Abstract
Migrating Schwann cells in developing or regenerating peripheral nerves are known to express dramatically increased levels of nerve growth factor (NGF) and the low-affinity NGF receptor (LNGFR). Schwann cells do not express detectable pp140trk, the NGF-activated receptor tyrosine kinase which is essential for neuronal responses to NGF. The temporal correlation observed in Schwann cells between migration and the enhanced expression of NGF and LNGFR suggests that NGF and LNGFR may promote Schwann cell migration. To test this possibility, we examined the effects of NGF on Schwann cell migration on cryostat sections of biologically relevant NGF-poor and NGF-rich substrates--normal or denervated peripheral (sciatic) nerve, untreated or pretreated with NGF. Results show that Schwann cells migrate more rapidly on denervated than on normal sciatic nerve. Antibodies to NGF or to LNGFR strongly, but incompletely, inhibit enhanced migration on denervated nerves. Pretreatment of denervated nerve sections with NGF increases further the rate of Schwann cell migration. The same antibodies to NGF or to LNGFR abolish this response. These results suggest that one function of the elevated levels of NGF known to be present in embryonic and regenerating peripheral nerves is to promote the migration of Schwann cells. In contrast to neurons, where pp140trk appears to be the functionally critical NGF receptor, NGF responses in Schwann cells depend on LNGFR.
Full text
PDF

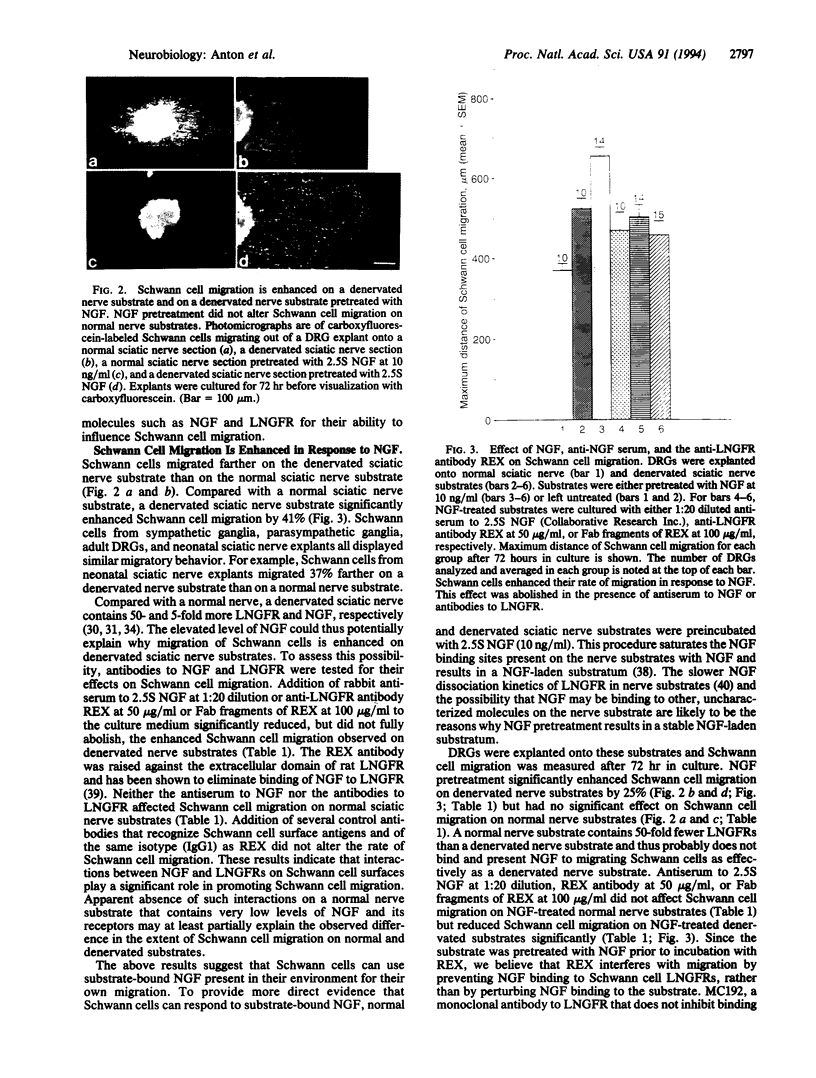


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assouline J. G., Pantazis N. J. Localization of the nerve growth factor receptor on fetal human Schwann cells in culture. Exp Cell Res. 1989 Jun;182(2):499–512. doi: 10.1016/0014-4827(89)90253-x. [DOI] [PubMed] [Google Scholar]
- Bailey S. B., Eichler M. E., Villadiego A., Rich K. M. The influence of fibronectin and laminin during Schwann cell migration and peripheral nerve regeneration through silicon chambers. J Neurocytol. 1993 Mar;22(3):176–184. doi: 10.1007/BF01246356. [DOI] [PubMed] [Google Scholar]
- Bandtlow C. E., Heumann R., Schwab M. E., Thoenen H. Cellular localization of nerve growth factor synthesis by in situ hybridization. EMBO J. 1987 Apr;6(4):891–899. doi: 10.1002/j.1460-2075.1987.tb04835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Van Evercooren A., Kleinman H. K., Seppä H. E., Rentier B., Dubois-Dalcq M. Fibronectin promotes rat Schwann cell growth and motility. J Cell Biol. 1982 Apr;93(1):211–216. doi: 10.1083/jcb.93.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings-Gagliardi S., Webster H. F., O'Connell M. F. In vivo and electron microscopic observations on Schwann cells in developing tadpole nerve fibers. Am J Anat. 1974 Nov;141(3):375–391. doi: 10.1002/aja.1001410308. [DOI] [PubMed] [Google Scholar]
- Blakemore W. F., Franklin R. J. Transplantation of glial cells into the CNS. Trends Neurosci. 1991 Aug;14(8):323–327. doi: 10.1016/0166-2236(91)90155-n. [DOI] [PubMed] [Google Scholar]
- Bradley W. G., Jaros E. Involvement of peripheral and central nerves in murine dystrophy. Ann N Y Acad Sci. 1979;317:132–142. [PubMed] [Google Scholar]
- Chandler C. E., Parsons L. M., Hosang M., Shooter E. M. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J Biol Chem. 1984 Jun 10;259(11):6882–6889. [PubMed] [Google Scholar]
