ABSTRACT
In this report, we present a simple and rapid method for analysis of 21 kinds of bile acids and the conjugates in rat serum and liver samples by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS-MS) in the negative ionization mode, using cholic-2, 2, 4, 4-d4 acid as internal standard. After liquid-liguid extraction from serum and liver samples, specimens were analyzed by UPLC equipped with an Acquity TQD tandem quadrupole mass spectrometer. All of the 21 bile acids were sufficiently separated within 5 min. For most bile acids, calibration curves showed good linearities in the range of 0.25 to 5000 ng/mL for serum samples, 2.5 ng/g to 50 μg/g for liver samples. The limits of detection (LOD) were estimated to be less than 0.25 to 7.5 ng/mL in serum, less than 2.5 to 10 ng/g in liver samples. The present method was validated with respect to repeatability; the coefficient of variation (CV) values were less than 26.7% in the serum and 25.9% in the liver. In the animal study, we compared 21 bile acids in the serum and liver samples of the stroke-prone spontaneously hypertensive (SHRSP) rats fed with control (SP) diet or high-fat and high-cholesterol-containing (HFC) diet. By feeding with HFC diet, the glycine conjugates of some bile acids significantly increased and the taurine conjugate of ulsodeoxicolate (TUDC) decreased in serum and liver samples. Our results suggest that the change of bile acid profiles could be applied for the diagnosis of non-alcoholic fatty liver disease (NAFLD).
Key Words: UPLC, tandem MS, Bile acids, Glycine conjugates, Taurine conjugates
INTRODUCTION
Bile acids (BAs), which are synthesized from cholesterol in the liver, play different physiological functions. BAs help the absorption of lipophilic nuturients in the intestine and regulate cholesterol homeostasis, they control glucose, lipid and energy homeostatis.1-3) There are two primary BAs, cholic acid (C), chenodeoxycholic acid (CDC) synthesized in the hepatocytes, and secondary bile acids, ursodeoxycholic acid (UDC), deoxycholic acid (DC), lithocholic acid (LC), are generated by the intestinal bacteria from primary BAs. BAs are regulated by different mechanisms; their kinetics are controlled by cholesterol 7α-hydroxylase involved in the rate-limiting step and other P450 isozymes, transporters such as bile salt excretion pump and apical bile salt transporter, receptors such as farnesoid X receptor, liver orphan receptor-α, and other molecules.4-8)
It is known that the amounts of total bile acids in body fluids are altered and reflect to hepatotoxicity after the intake of high fat diet, alcohol and hepatic toxicants.6,9,10)
It is thus needed to develop a highly sensitive and rapid analysis method for detecting and measuring bile acids and their conjugates in biological specimen; high performance liquid chromatography-mass spectrometry (HPLC-MS) or -tandem mass spectrometry (HPLC-MS-MS) is most suitable for determination of bile acids in biological fluids and tissues.11) Although several papers have been reported to analyze bile acids in biological fluids or tissues of human or other animals by HPLC-MS or HPLC-MS-MS,12-20) it took more than 10 minutes for MS analyses or tedious procedures for the extraction of bile acids were needed. In this report, we have presented a simple analysis method of 21 kinds of bile acids and the metabolites by ultra-performance liquid chromatography (UPLC)-MS-MS, which has enabled the analysis rapidly. Also, we have found the change of bile acids profile in rat serum and liver samples by a high fat-cholesterol (HFC) diet.
