ABSTRACT
Glial cells were investigated to elucidate their involvement in mechanisms underlying oral cancer pain. Squamous cell carcinoma (SCC-158) was inoculated into the lower gingiva of male Fisher rats. Pharmacological and immunohistochemical studies were performed to examine the roles played by TRPV1 and TRPV2 expressed in neurons and satellite glia in trigeminal ganglia (TG), and microglia and astrocytes in trigeminal spinal nucleus caudalis. Inoculation of SCC-158 into the lower gingiva induced marked mechanical allodynia in the whisker-pad skin area on days 16 through 28, and in the submandibular skin area on days 10 through 20. Cutaneous allodynia was diminished by systemic morphine administration. The number of TRPV1 and TRPV2-positive neurons in trigeminal ganglia increased in the medium and large cell groups on day 14 after tumor inoculation. The number of satellite glial cells encircling the medium and large trigeminal ganglion neurons increased on day 28 after tumor inoculation. In this gingival cancer pain model, microglia and astrocytes in trigeminal spinal nucleus caudalis were not activated, although they were reported to be activated in neuropathic and inflammatory pain models. These results suggest that TRPV1 and TRPV2 upregulation in trigeminal ganglion neurons may play an important role in inducing the mechanical allodynia observed in experimental models of oral squamous cell carcinoma. In addition, activation of satellite cells seems to be involved in the maintenance of mechanical allodynia, which could be the potential therapeutic target for oral cancer pain.
Key Words: Oral cancer, GFAP, TRPV1, TRPV2, Hyperalgesia
INTRODUCTION
Pain due to malignant tumors occurs in cancer patients. Oral cancer pain impairs a patient’s speech, swallowing, eating, drinking, and interpersonal relations, and has a significant impact on his or her overall quality of life.1) Most oral cancers are squamous cell carcinomas generated from oral mucosal epithelium. However, previous studies have scantly reported on the mechanism of pain using models of squamous cell carcinoma, except for our series of studies which have suggested several phenotypic changes associated with cancer pain in primary afferent neurons.2-4)
Recent studies have exposed the critical importance of glial cells for a variety of biological functions, including pain perception and modulation.5) Astrocytes and microglia in the spinal and medullary dorsal horn participate in the initiation and maintenance of persistent pain induced by tissue inflammation, nerve injury, and cancer, by the release of various chemical mediators. In addition, the role of satellite glial cells (SGCs) of sensory ganglia in chronic pain has begun to draw interest. SGCs are activated in animal models of both neuropathic and inflammatory pain.5) Activated satellite glial cells release several inflammatory and immune mediators in response to inflammation and nerve damage, and influence other sensory neurons within the ganglion.6-10) The objective of our study was to determine the involvement of glia cells in the CNS and PNS in cancer pain in a rat model of oral squamous cell carcinoma, a disease known to cause significant morbidity and mortality.
MATERIALS AND METHODS
Animals
Seventy-nine male Fisher rats (SLC, Hamamatsu, Japan) weighing around 250 g were used in this study. This investigation was conducted under the auspices of the local animal ethics committee in accordance with the Regulations for Animal Experiments of Nagoya University (permission No. 25352), the Animal Protection and Management Law of the Japanese Government (No. 105), and the Ethical Issues of the International Association for the Study of Pain.11)
Inoculation of tumor cells
The SCC-158 cells (squamous cell carcinoma derived from the external acoustic meatus of the Fisher rat, JCRB, Tokyo, Japan) were prepared as in a previous study.3) Under anesthesia with an intraperitoneal injection of pentobarbital (50 mg/kg, Nembutal, Abbot Laboratories, Chicago, IL, USA), the tumor cells (2.5 × 106 cells in 0.025 mL saline) were inoculated into the subperiosteal tissue of the rats on the lateral side of the lower gingiva with a 24 gauge needle. Control groups were injected with 0.025 mL saline.
Assessment of mechanical sensitivity
Mechanical sensitivity of the facial skin was assessed by the use of von Frey hairs (North Coast Medical Inc., Morgan Hill, CA, USA) with a group of SCC-158 injected animals and a control groups as in a previous study.3) The von Frey filaments were applied to the forehead (ophthalmic nerve area), whisker-pad (maxillary nerve area), and submandibular (mandibular nerve area) skin areas. The frequency of nociceptive response (head withdrawal or vocalization) was counted from 5 trials. The nociceptive threshold was defined as the minimum pressure needed to evoke nociceptive responses in at least 60% of the trials.