- Cravioto H. The role of Schwann cells in the development of human peripheral nerves. An electron microscopic study. J Ultrastruct Res. 1965 Jun;12(5):634–651. doi: 10.1016/s0022-5320(65)80053-3. [DOI] [PubMed] [Google Scholar]
- Curtis R., Stewart H. J., Hall S. M., Wilkin G. P., Mirsky R., Jessen K. R. GAP-43 is expressed by nonmyelin-forming Schwann cells of the peripheral nervous system. J Cell Biol. 1992 Mar;116(6):1455–1464. doi: 10.1083/jcb.116.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniloff J. K. A novel assay for the in vivo study of Schwann cells. Exp Neurol. 1991 Oct;114(1):140–143. doi: 10.1016/0014-4886(91)90092-q. [DOI] [PubMed] [Google Scholar]
- Davis J. B., Stroobant P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells. J Cell Biol. 1990 Apr;110(4):1353–1360. doi: 10.1083/jcb.110.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano P. S., Johnson E. M., Jr Nerve growth factor receptors on cultured rat Schwann cells. J Neurosci. 1988 Jan;8(1):231–241. doi: 10.1523/JNEUROSCI.08-01-00231.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein D. L., Larhammar D. Identification of a conserved protein motif in a group of growth factor receptors. FEBS Lett. 1990 Oct 15;272(1-2):7–11. doi: 10.1016/0014-5793(90)80437-n. [DOI] [PubMed] [Google Scholar]
- Feneley M. R., Fawcett J. W., Keynes R. J. The role of Schwann cells in the regeneration of peripheral nerve axons through muscle basal lamina grafts. Exp Neurol. 1991 Dec;114(3):275–285. doi: 10.1016/0014-4886(91)90153-4. [DOI] [PubMed] [Google Scholar]
- Fields R. D., Le Beau J. M., Longo F. M., Ellisman M. H. Nerve regeneration through artificial tubular implants. Prog Neurobiol. 1989;33(2):87–134. doi: 10.1016/0301-0082(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Franz T. Defective ensheathment of motoric nerves in the Splotch mutant mouse. Acta Anat (Basel) 1990;138(3):246–253. doi: 10.1159/000146947. [DOI] [PubMed] [Google Scholar]
- Griffin J. W., Drucker N., Gold B. G., Rosenfeld J., Benzaquen M., Charnas L. R., Fahnestock K. E., Stocks E. A. Schwann cell proliferation and migration during paranodal demyelination. J Neurosci. 1987 Mar;7(3):682–699. doi: 10.1523/JNEUROSCI.07-03-00682.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guénard V., Kleitman N., Morrissey T. K., Bunge R. P., Aebischer P. Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J Neurosci. 1992 Sep;12(9):3310–3320. doi: 10.1523/JNEUROSCI.12-09-03310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. M. Regeneration in cellular and acellular autografts in the peripheral nervous system. Neuropathol Appl Neurobiol. 1986 Jan-Feb;12(1):27–46. doi: 10.1111/j.1365-2990.1986.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Hall S. M. The effect of inhibiting Schwann cell mitosis on the re-innervation of acellular autografts in the peripheral nervous system of the mouse. Neuropathol Appl Neurobiol. 1986 Jul-Aug;12(4):401–414. doi: 10.1111/j.1365-2990.1986.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Heumann R., Korsching S., Bandtlow C., Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987 Jun;104(6):1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R., Lindholm D., Bandtlow C., Meyer M., Radeke M. J., Misko T. P., Shooter E., Thoenen H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8735–8739. doi: 10.1073/pnas.84.23.