MATERIAL AND METHODS
Chemicals and reagents
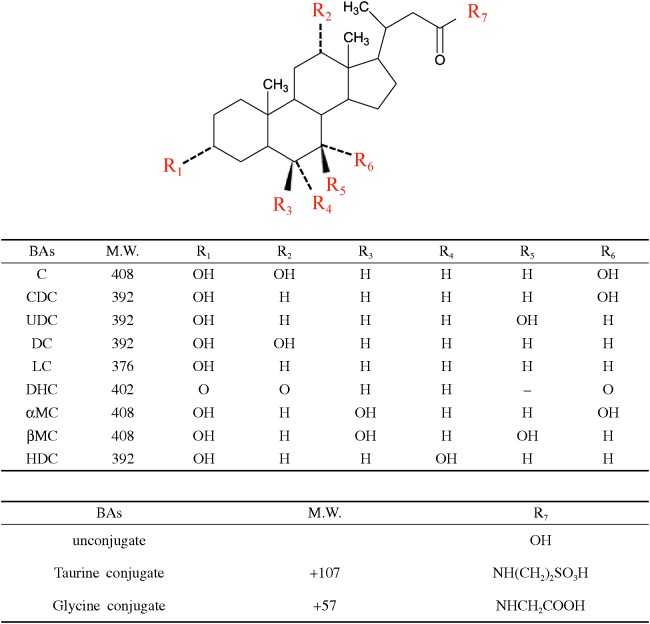
Cholic acid (C), chenodeoxycholic acid (CDC), ursodeoxycholic acid (UDC), lithocholic acid (LC), glycolithocholic acid (GLC), and dehydrocholic acid (DHC) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), taurocholic acid (TC), glycocholic acid (GC), taurochenodeoxycholic acid (TCDC), tauroursodeoxycholic acid (TUDC), deoxycholic acid (DC), taurodeoxycholic acid (TDC), and glycodeoxycholic acid (GDC) were purchased from Merck (Darmstadt, Germany), glycochenodeoxycholic acid (GCDC), and glycoursodeoxycholic acid (GUDC) from Santa Cruz Biotechnology, Inc. (Santa Cruz, California, USA), taurolithocholic acid (TLC), hyodeoxycholic acid (HDC), ammonium sulfate, and charcoal activated from Sigma-Aldrich (St. Louis, MI), and taurodehydrocholic acid (TDHC), glycodehydrocholic acid (GDHC), alpha-muricholic acid (α-MC), and beta-muricholic acid (β-MC) from Steraloids, Inc. (Newport, RI). The chemical structures of the above 21 bile acids were shown in Fig. 1. Cholic-2, 2, 4, 4-d4 acid was obtained from CDN (Pointe-Claire, Quebec, Canada). Acetonitrile (HPLC grade), distilled water (LC-MS grade), disodium hydrogen phosphate 12-water, and sodium dihydrogen phosphate dehydrate were obtained from Kanto Chemical Co., Inc. (Tokyo, Japan). Formic acid (abt. 99%) was obtained from Wako (Osaka, Japan). Other common chemicals used in this study were of analytical grade.
Fig. 1.
Chemical structures of 9 bile acids and their glycine and taurine conjugates.
UPLC-MS-MS conditions
The LC instrument used in combination with an MS-MS detector was a Waters Acquity UPLC system, including an Acquity UPLC binary pump and a sample manager (Waters, Milford, MA). The column used for chromatographic separation was a Poroshell 120 EC-C8 column (2.1 x 50 mm, particle size 2.7 μm; Agilent Technologies, Santa Clara, CA). The column temperature was maintained at 30°C, and the gradient system was used with a mobile phase A (0.2% formic acid aqueous solution) and mobile phase B (0.2% formic acid in acetonitrile), at a total flow rate of 0.5 mL/min. The gradient program was started at 70% mobile phase A and 30% mobile phase B, increased linearly to 62% mobile phase A and 38% mobile phase B for 2.6 min, increased linearly to 2% mobile phase A and 98% mobile phase B for 6.0 min, held at 2% mobile phase A and 98% mobile phase B for 1.0 min, and brought back to 70% mobile phase A and 30% mobile phase B for 0.1 min followed by 0.9 min re-equilibration. The total run time for each sample analysis was 8.0 min. The samples for analysis were maintained at 4°C and injection volume to UPLC-MS-MS analysis was 10 μL.
The MS-MS detection was performed in the negative ionization modes on a tandem quadrupole mass spectrometer (Acquity TQD; Waters) equipped with an electrospray ionization interface. Quantitation was performed using multiple reaction monitoring (MRM) mode using the peak areas. The optimal MS parameters were as follows: capillary voltage, 1.9 kV; source temperature, 120°C; desolvation temperature, 400°C; nitrogen gas with flow rates of desolvation and cone gas, 1000 and 10 L/hr, respectively; argon was used as collision gas with flow rates of 0.14 mL/min. The MRM transitions for the analytes and IS, their optimal MS parameters, such as the cone voltages and collision energies, were summarized in Table 1.
Table 1.
