ABSTRACT
Central nervous system (CNS) manifestations are rare complications of relapsing polychondritis (RP). The majority of patients respond well to glucocorticoid therapy, but need to maintain it. Some patients are refractory to initial glucocorticoid therapy and to additional immunosuppressants, and end up with an outcome worse than at therapy initiation. The standardized therapeutic protocol for this condition has not been established. The effects of anti-tumor necrosis factor (TNF) -α agents have been reported recently. We experienced a patient with RP and limbic encephalitis who was refractory to initial high-dose glucocorticoid, but subsequently responded to infliximab and did not show deterioration of signs and symptoms after stopping therapy. We report this case together with a systematic literature review. This is the first case report of RP with CNS manifestations successfully treated by an anti-TNF-α agent without recurrence after discontinuation.
Key Words: relapsing polychondritis, limbic encephalitis, infliximab, anti-tumor necrosis factor-alpha agent, therapy discontinuation
INTRODUCTION
Relapsing polychondritis (RP) is an uncommon disorder of unknown etiology that is characterized by recurrent and progressive inflammation of cartilaginous structures. A minority of patients with RP develop central nervous system (CNS) manifestations,1) and limbic encephalitis has also been reported.1-5) Glucocorticoid has been used as the first-line therapeutic agent,1-22) but a standardized second-line therapeutic protocol for RP with CNS manifestations has not been established. The effects of anti- tumor necrosis factor (TNF) -α agents have been reported recently.5,9) We report a patient with limbic encephalitis associated with RP who was refractory to initial high-dose glucocorticoid therapy, but subsequently responded to infliximab and discontinued therapy without recurrence. We also reviewed cases of RP with CNS manifestations using PubMed with regard to clinical manifestations and treatment.
CASE PRESENTATION
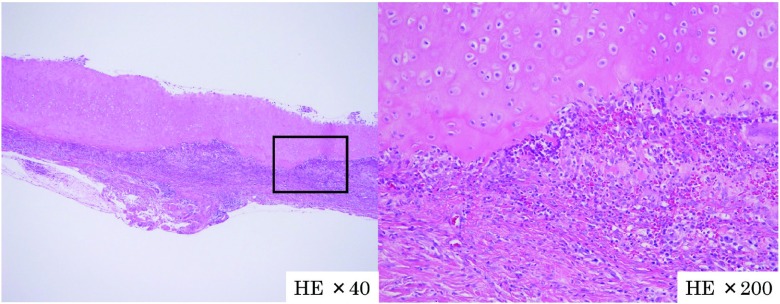
A 58-year-old Japanese male architect was brought to our institution by his wife, presenting with amnesia, disorientation, emotional liability and urinary incontinence. One year prior to admission, he had bilateral ear pain with swelling and erythema which improved without any treatment over a 4-week period. Nine months prior to admission, he experienced iritis and scleritis in addition to recurrent pain in bilateral auricles. Subsequent biopsy of the left auricle revealed infiltration of inflammatory cells in the perichondrium (Fig. 1). Diagnosis of RP was made based on McAdam’s criteria, modified by Damiani and Levine.23,24) No other organs were affected. Inflammation of bilateral auricles disappeared without any treatment, while iritis and scleritis were controlled by topical glucocorticoid therapy. Around 2 months prior to admission, he showed amnesia with gradual progression. One month prior to admission, he developed difficulty with drawing architectural drafts and finding his way home, together with emotional liability and urinary incontinence. Past medical history revealed well-controlled diabetes mellitus by diet and dipeptidyl peptidase-4 inhibitor (HbA1c was 6.4 to 6.7%).
Fig. 1.
Histopathological examination of ear biopsy (hematoxylin-eosin stain) showed infiltration of inflammatory cells (histiocytes, lymphocytes, neutrophils and eosinophils) in perichondrium and chondrium.