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Jr, Taniuchi M., DiStefano P. S. Expression and possible function of nerve growth factor receptors on Schwann cells. Trends Neurosci. 1988 Jul;11(7):299–304. doi: 10.1016/0166-2236(88)90090-2. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Hempstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991 Apr 26;252(5005):554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Klein R., Conway D., Parada L. F., Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990 May 18;61(4):647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- Klein R., Jing S. Q., Nanduri V., O'Rourke E., Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991 Apr 5;65(1):189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Kromer L. F., Cornbrooks C. J. Transplants of Schwann cell cultures promote axonal regeneration in the adult mammalian brain. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6330–6334. doi: 10.1073/pnas.82.18.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Beau J. M., LaCorbiere M., Powell H. C., Ellisman M. H., Schubert D. Extracellular fluid conditioned during peripheral nerve regeneration stimulates Schwann cell adhesion, migration and proliferation. Brain Res. 1988 Aug 30;459(1):93–104. doi: 10.1016/0006-8993(88)90289-2. [DOI] [PubMed] [Google Scholar]
- Lee K. F., Li E., Huber L. J., Landis S. C., Sharpe A. H., Chao M. V., Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992 May 29;69(5):737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- Letourneau P. C., Roche F. K., Shattuck T. A., Lemmon V., Takeichi M. Interactions of Schwann cells with neurites and with other Schwann cells involve the calcium-dependent adhesion molecule, N-cadherin. J Neurobiol. 1991 Oct;22(7):707–720. doi: 10.1002/neu.480220706. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Barbacid M., Parada L. F. Expression of the trk proto-oncogene is restricted to the sensory cranial and spinal ganglia of neural crest origin in mouse development. Genes Dev. 1990 May;4(5):683–694. doi: 10.1101/gad.4.5.683. [DOI] [PubMed] [Google Scholar]
- McCarthy J. B., Palm S. L., Furcht L. T. Migration by haptotaxis of a Schwann cell tumor line to the basement membrane glycoprotein laminin. J Cell Biol. 1983 Sep;97(3):772–777. doi: 10.1083/jcb.97.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes P. G., Bennett M. R. Growth of axons into developing muscles of the chick forelimb is preceded by cells that stain with Schwann cell antibodies. J Comp Neurol. 1987 May 15;259(3):330–347. doi: 10.1002/cne.902590303. [DOI] [PubMed] [Google Scholar]
- Noakes P. G., Bennett M. R., Stratford J. Migration of Schwann cells and axons into developing chick forelimb muscles following removal of either the neural tube or the neural crest. J Comp Neurol. 1988 Nov 8;277(2):214–233. doi: 10.1002/cne.902770205. [DOI] [PubMed] [Google Scholar]
- PETERS A., MUIR A. R. The relationship between axons and Schwann cells during development of peripheral nerves in the rat. Q J Exp Physiol Cogn Med Sci. 1959 Jan;44(1):117–130. doi: 10.1113/expphysiol.1959.sp001366. [DOI] [PubMed] [Google Scholar]
- Perkins C. S., Bray G. M., Aguayo A. J. Ongoing block of Schwann cell differentiation and deployment in dystrophic mouse spinal roots. Brain Res. 1981 Apr;227(2):213–220. doi: 10.1016/0165-3806(81)90109-7. [DOI] [PubMed] [Google Scholar]