Multiple reaction monitoring (MRM) transitions and parameters for each detected compounds
| Analyte | MRM transition | Cone voltage (V) |
Collision energy (eV) |
|---|---|---|---|
| C | 407 → 407 | 70 | 10 |
| TC | 514 → 514 | 70 | 20 |
| GC | 464 → 464 | 70 | 20 |
| CDC | 391 → 391 | 70 | 10 |
| TCDC | 498 → 498 | 70 | 20 |
| GCDC | 448 → 448 | 60 | 10 |
| UDC | 391 → 391 | 70 | 10 |
| TUDC | 498 → 498 | 70 | 20 |
| GUDC | 448 → 448 | 60 | 10 |
| DC | 391 → 391 | 70 | 10 |
| TDC | 498 → 498 | 70 | 20 |
| GDC | 448 → 448 | 60 | 10 |
| LC | 375 → 375 | 60 | 20 |
| TLC | 482 → 482 | 70 | 20 |
| GLC | 432 → 73.9 | 70 | 30 |
| DHC | 401 → 401 | 70 | 10 |
| TDHC | 508 → 508 | 70 | 20 |
| GDHC | 458 → 458 | 60 | 10 |
| αMC | 407 → 407 | 70 | 10 |
| βMC | 407 → 407 | 70 | 10 |
| HDC | 391 → 391 | 70 | 10 |
| IS | 411 → 411 | 70 | 10 |
Sample preparation
For serum samples, to a 100 μL sample of rat serum, mixed with 20 ng IS (2 μL of 10 μg/mL sample solution) and 200 μL of 0.5 M phosphate buffer (pH6.0), 700 μL of acetonitrile was added; the mixture was vortexed for 1 min, and centrifuged at 3,500 g for 1 min. The supernatant collected was evaporated under vacuum at room temperature, and the residue was reconstituted with 70 μL of 0.2% fomic acid and 30 μL of 0.2% formic acid in acetonitrile, and filtrated through a Millex®-GV 0.22 μm filter (Millipore, Billerica, MA) before subjecting to a UPLC-MS-MS analysis.
For liver samples, after approximately 100 mg of liver was homogenized with 9 volumes of 10 mM phosphate buffer (pH6.0), the homogenate was centrifuged at 3,500 g for 5 min. A 200-μL aliquot of the liver homogenate was mixed with 40 ng IS (4 μL of 10 μg/mL sample solution) and 20 μL saturated ammonium sulfate. To the homogenate, 800 μL of acetonitrile was added; the mixture was vortexed for 1 min, and centrifuged at 3,500 g for 1 min. The supernatant collected was evaporated under vacuum at room temperature, the residue was reconstituted with 70 μL of 0.2% fomic acid and 30 μL of 0.2% formic acid in acetonitrile, and filtrated through the 0.22 μm filter.
Method validation
For obtaining all calibration curves, intra-day and inter-day variations, matrix effects, and recoveries, the matrices in which endogenous bile acids were deprived with the treatment using activated charcoal, were used; the procedure was based on the method previously described with slight modification.18) In brief, a 1-mL aliquot of the serum or supernatant of liver homogenate sample was mixed with 50 mg of activated charcoal, and the mixture was shaken moderately on an orbital shaker overnight (for about 17 hr) at room temperature; after centrifugation at 3,500 g for 5 min, the supernatants were subjected for experiments of method validations. The calibration curves were drawn in the range of 0.25–7.5 ng/mL and 5 μg/mL for serum samples (8 to 14 points), and in the range of 2.5–25 ng/g liver and 50 μg/g liver for liver samples (10 to 14 points); at each concentration, triplicate samples were prepared. The value of limit of detection (LOD) and limit of quantitation (LOQ) for each bile acid in serum and liver samples were defined as the lowest concentrations which could provide a signal-to-noise ratio of 3:1 and 10:1,21) respectively. The values of intra- and inter-day variations, matrix effects, and recoveries were validated at 3 quality control points that were 5, 50, 500 ng/mL for serum samples, and 50, 500 and 5000 ng/g liver for liver samples, respectively. Five or six replicates of each quality control point were analyzed each day to determine the intra- and inter-day accuracy and precision, as well as matrix effect and recovery. The precision of the assay was determined as a coefficient of variations (CV, %). The matrix effect and recovery were determined by the following methods. For the matrix effect, two sets of samples were prepared by directly spiking the analytes into the reconstituted solutions with or without the presence of the residue extracted from bile acids-free serum and liver samples. The matrix effect was calculated by using the following equation: matrix effect =Aep/Ans x 100, where Aep and Ans represent the analyte peak area/IS peak area ratio of the extracted serum or liver sample, and the ratio of the peak areas of the neat solution, respectively.16) The recovery value was calculated by using the equation: recovery =Aex/Aep x 100, where Aex and Aep represent the analyte peak area/IS peak area ratio of the extracted serum or liver samples, and the ratio of the peak areas of the extracted blank serum or liver samples, spiked with the bile acids, respectively.