On admission, his body temperature was 36.7°C, blood pressure was 112/66 mmHg and heart rate was 68 beats per minute. Physical examination revealed flared ears. Head, eye, ear, nose, chest and abdominal examinations were unremarkable. Neurological examination revealed poor tandem gait and poor finger-nose-finger test, but other examinations such as the cranial nerve, sensory and motor systems were unremarkable. He was euphoric and disoriented. His Mini-Mental States Examination (MMSE) result was 16 out of 30. Complete blood cell count, serum chemistry screening and endocrine function were unremarkable. Antinuclear antibodies, anti-neutrophil cytoplasmic antibodies, rheumatoid factor, anti-thyroid peroxydase antibody, anti-thyroglobulin antibody, urinalysis and serological tests for human immunodeficiency virus (HIV) and treponema pallidum were all normal or negative. Cerebrospinal fluid (CSF) analysis showed 33 cells/µl with 32 polymorphonuclear leukocytes, glucose 81 mg/dl and protein 92 mg/dl. CSF smear for Gram stain and acid-fast organisms stain were negative. CSF cultures for bacteria and Mycobacterium tuberculosis and polymerase chain reaction of Herpes simplex virus and cytology were also negative. Both anti-N-methyl-D-aspartate type glutamate receptor (GluR) N2B antibody and anti-GluR δ2 antibody were positive in CSF, but neither were positive in serum.
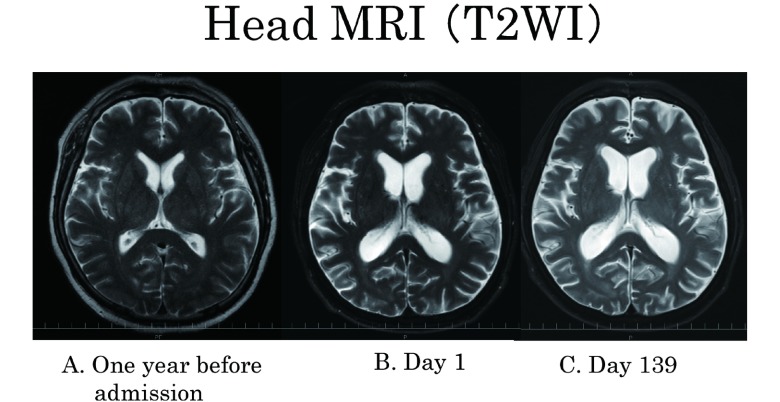
Whole body fluorine-18 fludeoxyglucose positron emission tomography ([18F]FDG-PET) with CT to detect tumor revealed no abnormal uptake. Comparing current brain magnetic resonance imaging (MRI) result with the previous ones indicated limbic system atrophy resulting in ventricular enlargement (Fig. 2-A, B). Diffusion weighted image, fluid-attenuated inversion recovery image (FLAIR) and gadolinium enhancement showed no abnormality. Electroencephalogram showed diffuse dominant theta waves with no spike.
Fig. 2.
(A) T2WI one year before admission; (B) T2WI of Day 1 showed ventricular enlargement compared to one year before admission; (C) there was no change after 6 months.
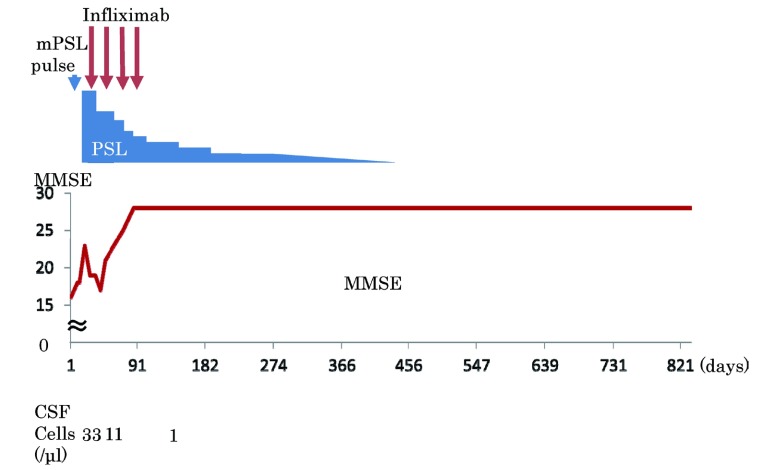
Considering his clinical symptoms like emotional lability and amnesia, limbic system atrophy in MRI and increased number of CSF cells limbic encephalitis was diagnosed. Because other causes such as HIV encephalitis, herpes simplex encephalitis, tumor-associated limbic encephalitis or Hashimoto encephalopathy were ruled out, limbic encephalitis associated with RP was diagnosed, clinically. A course of intravenous 1 g methylprednisolone for 3 days was administered, followed by oral prednisolone 1 mg/kg per day. His cognitive function improved temporarily, but worsened again (Fig. 3). Subsequently infliximab 3 mg/kg was added to the prednisolone. His head MRI had no change but MMSE score was improved gradually, ataxia disappeared through 4 doses of infliximab over a 3-month period, and problematic behavior disappeared. Because of his stable condition as well as the high cost of infliximab, he and his wife refused further infliximab therapy. His condition continued to be stable without infliximab. Prednisolone was tapered down over a 16-month period and finally stopped. The patient was followed up for an additional 9 months after stopping prednisolone without recurrence (Fig. 3). At the end, he could continue active daily living independently, but could not resume his work.