- Plantinga L. C., Verhaagen J., Edwards P. M., Hol E. M., Bär P. R., Gispen W. H. The expression of B-50/GAP-43 in Schwann cells is upregulated in degenerating peripheral nerve stumps following nerve injury. Brain Res. 1993 Jan 29;602(1):69–76. doi: 10.1016/0006-8993(93)90243-g. [DOI] [PubMed] [Google Scholar]
- Raivich G., Hellweg R., Kreutzberg G. W. NGF receptor-mediated reduction in axonal NGF uptake and retrograde transport following sciatic nerve injury and during regeneration. Neuron. 1991 Jul;7(1):151–164. doi: 10.1016/0896-6273(91)90083-c. [DOI] [PubMed] [Google Scholar]
- Rush R. A. Immunohistochemical localization of endogenous nerve growth factor. Nature. 1984 Nov 22;312(5992):364–367. doi: 10.1038/312364a0. [DOI] [PubMed] [Google Scholar]
- Sandrock A. W., Jr, Matthew W. D. Substrate-bound nerve growth factor promotes neurite growth in peripheral nerve. Brain Res. 1987 Nov 10;425(2):360–363. doi: 10.1016/0006-8993(87)90520-8. [DOI] [PubMed] [Google Scholar]
- Scarpini E., Ross A. H., Rosen J. L., Brown M. J., Rostami A., Koprowski H., Lisak R. P. Expression of nerve growth factor receptor during human peripheral nerve development. Dev Biol. 1988 Feb;125(2):301–310. doi: 10.1016/0012-1606(88)90213-8. [DOI] [PubMed] [Google Scholar]
- Seilheimer B., Schachner M. Regulation of neural cell adhesion molecule expression on cultured mouse Schwann cells by nerve growth factor. EMBO J. 1987 Jun;6(6):1611–1616. doi: 10.1002/j.1460-2075.1987.tb02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M., Clark H. B., Schweitzer J. B., Johnson E. M., Jr Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. J Neurosci. 1988 Feb;8(2):664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Valenzuela D. M., Maisonpierre P. C., Glass D. J., Rojas E., Nuñez L., Kong Y., Gies D. R., Stitt T. N., Ip N. Y., Yancopoulos G. D. Alternative forms of rat TrkC with different functional capabilities. Neuron. 1993 May;10(5):963–974. doi: 10.1016/0896-6273(93)90211-9. [DOI] [PubMed] [Google Scholar]
- Weskamp G., Reichardt L. F. Evidence that biological activity of NGF is mediated through a novel subclass of high affinity receptors. Neuron. 1991 Apr;6(4):649–663. doi: 10.1016/0896-6273(91)90067-a. [DOI] [PubMed] [Google Scholar]
- Williams L. R., Longo F. M., Powell H. C., Lundborg G., Varon S. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: parameters for a bioassay. J Comp Neurol. 1983 Aug 20;218(4):460–470. doi: 10.1002/cne.902180409. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Reynolds M. L., Chong M. S., Emson P., Irwin N., Benowitz L. I. Denervation of the motor endplate results in the rapid expression by terminal Schwann cells of the growth-associated protein GAP-43. J Neurosci. 1992 Oct;12(10):3999–4010. doi: 10.1523/JNEUROSCI.12-10-03999.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Johnson E. M., Jr An immunohistochemical study of the nerve growth factor receptor in developing rats. J Neurosci. 1988 Sep;8(9):3481–3498. doi: 10.1523/JNEUROSCI.08-09-03481.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L. Physiological and morphological changes in developing peripheral nerves of rat embryos. Brain Res. 1988 Jul 1;470(1):15–28. doi: 10.1016/0165-3806(88)90198-8. [DOI] [PubMed] [Google Scholar]