Animal study
The animal study was conducted according to the Guidelines for Animal Experiments of the Nagoya University Animal Center. In the experiments, the Stroke-prone spontaneously hypertensive (SHRSP) rats were used for the experiment; the rats had been fed with the SP and HFC diet (those compositions were shown in Table 2), for 2 and 8 weeks.22)
Table 2.
Nutrient components of SP and HFC diets (weight %)
| SP diet | HFC diet | |
|---|---|---|
| Feed formulation rate | ||
| SP diet | 100 | 68 |
| Palm oil | 25 | |
| Cholesterol | 5 | |
| Cholic acid | 2 | |
| Interdients | ||
| Crude protein | 20.8 | 14.1 |
| Crude lipid | 4.8 | 35.3 |
| Crude fiber | 3.2 | 2.2 |
| Crude ash | 5 | 3.4 |
| Moisture | 8 | 5.4 |
| Carbohydrate | 58.2 | 39.6 |
Statistical analysis
In the method varidations and the animal studies, data of bile acids were shown as mean ± standard deviation of the mean (SD). Results from different groups were compared using Student’s t-test. If the variance was heterogeneous, logarithm or square root transformation was performed before analysis. P values less than 0.05 were considered to be statistically significant. All analyses were performed with SPSS 17.0 software.
RESULTS AND DISCUSSION
Product ion mass spectra and a multiple reaction monitoring (MRM) chromatograms
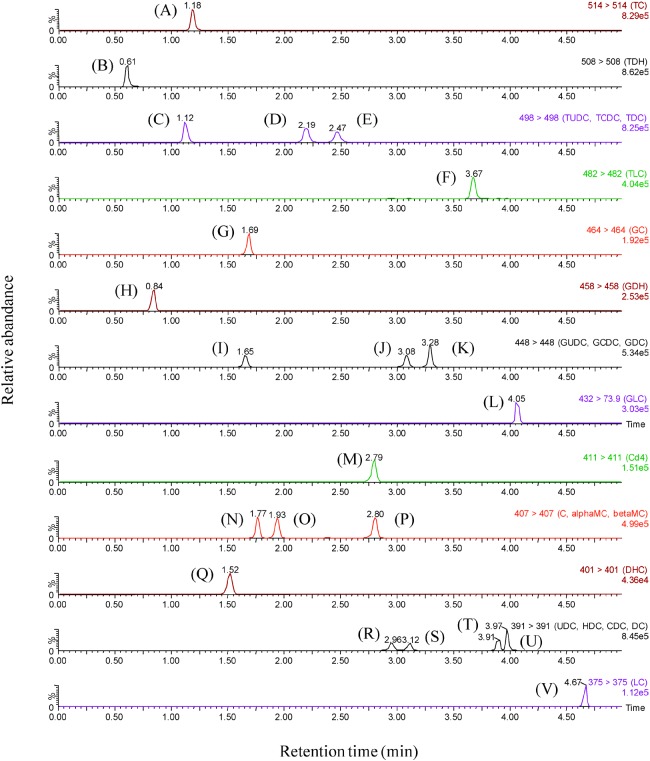
After optimization, the protonated precursor molecular ions were chosen to produce product ions most efficiently. The conditions of MRM transition reactions for the bile acids and IS were shown in Table 1. In our conditions, the m/z values of the product ions were the same as those of the precursor ions for most compounds; a similar phenomenon was observed in the analysis for amanitins.23) As shown in Fig. 2, all the bile acids were sufficiently separated within 5 min. In the previous reports, the analyses took more than 10 min;12-20) our present method has enabled high throughput analysis of different bile acids in biological specimens.
Fig. 2.