Fig. 3.
Clinical course. Cognitive function improved temporarily after methylpredonisolone pulse, but worsened again. MMSE score was improved gradually after infliximab.
DISCUSSION
RP, a rare episodic and progressive inflammatory disease presumed to have autoimmune etiology, was first described in 1923.25) RP affects cartilage in multiple organs, such as the ear, nose, larynx, trachea, bronchi, and joints.25) In addition, it can affect proteoglycan-rich tissues such as the eyes, aorta, heart and skin.25) The diagnosis of RP is usually made on the basis of clinical findings.25) McAdam criteria23) modified by Damiani and Levine,24) which is commonly used as a criterion to confirm the diagnosis of RP, consists of: a) at least 3 of 6 clinical criteria (bilateral auricular chondritis, nonerosive seronegative inflammatory polyarthritis, nasal chondritis, ocular inflammation, respiratory chondritis and audiovestibular damage); b) 1 or more of the previously-mentioned clinical criteria and biopsy confirmation of cartilage inflammation; or c) chondritis at 2 or more separate anatomic locations with response to steroids and/or dapsone. This case fits criterion b).
RP with CNS manifestations is rare.8,22) We searched MEDLINE in March 2014 using ("Polychondritis, Relapsing" [Mesh] OR "Relapsing polychondritis") AND ("Encephalitis" [Mesh] OR "Limbic Encephalitis" [Mesh] or encephalitis or encephalopathy or "Limbic Encephalitis" OR "Meningoencephalitis" [Mesh] OR Meningoencephalitis or "nervous system") as keywords. We retrieved a total of 54 articles, 26 of them including 31 cases that met inclusion criteria (case report or case series written in English or Japanese) (Table 1).1-22,26-28)
Table 1.
Abbreviations: T2WI, T2 weighted image. mPSL, methylpredonisolone. PSL, predonisolone. AZP, azathioprine. MONO, monocytes. PMN, polymorphonuclear leukocytes. CYC, cyclophosphamide. MTX, methotrexate. IVIG, intravenous immunoglobulin. nr, not reported.
Cases filled in red were refractory to initial glucocorticoid therapy. Cases filled in blue had good response to initial glucocorticoid therapy. Other patients received no treatment or the results were unknown.5,7,21,27,28,30)
*The clinical course after the second pulse is not shown.