Intraperitoneal administration of morphine
Animals were tested with morphine (i.p.) to see whether the alteration in mechanical sensitivity assessed by von Frey hairs reflected mechanical hyperalgesia. Either vehicle or morphine (5, 7.5, 10 mg/kg) was intraperitoneally injected into tumor inoculation or control animals. Assessments of mechanical sensitivity for submandibular (on days 12 through 16), and whisker-pad skin areas (on days 19 through 23) were determined both before and 30 minutes after morphine treatment.
Tissue preparation and immunohistochemistry
Rats were perfused with physiological salines followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) 14 days and 28 days after either tumor cell inoculation or saline injection, under deep anesthesia with pentobarbital (i.p. 50 mg/kg). Frontal sections (15 μm thickness) of mandibles were stained by hematoxylin-eosin.
For immunohistochemical staining of the trigeminal ganglion (TG), serial cryostat sections of TG (10 µm thickness) were reacted with either rabbit anti-GFAP anti-serum (1:2,000; DAKO, Kyoto, Japan), rabbit anti-TRPV1 anti-serum (1:1,000: Trans Genic Inc., Kumamoto, Japan), or rabbit anti-TRPV2 anti-serum (1:1,000: Sigma, St. Louis, Mo, USA) after dilution in 0.1 mol/L PBS containing 4% normal goat serum and 0.3% Triton-X 100 (Sigma). GFAP is a marker for satellite glial cells. TRPV1 and TRPV2 are transducer molecules involved in the generation of pain sensation. Sections were then reacted with biotinylated goat antirabbit IgG (Vector Laboratories, Burlingame, CA, USA) and avidin andbiotin-peroxidase complex (Vector Laboratories), and visualized with 0.1% 3.3’-diaminobenzidine tetrahydrochloride (Sigma).
For immunohistochemical staining of the trigeminal spinal nucleus, serial frozen transverse sections (30 µm thickness) of the caudal medulla and upper cervical spinal cord were made from 2 mm rostral through 6 mm caudal to the obex. The free-floating sections were reacted with either rabbit anti-Iba1 anti-serum (1:1,000; WAKO, Osaka, Japan) or rabbit anti-GFAP anti-serum (1:5,000; DAKO, Kyoto, Japan), to label microglia and astrocytes, respectively. Sections were then incubated with biotinylated goat antirabbit IgG (Vector Laboratories), avidins, and biotinylated HRP complex (Vector Laboratories), and visualized with 0.1% DAB (Sigma).
The neurons framed by GFAP-positive satellite cells were evaluated. A neuron framed by GFAP-ir SGCs was counted as positive if more than half of its circumference was framed by GFAP-ir SGCs. TRPV1-positive neurons and TRPV2-positive neurons were also counted, but only those neurons with visible nuclei. The area of neurons was measured by a computer-aided image-analyzing system (NeuroLucida; Microbrightfield, Colchester, VT, USA). Adjacent sections were compared to examine whether neurons framed by GFAP-ir SGCs expressed TRPV1 or TRPV2.
The area of either GFAP-positive astrocytes or Iba1-positive microglia in a given area (680 × 512 pixels: 1 pixel = 0.38 µm2) of a trigeminal spinal nucleus was calculated using a computer-assisted image analyzing system (ImageJ software, ver.1.38, National Institutes of Health, USA).
Statistical analysis
Statistical analyses were performed by one-way or two-way analysis of variance with repeated or non-repeated measures, followed by Holm-Sidak multiple comparison tests if warranted, or Student’s t-test where appropriate. Data from the von Frey test were analyzed using nonparametric two-way repeated-measures analysis of variance on ranks, followed by a Holm-Sidak comparison if warranted. A P value of < 0.05 was considered significant.
RESULTS
Tumor histology
Similar to a previous study,3) SCC-158 cells produced mandibular bone degeneration fourteen days after inoculation. Twenty-eight days after inoculation, the inferior alveolar nerve was surrounded by invaded tumor tissue (date not shown).
