computed tomography (CT) and bone marrow biopsy of the iliac bone did not show abnormalities. We diagnosed this patient with primary cauda equina lymphoma.
To obtain more detailed pathological information, we performed surgical open biopsy within the cauda equina area. After fenestration at the L3–4 left side and incision of the dural sac, the cauda equina was observed as extremely swollen with dull red tumors present. The tumors infiltrated extensively between the cauda equina and adhered strongly to the nerve root. We performed biopsy by cutting the nerve sheath of one of the cauda equina nerves. After surgery, the patient’s severe leg pain did not worsen. A short course of methylprednisolone was administered, with transient improvement in the immediate postoperative period.
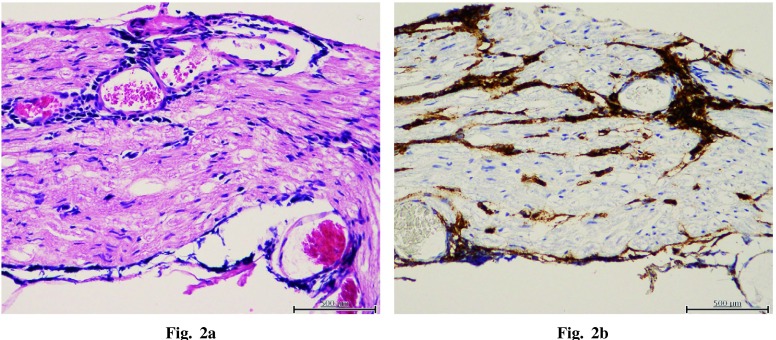
A diagnosis of diffuse large B-cell lymphoma (DLBCL) of the cauda equina was made on the basis of morphological and immunohistochemical assessment. Histologically, atypical lymphoid cells infiltrate the nerve tissue. Tumor cells were positive for CD20 and BCL-2 and negative for CD3, CD5, CD10, BCL-6, MUM-1, and PAX5 (Figure 2). Lymphoma cells were observed to infiltrate into nerve tissues.
Fig. 2.
(a) Diffuse proliferation of lymphoma cells infiltrating in nerve tissue of cauda equina (hematoxylin-eosin stain). (b) Lymphoma cells are positive for CD20
The patient received radiotherapy to the lumbosacral area with a dose of 50 Gy and methotrexate (MTX) after surgery. One year after surgery, lower leg pain was relieved and walking unaided was possible. MRI performed 1 year after surgery showed that swelling of the cauda equina was diminished (Figure 1e). Furthermore, metastasis was not found on radiographical examination.
DISCUSSION
PCNSL is a form of extranodal lymphoma and accounts for 5%–7% of primary brain tumors4). Most PCNSLs (almost 90%) are diffuse large B-cell non-Hodgkin lymphomas5) and account for 1%–2% of all cases of non-Hodgkin lymphoma4). PCNSLs are often associated with immunocompromized patients.
In the CNS, the spinal cord is the rarest site of involvement in patients with PCNSLs (<1% of PCNSLs)2). Moreover, among spinal cord lymphomas, cauda equina lesions are rarest. To the best our knowledge, only 15 cases of primary cauda equina lymphoma have been reported (Table 1)6-19,23). Moreover, there were no case resembling the present case, with primary lymphoma in the whole cauda equina area and the disappearance of the cauda equina nerves on MRI. Our patient had a long history (7 months) of clinical symptoms prior to diagnosis, which may be unusual in patients with PLML of the cauda equina (Table 1). This long clinical history might give the largest tumor extent. Examination of spinal MRI with intravenous Gd has been shown to be useful in detecting lymphoma in the cauda equina, and thickened cauda equina and nerve roots are detected after Gd enhancement. The differential diagnosis of intradural non-solid tumor in the cauda equina is metastasis, infections (tuberculosis, toxoplasmosis, and cryptococcal granulomatas), and arachnoiditis19). Open biopsy is needed for definitive diagnosis, but some authors suggest the collection of cerebrospinal fluid for diagnosis6,20). In the present case, we also obtained cerebrospinal fluid before biopsy. If the cerebrospinal fluid test results identify a metastatic tumor, open biopsy should not be performed in order to reduce the risk of paralysis after cauda equina biopsy. Whole-body CT should therefore be used to identify the origin of the tumor. If the cerebrospinal fluid test results identify infection or arachnoiditis, there is no need for further biopsy.
Table 1.