| Year | Age | Sex | Associated neurologic disorders (most patients had fever, headache or meningeal irritation signs) | CSF Leucocytes (/mm 3) | MRI | Treatment | neurological response to initial therapy | neurological response at end of follow up | Outcome | treatment successfully discontinued | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| this case | 58 | M | amnesia, cognitive impairment, emotional liability, urinary incontinence, euphoria |
33 (32 PMN and 1 MONO) |
ventricular enlargement | mPSL 1 g/day 3 days→ PSL 1 mg/kg/day→ +infliximab→PSL →stopped |
transitory | good | alive | yes | |
| 2011 | 9) | 57 | M | generalized seizure, confusion | 700 (MONO 686) |
T2WI high, gadolinium-enhanced |
high dose i.v. mPSL→ high dose i.v. mPSL+CYC →PSL+CYC→ PSL+MTX→infliximab |
transitory | good | alive | no |
| 2011 | 7) | 52 | M | amnesia, gait disorders and urinary incontinence, acalculia | 231 (PMN 161, MONO 69) |
ventricular enlargement | mPSL 500 mg+IVIG 30 g/day 5 days→ PSL 20 mg/day→ Steroid Pulse |
transitory | good or transitory* | alive | no |
| 2009 | 3) | 62 | M | delirium, hallucinations, agitation, disinhibition, cognitive impairment, seizure, disturbed consciousness, recurrent clonic convulsion | 39 (MONO 23) | FLAIR high | iv mPSL 3 days a week 3 weeks→iv mPSL 3 days a week 4 weeks→ PSL 20 mg/day+tacrolimus 3 mg/day |
transitory | worsend | alive | no |
| 2008 | 12) | 51 | M | coordination disorder, distractibility, emotional lability, insomnia, nocturnal myoclonic jerks, perseveration, attention and concentration deficits, confusion, speech latency, word-finding difficulty, myoclonus | 39 (MONO 59) | high signal abnormalities | PSL 80 mg/day→ Cyclophosphamide 150 mg/day |
worsened | worsend | died (after 10 months of neurological onset) | no |
| 2011 | 4) | 73 | M | transitory loss of consciousness, confusion, disorientation, confabulation, aphasia, hallucinations, cognitive impairment | 89 (MONO 89) | FLAIR high, T2 high | mPSL1500 mg 3 days→ 3500 mg→ mPSL po 24 mg/day→ mPSL 1500 mg+ plasmapheresis→+IVIG |
transitory | worsend | died (after 5 month of disease onset) | no |
| 2009 | 11) | 67 | F | bradykinesia, disturbed consciousness, reduced willingness, walking disorder | 73 (MONO 73) | FLAIR high | mPSL 1000 mg/day 3 days→PSL 40 mg/day→ PSL 60–50 mg/day +MTX 6–8 mg/w+CyA 100–200 mg/day |
transitory | worsend | died (after 6 months of neurological onset) | no |
| 1992 | 19) | 73 | F | decreased consciousness, slow in mention, right eyelid paresis, slight unilateral facial weakness | nr | nr | PSL 100 mg/day→ +Cyc 100 mg/day→ PSL 5 mg/day→both stopped |
good | good | alive | yes |
| 2011 | 7) | 44 | M | amnesia, irritated, anxious | 190 (PMN17 MONO 171) | T2WI high | mPSL 200 mg 5 days→ 120 mg/day one week→ PSL 60 mg/day +AZP 100 mg/day→PSL 25 mg/day+AZP 100 mg/day |
good | good | alive | no |
| 2011 | 7) | 44 | F | anxiety, insomnia | 70 (PMN 28 MONO 42) | normal | mPSL 500 mg iv→mPSL 120 mg/day→+AZP | good | good | alive | no |
| 2011 | 8) | 68 | F | dysarthria, disorientation, impaired language function, agraphia | 100 (PMN 33, MONO 67) | high intensity | mPSL 1 g 3 days→ PSL 1 mg/kg/day→10 mg/day |
good | good | alive | no |
| 2010 | 10) | 70 | M | confusion, hallucinations | 38 | T2WI high, gadolinium enhancement | mPSL 1 g 3 days→ PSL 1 mg/kg/day→15 mg/day |
good | good | alive | no |
| 2008 | 13) | 66 | F | bradykinesia, somnolence, urinary incontinence, mutism, disorientation | 90 | T1WI low, T2WI high, FLAIR high | mPSL 1000 mg/day 3 days | good | good | alive | no |
| 2008 | 12) | 68 | M | comprehension problems, emotional lability, confusion, language problems, amnesia, executive dysfunction, visuospatial impairment, mild anomia | 4 (MONO 2) | T2WI high | PSL 80 mg/day | good | good | alive | no |
| 2007 | 14) | 40 | M | confusion, somnolence | 1500 (PMN 1245) | T2WI high, FLAIR high | intravenous steroid therapy | good | good | alive | no |
| 2007 | 26) | 64 | M | amnesia, disorientation, acalculia, reduced willingness | 14 (MONO 14) | T2WI high, FLAIR high | PSL 30 mg po→ PSL 20 mg/day po |