Multiple reaction monitoring (MRM) chromatograms for (A) TC, (B) TDHC, (C) TUDC, (D) TCDC, (E) TDC, (F) TLC, (G) GC, (H) GDHC, (I) GUDC, (J) GCDC, (K) GDC, (L) GLC, (M) IS, (N) α-MC, (O) β-MC, (P) C, (Q) DHC, (R) UDC, (S) HDC, (T) CDC, (U) DC, and (V) LC, obtained by UPLC-MS-MS.
Reliability of the method
The peak area ratio of each bile acid to IS obtained from each MRM chromatogram was used method validation and quantitation of the samples. Calibration curves were plotted at different concentrations in the range of 0.25 to 5000 ng/ml for each bile acid in rat serum samples, except for CDC, GCDC, UDC, GUDC, DC, LC, DHC TDHC, GDHC, α-MC, β-MC and HDC; the LOQ values were estimated to be 0.5 ng/mL for CDC, UDC, GUDC, DC, TDHC, α-MC, β-MC and HDC, 0.75 ng/mL for LC and GDHC, and 7.5 ng/mL for DHC, respectively. Calibration curves were drawn in the range of 2.5 ng to 50 μg/g liver in rat liver samples, except for UDC, LC, DHC and GDHC; the LOQ values were estimated to be 7.5 ng/g for UDC and LC, 10 ng/g for GDHC, and 25 ng/g for DHC, respectively. In the calibration ranges of the compounds, good linearities were obtained for all bile acids with correlation coefficients (r 2) more than 0.990 as shown in Table 3. The limits of detection, defined as the concentration giving the signal-to-noise ratio of 3, were estimated to be in the range of less than 0.25 to 2.5 ng/mL for rat serum samples, and in the range of less than 2.5 to 10 ng/g for rat liver samples, respectively. The sensitivities obtained were poorest for LC and DHC; this phenomenon could be explained because of their hydrophobicity and consequent difficulties in the ionization. In previous methods by HPLC-MS-MS, the detection limits for bile acids were approximately 5 ng/ml14) and 0.5 to 2.0 ng/ml16) for rat serum, 0.001 to 0.008 nmol/ml for human plasma,18) 0.2 to 0.5 ng/ml for the biological fluids of mice.20) The detection limits of our present method seems almost comparable to those in the previous reports.
Table 3.
Linearity data, limits of quantitation (LOQ) and limits of detection (LOD) in serum and liver samples
| Calibration curves of serum samples | Calibration curves of liver samples | |||||||||
| BAs | calibration range | correlation coefficient | LOQ (S/N=10) |
LOD (S/N=3) |
BAs | calibration range | correlation coefficient | LOQ (S/N=10) |
LOD (S/N=3) |
|
| ng/mL | r2 | ng/mL | ng/mL | ng/g liver |
r2 | ng/g liver |
ng/g liver |
|||
| C | 0.25-5000 | 0.999 | 0.25 | < 0.25 | C | 2.5-50000 | 0.999 | 2.5 | < 2.5 | |
| TC | 0.25-5000 | 0.999 | 0.25 | < 0.25 | TC | 2.5-50000 | 0.999 | 2.5 | < 2.5 | |
| GC | 0.25-5000 | 0.999 | 0.25 | < 0.25 | GC | 2.5-50000 | 0.999 | 2.5 | < 2.5 | |
| CDC | 0.5-5000 | 0.998 | 0.5 | 0.25 | CDC | 2.5-50000 | 0.999 | 2.5 | < 2.5 | |
| TCDC | 0.25-5000 | 0.998 | 0.25 | < 0.25 | TCDC | 2.5-50000 | 0.999 | 2.5 | < 2.5 | |
| GCDC | 0.25-5000 | 0.999 | 0.25 | < 0.25 | GCDC | 2.5-50000 | 0.999 | 2.5 | < 2.5 | |
| UDC | 0.5-5000 | 0.999 | 0.5 | 0.25 | UDC | 7.5-50000 | 0.999 | 7.5 | 5 | |
| TUDC | 0.25-5000 | 0.999 | 0.25 | < 0.25 | TUDC | 2.5-50000 | 0.99 | 2.5 | < 2.5 | |