Literature review about primary lymphoma of cauda equina
| Reference | Age/Sex | Interval (Onset-Diagnosis) |
Pathology | Treatment | Outcome | Follow up |
|---|---|---|---|---|---|---|
| Mauney6) | 68/F | 1 wk | B cell lymphoma | RT | Alive | 3 months |
| Toner7) | 59/M | 3 mo | B cell lymphoma | RT, CT (intrathecal and intravenous) | Alive | 2 years |
| Klein8) | 29/F | 2 wk | B cell lymphoma | N.A. | Died | 5 weeks |
| Knopp9) | 69/F | 3 wk | N.A. | N.A. | N.A. | N.A. |
| Ooi10) | 16/M | 3 wk | T-lymohoblastic lymphoma | RT, CT (intrathecal and intravenous) | Died | 8 months |
| Giobbia11) | 30/F | 2 mo | B cell lymphoma | RT, CT (intrathecal) | Alive | 1 year |
| Zagami12) | 71/F | 3 wk | B cell lymphoma | CT (intrathecal) | Died | 16 months |
| Tajima13) | 67/F | 3 yr | B cell lymphoma | RT, CT (intrathecal) | Alive | 3 years |
| Khong14) | 16/M | 6 wk | B cell lymphoma | RT, CT (intravenous) | Alive | 1 year |
| Morita15) | 67/M | 2 mo | NK/T cell lymphoma | RT, CT (oral) | Died | 14 months |
| Beitzke23) | 69/M | 1 yr | B cell lymphoma | CT | Died | Soon after diagnosis |
| Teo16) | 58/M | 2 mo | B cell lymphoma | RT, CT (intravenous) | Alive | 2 years |
| Cugati17) | 11/M | 3 day | N.A. | RT, CT (intravenous) | Alive | 1 year |
| Iwasaki18) | 69/M | N.A. | B cell lymphoma | RT, CT (intravenous) | Died | 1.5 years |
| Nishida19) | 47/M | 10 day | B cell lymphoma | RT, CT (intrathecal and intravenous) | Alive | 1.5 years |
| Nakashima (this study) |
59/M | 7 mo | B cell lymphoma | RT, CT (intravenous) | Alive | 1 year |
N.A., not available; RT, radiation therapy; CT, chemotherapy
With regard to treatment of PCNSL, there is consensus that combined chemoradiotherapy is superior to radiotherapy or chemotherapy alone21,22). MTX is known to be the single most effective chemotherapeutic agent for PCNSL21,22). Standard systemic chemotherapy for nodal lymphomas, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), is ineffective because of the difficulty of penetration of the blood–brain barrier by chemotherapeutic drugs21). Among other chemotherapeutic drugs, rituximab, a chimeric monoclonal antibody against the CD20 antigen, has been reported as a potential drug to increase survival when used for patients with systemic CD20 diffuse large B-cell non-Hodgkin lymphoma. However, its ability to prevent CNS dissemination of DLBCL remains low21). Although it has not been addressed in a phase 3 clinical trial to date, MTX combined with ara-C has the potential to be an effective chemotherapeutic approach for de novo PCNSL21). While intrathecal chemotherapy has been investigated for PCNSL, including the spine area (Table 1), its effectiveness remains controversial21).
Radiation therapy alone is initially effective; however, the response is usually short-lived with median survival rates ranging from 10 to 18 months21,22). A dose of 40–50 Gy has been suggested, resulting in a 19% complete remission rate in the brain21). Doses of >50 Gy and the addition of a boost are associated with increased risk of neurotoxicity21). Aggressive surgical tumor resection is not effective for the treatment of PCNSL lesions22).
The International Extranodal Lymphoma Study Group presented prognostic scoring according to age, performance status, serum LDH level, cerebrospinal fluid protein level, and involvement of deep structures3). Although it is difficult to adapt this prognostic scoring system to the cauda equina lymphoma in the present case, because of the difference in tumor site, 3 risk factors (low performance status, elevated cerebrospinal fluid protein level, and lymphoma invasion to deep site of the cauda equina) were identified in the present case. This patient was classified in the middle risk group with high dose MTX, and the 2-year overall survival was estimated as 57% ± 8%3). The patient’s performance status is currently high 1 year after biopsy; however, further long-term follow-up is needed.