good | good | alive | no |
| 2006 | 15) | 71 | F | confuse, aphasia, weakness of right extremities | 110 (MONO 100) | enhanced | hydrocortisone 200 mg/day→ PSL 60 mg/day→ PSL 20 mg/day |
good | good | alive | no |
| 2004 | 17) | 38 | M | right-side weakness, diplopia, right side hemipregia, with hyperreflexia and clonus at the ankle, confuse | nr | T2WI high | corticosteroid therapy→ PSL 1 mg/kg/day+AZP |
good | good | alive | no |
| 2004 | 2) | 45 | M | confusion, euphoria, hyperactive behavior, disorientation, amnesia, fever, inappropriately jocular affect, disjointed speech, confabulation, attention deficits | 8000 (MONO 7520) | T2WI high | high dose mPSL→ PSL 40 mg/day |
good | good | alive | no |
| 2004 | 16) | 49 | M | disorientation, somnolent, ataxic, disorientation, gait disorder | 145 (PMN 55 MONO 81) | T2WI high | 1 g mPSL 3 days→ PSL 40 mg/day a week→ 20 mg→10 mg/day+ 200 mg hydroxychloroquine per day |
good | good | alive | no |
| 2004 | 1) | 57 | M | amnesia, anxiety, depressive state | 119 (MONO 105) | T2WI high, FLAIR high, gadolinium enhanced | mPSL 1 g/day 3 days 2 course →60 mg/day |
good | good | alive | no |
| 2004 | 2) | 62 | M | acalculia, confusion, euphoria, amnesia | 24000 (MONO 21360) | T2WI high, FLAIR high | methylprednisone pulse→ PSL 40 mg/day po |
good | good | alive | no |
| 1995 | 18) | 36 | M | horizontal diplopia | 5 (5 MONO) | T2WI high, gadolinium enhancement | PSL 20 mg taper over 2 weeks→ 30 mg/day→3 months→ 10 mg/day |
good | good | alive | no |
| 1991 | 20) | 64 | M | change in mental status, hallucination | 100 (2PMN, 96 MONO) | nr | PSL 100 mg iv→ 60 mg/day→ +Cyclophosphamide 125 mg/day |
good | good | alive | no |
| 1983 | 22) | 58 | F | unsteady in walking, confused, hallucination, disorientation, nystagmus, facial weakness | nr | nr | PSL 80 mg/day→ dapson 200 mg/day |
good | good | alive | no |
| 2012 | 6) | 60 | M | acalculia, dyslexia, right left agnosia, mild right hemiplesia |
138 (MONO 128 PMN 10) | FLAIR high, enhanced | PSL iv→PSL po 20 mg | good | good | improved | no |
| 1984 | 21) | 51 | M | left facial weakness, ataxia, dementia, confuse | normal | nr | steroid | no | no | alive | no |
| 2011 | 7) | 54 | M | bipolar disorder, fmemory loss, hallucinations, amnesia, disorientation, insomnia, irritability | 800 (MONO 800) | T2WI high | mPSL 1000 mg iv 3 days→ PSL 80 mg/day+AZP |
nr | good | alive | no |
| 2009 | 5) | 29 | M | nr | 32 (PMN 32) | T2WI high, FLAIR high | oral steroid→ azathioprine+adalimumab |
nr | nr | alive | no |
| 2000 | 28) | 75 | F | tremor | nr | T2WI high | nr | nr | nr | alive | no |
| 2008 | 30) | 61 | M | convulsions, dicreased interest, slurred speech, hallucinations, somnolent, rigidity | 312 (MONO 299) | T2WI high, FLAIR high, gadolinuim enhancement | no treatment (supportive therapy alone) | good | good | alive | no |
| 2006 | 27) | 53 | M | cognitive impairment, difficulties with problem solving, amnesia, uncharacteristically aggressive and abusive behavior, disorientation, psychomotor dysfunction | nr | DWI high | palliative care | no (no treatment) | no (no treatment) | died (after 18 months from onset) | no |
As shown in Table 1, 28 out of 31 patients have been treated with a high dose of glucocorticoid.1-22) Twenty-two out of those 28 patients had symptoms which were well-controlled by initial therapy, but only one could discontinue glucocorticoid therapy.19) Six patients were refractory to initial glucocorticoid therapy.3,4,7,9,11,12) Additional therapy (cyclophosphamide, intravenous immune globulin, tacrolimus, plasmapheresis, methotrexate and cyclosporin) showed no remarkable effect and 3 patients died.3,4,7,11,12 Only one who was treated with inflixmab9) had a good outcome, so we chose infliximab as a second-line agent.
This is the first case report of RP with CNS manifestations treated with an anti-TNF-α agent who did not show deterioration of signs and symptoms after stopping therapy.