Nociceptive responses after tumor cell inoculation
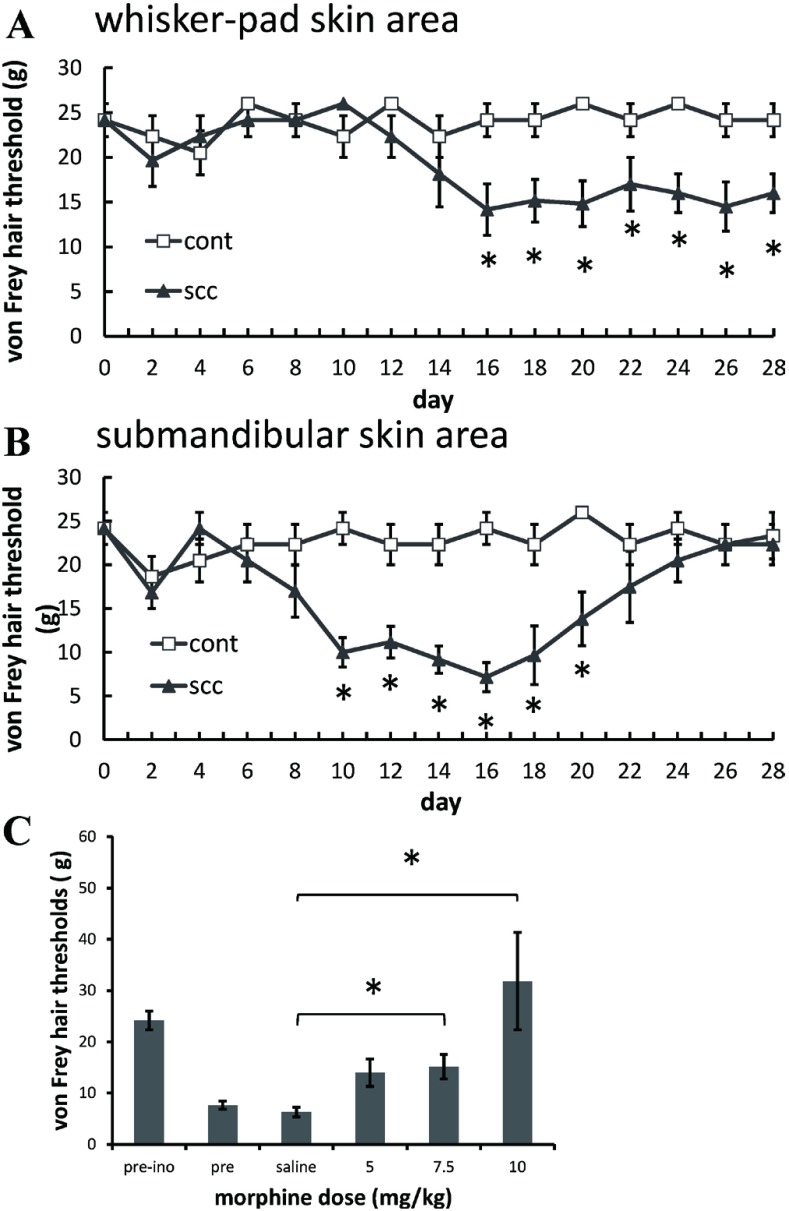
In the ipsilateral whisker-pad skin area, the withdrawal threshold of von Frey filament testing showed a significant decrease 16 through 28 days after the inoculation of tumor cells, compared with saline-injected control animals, indicating the development of mechanical allodynia (Figure 1A). In the ipsilateral submandibular skin area, the withdrawal threshold showed a significant decrease compared to the saline-injected control group on post inoculation days 10 through 20 (Figure 1B). In the forehead skin area, the withdrawal threshold showed no significant changes after the injection of either tumor cells or saline (data not shown).
Fig. 1.
Cutaneous allodynia observed in both whisker-pad skin (A) and submandibular skin (B) areas in rats inoculated with squamous cell carcinoma (SCC-158). (C) Effect of vehicle or morphine (5, 7.5 and 10 mg/kg i.p.) on mechanical allodynia observed in submandibular skin area 2 weeks after inoculation of tumor cells. *P < .05.
Effects of morphine
Intraperitoneal morphine (7.5, 10 mg/kg) significantly increased the von Frey hair thresholds of the tumor-inoculated submandibular skin area (Figure 1C) and whisker-pad skin area compared with saline treatment (data not shown).