Conflict of Interest
Nil
REFERENCES
- 1).Eichler AF, Batchelor TT. Primary central nervous system lymphoma: presentation, diagnosis and staging. Neurosurg Focus, 2006; 21: E15. [DOI] [PubMed]
- 2).Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg, 1988; 68: 835–853. [DOI] [PubMed]
- 3).Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, Calderoni A, Rossi A, Vavassori V, Conconi A, Devizzi L, Berger F, Ponzoni M, Borisch B, Tinguely M, Cerati M, Milani M, Orvieto E, Sanchez J, Chevreau C, Dell’Oro S, Zucca E, Cavalli F. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol, 2003; 21: 266–272. [DOI] [PubMed]
- 4).Iwamoto FM, DeAngelis LM. An update on primary central nervous system lymphoma. Hematol Oncol Clin North Am, 2006; 20: 1267–1285. [DOI] [PubMed]
- 5).Lukes RJ, Collins RD. Immunologic characterization of human malignant lymphomas. Cancer, 1974; 34: 1488–1503. [DOI] [PubMed]
- 6).Mauney M, Sciotto CG. Primary malignant lymphoma of the cauda equina. Am J Surg Pathol, 1983; 7: 185–190. [DOI] [PubMed]
- 7).Toner GC, Holmes R, Sinclair RA, Tang SK, Schwarz MA. Central nervous system lymphoma: primary lumbar nerve root infiltration. Acta Haematol, 1989; 81: 44–47. [DOI] [PubMed]
- 8).Klein P, Zientek G, VandenBerg SR, Lothman E. Primary CNS lymphoma: lymphomatous meningitis presenting as a cauda equina lesion in an AIDS patient. Can J Neurol Sci, 1990; 17: 329–331. [DOI] [PubMed]
- 9).Knopp EA, Chynn KY, Hughes J. Primary lymphoma of the cauda equina: myelographic, CT myelographic, and MR appearance. AJNR Am J Neuroradiol, 1994; 15: 1187–1189. [PMC free article] [PubMed]
- 10).Ooi G, Peh W, Fung C. Magnetic resonance imaging of primary lymphoma of the cauda equina. British journal of radiology, 1996; 69: 1057–1060. [DOI] [PubMed]
- 11).Giobbia M, Carniato A, Scotton PG, Vaglia A, Marchiori GC. Primary EBV-associated cauda equina lymphoma. J Neurol, 1999; 246: 739–740. [DOI] [PubMed]
- 12).Zagami AS, Granot R. Non-Hodgkin’s lymphoma involving the cauda equina and ocular cranial nerves: case reports and literature review. J Clin Neurosci, 2003; 10: 696–699. [DOI] [PubMed]
- 13).Tajima Y, Sudo K, Matumoto A. Malignant lymphoma originating in the cauda equina mimicking the inflammatory polyradiculoneuropathy. Internal Medicine, 2007; 46: 1029–1032. [DOI] [PubMed]
- 14).Khong P, Pitham T, Owler B. Isolated neurolymphomatosis of the cauda equina and filum terminale: case report. Spine (Phila Pa 1976), 2008; 33: E807–811. [DOI] [PubMed]
- 15).Morita M, Osawa M, Naruse H, Nakamura H. Primary NK/T-cell lymphoma of the cauda equina: a case report and literature review. Spine (Phila Pa 1976), 2009; 34: E882–885. [DOI] [PubMed]
- 16).Teo MK, Mathieson C, Carruthers R, Stewart W, Alakandy L. Cauda equina lymphoma-a rare presentation of primary central nervous system lymphoma: case report and literature review. British Journal of Neurosurgery, 2012; 26: 868–871. [DOI] [PubMed]
- 17).Cugati G, Singh M, Symss NP, Pande A, Vasudevan MC, Ramamurthi R. Primary spinal intradural extramedullary lymphoma causing cauda equina syndrome. J Craniovertebr Junction Spine, 2012; 3: 58–61. [DOI] [PMC free article] [PubMed]
- 18).Iwasaki M, Hida K, Yano S, Aoyama T, Kaneko S, Iwasaki Y. Primary Cauda Equina Lymphoma Treated With High-Dose Methotrexate. Neurologia medico-chirurgica, 2012; 52: 679–683. [DOI] [PubMed]
- 19).Nishida H, Hori M, Obara K. Primary B-cell lymphoma of the cauda equina, successfully treated with high-dose methotrexate plus high-dose cytarabine: a case report with MRI findings. Neurological Sciences, 2012; 33: 403–407. [DOI] [PubMed]
- 20).MacKintosh FR, Colby TV, Podolsky WJ, Burke JS, Hoppe RT, Rosenfelt FP, Rosenberg SA, Kaplan HS. Central nervous system involvement in non-Hodgkin’s lymphoma: an analysis of 105 cases. Cancer, 1982; 49: 586–595. [DOI] [PubMed]
- 21).Ferreri AJ. How I treat primary CNS lymphoma. Blood, 2011; 118: 510–522. [DOI] [PubMed]
- 22).Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. Journal of Clinical Oncology, 2000; 18: 3144–3150. [DOI] [PubMed]
- 23).Beitzke M, Enzinger C, Beitzke D, Neureiter D, Ladurner G, Fazekas F. Primary leptomeningeal lymphoma of the cauda equina: a rare cause of radiculopathy. J Neurol, 2010; 257: 1734–1737. [DOI] [PubMed]