Infliximab may be a good choice for RP with CNS manifestation refractory to initial glucocorticoid therapy.9) Infliximab has a large molecular weight, so it is impossible for it to permeate the blood-brain barrier. Then why does it work? One potential explanation is that breakdown of the blood-brain barrier by inflammation may permit infliximab to access cerebral parenchyma, resulting in the suppression of TNF-α mediated inflammatory processes.29) Although theoretically it may be reasonable to stop infliximab when neurologic symptoms are stable, if breakdown of the blood-brain barrier by inflammation is important for the effect of infliximab, it would be wise to closely observe the clinical course when discontinuing infliximab.
CONCLUSION
Anti-TNF-α agents may be a treatment of choice for RP with CNS manifestations refractory to initial glucocorticoid therapy. In addition, anti-TNF-α agents may be discontinued, but it would be prudent to closely observe the clinical course when stopping infliximab.
Footnotes
The authors declare no conflict of interest associated with this article.
REFERENCES
- 1).Ohta Y, Nagano I, Niiya D, Fujioka H, Kishimoto T, Shoji M, Abe K. Nonparaneoplastic limbic encephalitis with relapsing polychondritis. J Neurol Sci, 2004; 220: 85–88. [DOI] [PubMed]
- 2).Fujiki F, Tsuboi Y, Hashimoto K, Nakajima M, Yamada T. Non-herpetic limbic encephalitis associated with relapsing polychondritis. J Neurol Neurosurg Psychiatry, 2004; 75: 1646 [DOI] [PMC free article] [PubMed]
- 3).Kashihara K, Kawada S, Takahashi Y. Autoantibodies to glutamate receptor GluRepsilon2 in a patient with limbic encephalitis associated with relapsing polychondritis. J Neurol Sci, 2009; 287: 275–277. [DOI] [PubMed]
- 4).Storey K, Mateˇj R, Rusina R. Unusual association of seronegative, nonparaneoplastic limbic encephalitis and relapsing polychondritis in a patient with history of thymectomy for myasthenia: a case study. J Neurol, 2011; 258: 159–161. [DOI] [PubMed]
- 5).Kumar N, Leep Hunderfund AN, Kutzbach BR, Pulido JS, Miller GM. A Limbic encephalitis MR imaging in a patient with Behçet disease and relapsing polychondritis. Am J Neuroradiol, 2009; 30: e96. [DOI] [PMC free article] [PubMed]
- 6).Fujiwara S, Zenke K, Iwata S, Shouda D, Suehiro S, Kawano Y. Relapsing polychondritis presenting as encephalitis. No Shinkei Geka, 2012; 40: 247–253. [PubMed]
- 7).Wang ZJ, Pu CQ, Wang ZJ, Zhang JT, Wang XQ, Yu SY, Shi Q, Liu JX, Huang XL, Fu CJ, Liu AJ, Huang XS. Meningoencephalitis or meningitis in relapsing polychondritis: four case reports and a literature review. J Clin Neurosci, 2011; 18: 1608–1615. [DOI] [PubMed]
- 8).Choi HJ, Lee HJ. Relapsing polychondritis with encephalitis. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis, 2011; 17: 329–331. [DOI] [PubMed]
- 9).Garcia-Egido A, Gutierrez C, De la Fuente C, Gomez F. Relapsing polychondritis-associated meningitis and encephalitis: response to infliximab. Rheumatol Oxf Engl, 2011; 50: 1721–1723. [DOI] [PubMed]
- 10).Sampaio L, Silva L, Mariz E, Ventura F. Central nervous system involvement in relapsing polychondritis. Jt Bone Spine Rev Rheum, 2010; 77: 619–620. [DOI] [PubMed]
- 11).Imamura E, Yamashita H, Fukuhara T, Nagashima K, Kohriyama T, Tokinobu H. An autopsy case of perivasculitic meningoencephalitis associated with relapsing polychondritis presenting with central nervous system manifestation. Clin Neurol, 2009; 49: 172–178. [DOI] [PubMed]