Activation of SGCs in trigeminal ganglion
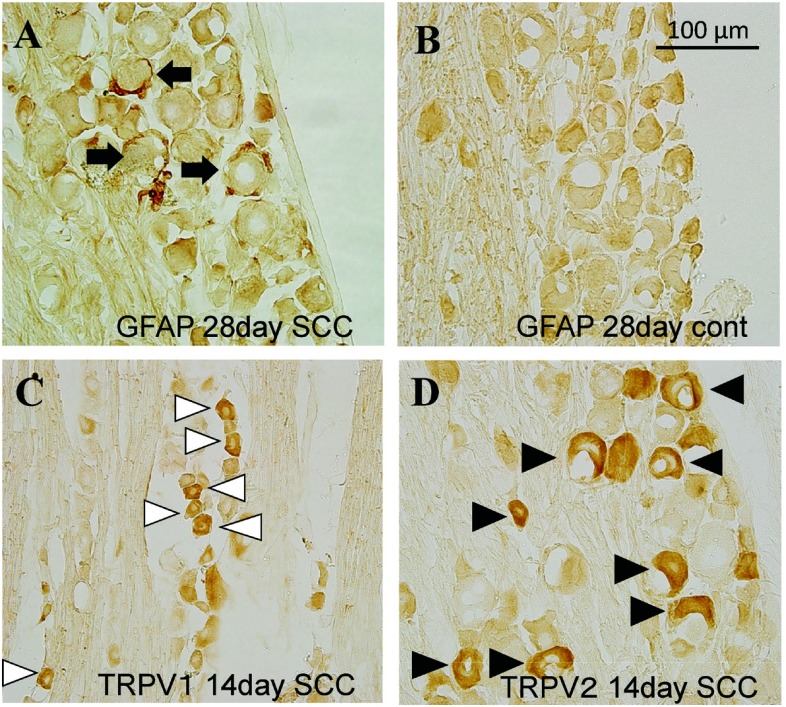
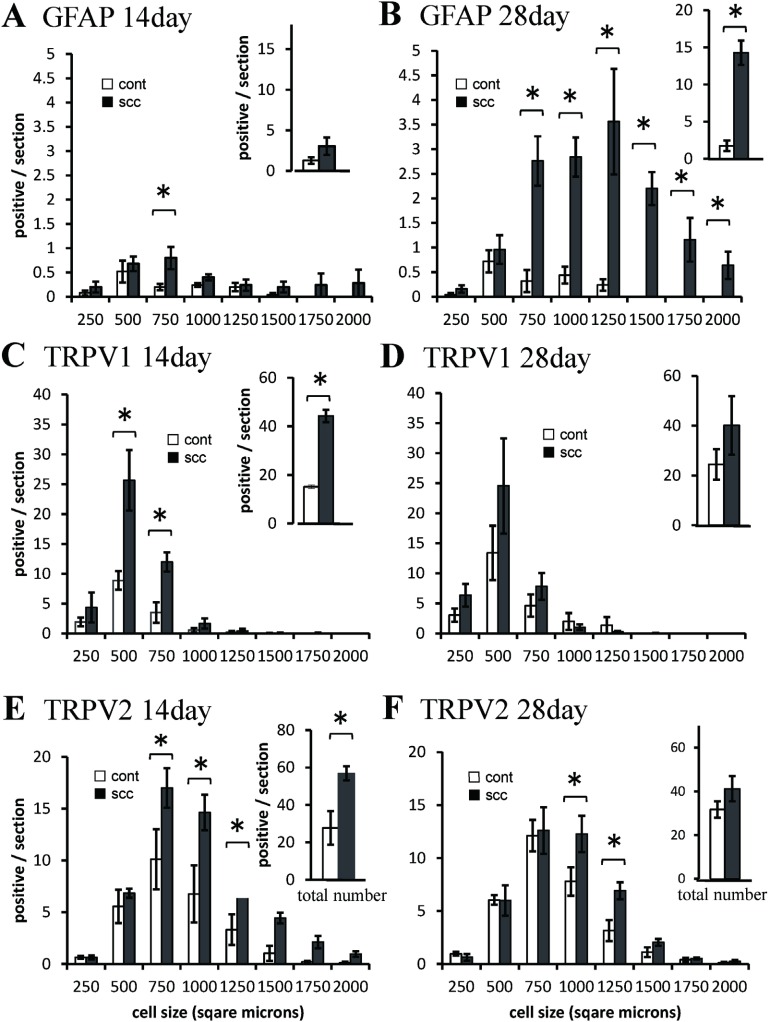
SGC activation in trigeminal ganglion was observed in tumor inoculated animals (Figure 2A). Neurons framed by GFAP-positive satellite cells slightly increased in the medium cell groups (500–750 µm2) 14 days after tumor inoculation (Figure 3A), and significantly increased in the medium and large cell groups (>500 µm2) on day 28 (Figure 3B). In total count, neurons framed by GFAP-positive satellite cells significantly increased on day 28 after tumor inoculation (Figure 3B).
Fig. 2.
GFAP (A, B), TRPV1 (C) and TRPV2 (D) -positive cells in trigeminal ganglion 14 days (C, D) and 28 days (A, B) after tumor innoculation. Black arrows: GFAP immunoreactive cells. White arrowheads: TRPV1 immunoreactive cells. Black arrowheads: TRPV2 immunoreactive cells.
Fig. 3.
Size-frequency histogram illustrating distribution and total number of neurons framed by GFAP positive satellite cells (A, B), TRPV1-positive cells (C, D), and TRPV2-positive cells (F, F) in the trigeminal ganglion, 14 days (A, C, E) and 28 days afterinoculation of tumor cells (B, D, F), compared with saline-injected control animals. *P < .05.
TPRV1-expression in TG
TRPV1 was expressed in small size neurons in TG, consistent with a previous study3) (Figure 2C). TRPV1-positive cells significantly increased in the small and medium cell groups (250–500 µm2 and 500–750 µm2) on day 14 after tumor inoculation (Figure 3C). In total count the TRPV1-positive cells significantly increased 14 days after tumor inoculation, but not on day 28 (Figure 3C, D).
TRPV2-expression in TG
TRPV2 was expressed in small, medium, and large size neurons in TG (Figure 2D). The TRPV2-positive cells remarkably increased in the medium and large cell groups (500–750 µm2, 750–1000 µm2, and 1000–1250 µm2) on day 14 (Figure 3E), and slightly increased 28 days after tumor inoculation (Figure 3F). In total count the TRPV2-positive cells significantly increased 14 days after tumor inoculation, but not 28 days after (Figure 3E, F).
Adjacent sections were compared to examine TRPV1 and TRPV2-expression in neurons framed by GFAP-positive satellite cells in TG. There was no TRPV1-expression in neurons framed by GFAP-positive satellite cells at 14 and 28 days after tumor inoculation. A few neurons framed by GFAP-positive SGCs expressed TRPV2 on day 14 (0.56±0.56%) and day 28 (5.98±2.68%).
Astrocytes and microglia in trigeminal spinal nucleus
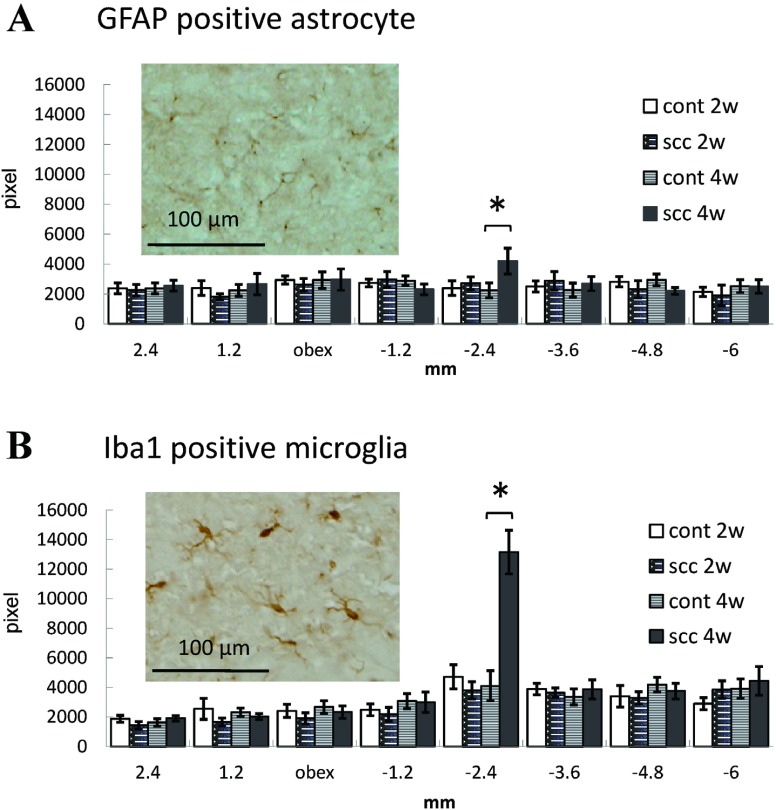
The total area of both GFAP-positive astrocytes and Iba1-positive microglia showed no changes on days 14 and 28, except at –2.4 mm from the obex on day 28 (Figure 4A, B).