- 12).Erten-Lyons D, Oken B, Woltjer RL, Quinn J. Relapsing polychondritis: an uncommon cause of dementia. J Neurol Neurosurg Psychiatry, 2008; 79: 609–610. [DOI] [PMC free article] [PubMed]
- 13).Fujioka S, Tsuboi Y, Mikasa M, Onozawa R, Saitoh N, Baba Y, Yamada T. A case of encephalitis lethargica associated with relapsing polychondritis. Mov Disord, 2008; 23: 2421–2423. [DOI] [PubMed]
- 14).Kao KT, Potrebic S, Evans JR. Relapsing polychondritis presenting as meningoencephalitis with valvular abnormality: a case report. Clin Rheumatol, 2007; 26: 1985–1988. [DOI] [PubMed]
- 15).Hsu K-C, Wu Y-R, Lyu R-K, Tang L-M. Aseptic meningitis and ischemic stroke in relapsing polychondritis. Clin Rheumatol, 2006; 25: 265–267. [DOI] [PubMed]
- 16).Yang S-M, Chou C-T. Relapsing polychondritis with encephalitis: JCR J Clin Rheumatol, 2004; 10: 83–85. [DOI] [PubMed]
- 17).Gertner E. Severe recurrent neurological disease in the MAGIC syndrome. J Rheumatol, 2004; 31: 1018–1019. [PubMed]
- 18).Massry GG, Chung SM, Selhorst JB. Optic neuropathy, headache, and diplopia with MRI suggestive of cerebral arteritis in relapsing polychondritis. J Neuro-Ophthalmol, 1995; 15: 171–175. [PubMed]
- 19).Wasserfallen JB, Schaller MD. Unusual rhombencephalitis in relapsing polychondritis. Ann Rheum Dis, 1992; 51: 1184. [DOI] [PMC free article] [PubMed]
- 20).Schindzielorz A, Edberg SC, Bia FJ. Strongyloides stercoralis hyperinfection and central nervous system involvement in a patient with relapsing polychondritis. South Med J, 1991; 84: 1055–1057. [DOI] [PubMed]
- 21).Hull RG, Morgan SH. The nervous system and relapsing polychondritis. Neurology, 1984; 34: 557. [DOI] [PubMed]
- 22).Sundaram MB, Rajput AH. Nervous system complications of relapsing polychondritis. Neurology, 1983; 33: 513–515. [DOI] [PubMed]
- 23).McAdam LP, O’Hanlan MA, Bluestone R, Pearson CM. Relapsing polychondritis: prospective study of 23 patients and a review of the literature. Medicine (Baltimore), 1976; 55: 193–215. [PubMed]
- 24).Damiani JM, Levine HL. Relapsing polychondritis--report of ten cases. Laryngoscope, 1979; 89: 929–946. [PubMed]
- 25).Letko E, Zafirakis P, Baltatzis S, Voudouri A, Livir-Rallatos C, Foster CS. Relapsing polychondritis: a clinical review. Semin Arthritis Rheum, 2002; 31: 384–395. [DOI] [PubMed]
- 26).Ochi M, Kawajiri M, Igase M, Takada K, Kohara K, Miki T. Case of relapsing polychondritis followed by cognitive impairment. Rinshoˉ Shinkeigaku Clin Neurol, 2007; 47: 353–355. [PubMed]
- 27).Yan M, Cooper W, Harper C, Schwartz R. Dementia in a patient with non-paraneoplastic limbic encephalitis associated with relapsing polychondritis. Pathology (Phila), 2006; 38: 596–599. [DOI] [PubMed]
- 28).Dreher A, Aigner J, Fuchshuber S, Kastenbauer E. Relapsing polychondritis: a course over 20 years with cerebral involvement. Arch Otolaryngol Head Neck Surg, 2000; 126: 1495–1498. [DOI] [PubMed]
- 29).Pipitone N, Olivieri I, Padula A, D’angelo S, Nigro A, Zuccoli G, Boiardi L, Salvarani C. Infliximab for the treatment of Neuro-Behçet’s disease: a case series and review of the literature. Arthritis Rheum, 2008; 59: 285–290. [DOI] [PubMed]
- 30).Kuwabara M, Shimono T, Toyomasu M, Shioyama M, Mitsui Y, Yoshinaga E, Kawada A, Hosono M, Murakami T, Kusunoki S. "Prominent ear sign" on diffusion-weighted magnetic resonance imaging in relapsing polychondritis. Radiat Med, 2008; 26: 438–441. [DOI] [PubMed]