Fig. 4.
Alteration of GFAP-positive astrocytes and Iba1-positive microglia in the ipsilateral trigeminal spinal nucleus after tumor inoculation into the lower gingiva. (A): The total area of GFAP-positive astrocytes in a given area identified with an altitude (rostral-caudal +2.4 mm, +1.2 mm, 0 mm, –1.2 mm, –2.4 mm, –3.6 mm, –4.8 mm, –6.0 mm from obex) of trigeminal spinal nucleus. Photograph shows GFAP-positive astrocytes in the trigeminal spinal nucleus 28 days after tumor inoculation. (B): The total area of Iba-1-positive microglia in trigeminal spinal nucleus and photograph of Iba-1-positive microglia. *P < .05.
DISCUSSION
MODEL OF ORAL CANCER PAIN
In this study, tumor growth after the inoculation of SCC cells into the lower gingiva produced mechanical allodynia of both whisker-pad skin (maxillary nerve area) and submandibular skin (mandibular nerve area).
The mechanical allodynia was suppressed by 7.5 mg/kg morphine, the dose at which normal mechanical sensitivity is not affected. These findings suggested that the decreased von Frey threshold observed was an indicator of mechanical allodynia induced by the earlier inoculation of squamous cell carcinoma cells (SCC-158) into the lower gingiva.
Glial changes in trigeminal ganglion
Satellite glial cells of sensory ganglia participate in the development and maintenance of chronic pain conditions.5) Following stimulation of neurons in the dorsal root ganglia (DRG), SGCs wrapped around neuronal bodies became activated and increased neuronal excitability by releasing inflammatory mediators such as tumor necrosis factor-α (TNFα).12,13) SGCs in DRGs and TGs are tightly associated with sensory neurons via gap junction, and gap junction communication between SGCs and neurons is greatly enhanced in persistent pain conditions.7,8,14)
In the present study of TG, GFAP-positive satellite cells encircling neurons slightly increased on day 14 after tumor inoculation, when apparent mechanical allodynia was observed in the submandibular skin area. The satellite cells encircling medium and large neurons strikingly increased on day 28 after tumor inoculation, when mechanical allodynia was observed in the whisker-pad skin area. This suggested that activation of GFAP-positive SGCs might be involved in mechanical allodynia not only in the submandibular area, but also in the maxillary nerve area where the tumor was not inoculated. As large size neurons were mostly surrounded by the activated GFAP-positive satellite cells, they might have some roles in maintaining mechanical allodynia through activation of tactile neurons.15)
Increased expression of TRPV1 and TRPV2 in TG neuron
The transient receptor potential vanilloid 1 (TRPV1) and its homologue TRPV2, which belong to the TRPV subfamily of the large TRP ion channel super family, are critical contributors to normal and pathological pain.16) In a model of bone cancer pain, administration of a TRPV1 antagonist or disruption of the TRPV1 gene was reported to result in a significant attenuation of both ongoing and movement-evoked nocifensive behaviors.17) In the hind-paw model of SCC, the TRPV1-expressing neurons increased in the small-, medium-, and large-cell groups in the dorsal root ganglia (DRGs), and administration of the TRPV1 antagonist inhibited hyperalgesic response.2,4)
In our study, we observed hyperalgesia in the submandibular skin area on day 14 and in the whisker-pad skin area 28 days after tumor inoculation. Fourteen days after tumor inoculation, medium and small cells expressed TRPV1, and medium and large cells expressed TRPV2 significantly more in TG, suggesting that TRPV1 and TRPV2 upregulation in neurons may play an important role in inducing mechanical allodynia in submandibular skin. On day 28 after tumor inoculation, upregulation of TRPV1 and TRPV2 did not have a significant total number. TRPV1 and TRPV2 expression may not have had an important role in maintaining the mechanical allodynia observed in this model. GFAP-positive satellite cells encircling neurons significantly increased on day 28, when upregulation of TRPV1 and TRPV2 were no longer apparent. Immunohistochemical study using serial sections revealed that no TRPV1-expressed neurons and only a few TRPV2-expressed neurons were framed by GFAP-positive satellite cells after tumor inoculation. These results suggested that both activation of GFAP-positive satellite cells and upregulation of TRPV1/2 were independently involved in mechanical allodynia observed in this model.
Glial changes in trigeminal spinal nucleus
During pathological pain states including cancer pain, CNS microglia and/or astrocytes become active and release proinflammatory signals which are responsible for the hyperexcitability of nociceptive pathways, leading to the development of hyperalgesia and allodynia. The pharmacological inhibition of glial cell function effectively attenuates the development of neuropathic, inflammatory and cancer pain. In the present study, the apparent activation of microglia and astrocytes, which occurred commonly in the trigeminal spinal nucleus of inflammatory, neuropathic and cancer pain models,18-20) was not developed. Twenty-eight days after tumor inoculation, activation of microglia and astrocytes developed in the caudal part of the trigeminal spinal nucleus caudalis (–2.4 mm from the obex), which was involved in nociception in the mental cutaneous area. Glial activation was not observed in the trigeminal spinal nucleus interpolaris and caudalis transition zone at the periobex level, which were involved instead in nociception in lower molar teeth and gum.21) The activation of microglia and astrocytes –2.4 mm from the obex might therefore be produced by cutaneous rupture associated with tumor development. Femoral bone cancer pain and sciatic nerve proximal cancer pain in mice resulted in severe spinal astrogliosis without activation of microglia.22) Furthermore, a rat bone cancer pain model produced by injecting rat mammary gland carcinoma cells into the tibia showed no activation of either microglia or astrocytes.23) The response of glia in CNS might differ depending on animal species, the type of tumor, and its location.
In the present study, inoculating SCC into the lower gingiva induced mechanical allodynia in the facial skin area. In addition to the increase of TRPV1 and TRPV2 in TG after tumor inoculation, we could see activation of GFAP-positive satellite cells in TG at a later stage. GFAP-positive satellite cells seem to be involved in the maintenance of mechanical allodynia associated with inoculation of SCC into the lower gingiva.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Masamichi Shinoda for his valuable suggestions. They have no conflicts of interest.
REFERENCES
- 1).Viet CT, Schmidt BL. Biologic mechanisms of oral cancer pain and implications for clinical therapy. J Dent Res, 2012; 91: 447–453. [DOI] [PMC free article] [PubMed]
- 2).Shinoda M, Ogino A, Ozaki N, Urano H, Hironaka K, Yasui M, Sugiura Y. Involvement of TRPV1 in nociceptive behavior in a rat model of cancer pain. J Pain, 2008; 9: 687–699. [DOI] [PubMed]
- 3).Nagamine K, Ozaki N, Shinoda M, Asai H, Nishiguchi H, Mitsudo K, Tohnai I, Ueda M, Sugiura Y. Mechanical allodynia and thermal hyperalgesia induced by experimental squamous cell carcinoma of the lower gingiva in rats. J Pain, 2006; 7: 659–670. [DOI] [PubMed]
- 4).Asai H, Ozaki N, Shinoda M, Nagamine K, Tohnai I, Ueda M, Sugiura Y. Heat and mechanical hyperalgesia in mice model of cancer pain. Pain, 2005; 117: 19–29. [DOI] [PubMed]
- 5).Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain, 2013; 154 Suppl 1: S10–28. [DOI] [PMC free article] [PubMed]
- 6).Chen Y, Zhang X, Wang C, Li G, Gu Y, Huang LY. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc Natl Acad Sci U S A, 2008; 105: 16773–16778. [DOI] [PMC free article] [PubMed]
- 7).Dublin P, Hanani M. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav Immun, 2007; 21: 592–598. [DOI] [PubMed]
- 8).Hanani M, Huang TY, Cherkas PS, Ledda M, Pannese E. Glial cell plasticity in sensory ganglia induced by nerve damage. Neuroscience, 2002; 114: 279–283. [DOI] [PubMed]
- 9).Jasmin L, Vit JP, Bhargava A, Ohara PT. Can satellite glial cells be therapeutic targets for pain control? Neuron Glia Biol, 2010; 6: 63–71. [DOI] [PMC free article] [PubMed]
- 10).Liu FY, Sun YN, Wang FT, Li Q, Su L, Zhao ZF, Meng XL, Zhao H, Wu X, Sun Q, Xing GG, Wan Y. Activation of satellite glial cells in lumbar dorsal root ganglia contributes to neuropathic pain after spinal nerve ligation. Brain Res, 2012; 1427: 65–77. [DOI] [PubMed]
- 11).Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain, 1983; 16: 109–110. [DOI] [PubMed]
- 12).Pannese E. The structure of the perineuronal sheath of satellite glial cells (SGCs) in sensory ganglia. Neuron Glia Biol, 2010; 6: 3–10. [DOI] [PubMed]
- 13).Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A, 2007; 104: 9864–9869. [DOI] [PMC free article] [PubMed]
- 14).Hanani M. Intercellular communication in sensory ganglia by purinergic receptors and gap junctions: implications for chronic pain. Brain Res, 2012; 1487: 183–191. [DOI] [PubMed]
- 15).Ichikawa H, Mo Z, Xiang M, Sugimoto T. Effect of Brn-3a deficiency on nociceptors and low-threshold mechanoreceptors in the trigeminal ganglion. Brain Res Mol Brain Res, 2002; 104: 240–245. [DOI] [PubMed]
- 16).Sousa-Valente J, Andreou AP, Urban L, Nagy I. Transient receptor potential ion channels in primary sensory neurones as targets for novel analgesics. Br J Pharmacol, 2013 Nov 27. [DOI] [PMC free article] [PubMed]
- 17).Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE, Carruthers NI, Swanson D, Kuskowski M, Flores CM, Julius D, Mantyh PW. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci, 2005; 25: 3126–3131. [DOI] [PMC free article] [PubMed]
- 18).Hidaka K, Ono K, Harano N, Sago T, Nunomaki M, Shiba S, Nakanishi O, Fukushima H, Inenaga K. Central glial activation mediates cancer-induced pain in a rat facial cancer model. Neuroscience, 2011; 180: 334–343. [DOI] [PubMed]
- 19).Villa G, Ceruti S, Zanardelli M, Magni G, Jasmin L, Ohara PT, Abbracchio MP. Temporomandibular joint inflammation activates glial and immune cells in both the trigeminal ganglia and in the spinal trigeminal nucleus. Mol Pain, 2010; 6: 89. [DOI] [PMC free article] [PubMed]
- 20).Dauvergne C, Molet J, Reaux-Le Goazigo A, Mauborgne A, Mélik-Parsadaniantz S, Boucher Y, Pohl M. Implication of the chemokine CCL2 in trigeminal nociception and traumatic neuropathic orofacial pain. Eur J Pain, 2013 Aug 5. [DOI] [PubMed]
- 21).Noma N, Tsuboi Y, Kondo M, Matsumoto M, Sessle BJ, Kitagawa J, Saito K, Iwata K. Organization of pERK-immunoreactive cells in trigeminal spinal nucleus caudalis and upper cervical cord following capsaicin injection into oral and craniofacial regions in rats. J Comp Neurol, 2008 Mar 20; 507(3): 1428–1440. [DOI] [PubMed]
- 22).Hald A, Nedergaard S, Hansen RR, Ding M, Heegaard AM. Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur J Pain, 2009; 13: 138–145. [DOI] [PubMed]
- 23).Ducourneau VR, Dolique T, Hachem-Delaunay S, Miraucourt LS, Amadio A, Blaszczyk L, Jacquot F, Ly J, Devoize L, Oliet SH, Dallel R, Mothet JP, Nagy F, Fénelon VS, Voisin DL. Cancer pain is not necessarily correlated with spinal overexpression of reactive glia markers. Pain, 2014; 155: 275–291. [DOI] [PubMed]