| GUDC | 0.5-5000 | 0.999 | 0.5 | 0.25 | GUDC | 2.5-50000 | 0.996 | 2.5 | < 2.5 | |
| DC | 0.5-5000 | 0.999 | 0.5 | 0.25 | DC | 2.5-50000 | 0.999 | 2.5 | < 2.5 | |
| TDC | 0.25-5000 | 0.998 | 0.25 | < 0.25 | TDC | 2.5-50000 | 0.999 | 2.5 | < 2.5 | |
| GDC | 0.25-5000 | 0.999 | 0.25 | < 0.25 | GDC | 2.5-50000 | 0.999 | 2.5 | < 2.5 | |
| LC | 0.75-5000 | 0.996 | 0.75 | 0.5 | LC | 7.5-50000 | 0.999 | 7.5 | 5 | |
| TLC | 0.25-5000 | 0.996 | 0.25 | < 0.25 | TLC | 2.5-50000 | 0.998 | 2.5 | < 2.5 | |
| GLC | 0.25-5000 | 0.999 | 0.25 | < 0.25 | GLC | 2.5-50000 | 0.999 | 2.5 | < 2.5 | |
| DHC | 7.5-5000 | 0.993 | 7.5 | 2.5 | DHC | 25-50000 | 0.99 | 25 | 10 | |
| TDHC | 0.5-5000 | 0.999 | 0.5 | 0.25 | TDHC | 2.5-50000 | 0.998 | 2.5 | < 2.5 | |
| GDHC | 0.75-5000 | 0.999 | 0.75 | 0.5 | GDHC | 10-50000 | 0.999 | 10 | 5 | |
| αMC | 0.5-5000 | 0.999 | 0.5 | 2.5 | αMC | 2.5-50000 | 0.999 | 2.5 | < 2.5 | |
| βMC | 0.5-5000 | 0.999 | 0.5 | 0.25 | βMC | 2.5-50000 | 0.999 | 2.5 | < 2.5 | |
| HDC | 0.5-5000 | 0.999 | 0.5 | 0.25 | HDC | 2.5-50000 | 0.996 | 2.5 | < 2.5 | |
Table 4.1 and 4.2 show the values of coefficient of variation (CV), accuracy in intra- and inter-day variations (n=5–6), matrix effect, and recovery for each bile acid in rat serum and liver samples, respectively. Using liquid-liquid extraction, bile acids were recovered more than 65.3% in the serum, more than 69.0% in the liver. The CV values were not greater than 26.7% in the serum and 25.9% in the liver. The values of accuracy ranged from 53.1 to 186% in serum, and from 78.6 to 180% in liver. The CV values in rat live samples were much better than those of rat serum samples. Especially the values for DHC and its conjugates in rat serum samples were poor, due to their low sensitivities; the problem remains to be solved in the further research. The matrix effects were observed in some specimen; the values were 52.9 to 330% for the serum, and 28.5 to 368% for the liver. Altough most values ranged from approximately 80 to 130%, highly positive matrix effects were observed in TLC of rat serum and liver, and negative matrix effect observed in DHC of rat liver at 50 ng/g; we observed a similar phenomenon in the detection of amanitins in human or rat urine samples using UPLC-MS-MS.23) We thus recommend that the calibration curves for bile acids should be prepared using the same biological samples.
Table 4.1.
Precision, accuracy, matrix effect, and recovery data of 21 bile acids in rat serum samples
| BAs | concentration | intra-day (n=6) | inter-day (n=5-6) | matrix effect | recovery | |||
|---|---|---|---|---|---|---|---|---|
| ng/mL | CV (%) |
accuracy (%) |
CV (%) |
accuracy (%) |
(n=6, %) | (n=6, %) | ||
| C | 5 | 16.4 | 95.3 | 3.9 | 82.0 | 105±14.9 | 109±8.4 | |
| 50 | 4.3 | 95.9 | 4.7 | 94.3 | 97.2±3.2 | 109±1.4 | ||
| 500 | 5.2 | 103 | 4.2 | 104 | 91.7±1.6 | 110±4.1 | ||
| TC | 5 | 22.8 | 102 | 26.4 | 63.2 | 110±30.2 | 141±24.2 | |
| 50 | 4.5 | 104 | 24.2 | 91.4 | 126±12.7 | 113±9.8 | ||
| 500 | 3.3 | 100 | 17.2 | 87.1 | 132±9.0 | 110±13.5 | ||
| GC | 5 | 16.3 | 122 | 20.8 | 80.2 | 220±35.4 | 65.3±10.4 | |
| 50 | 7.4 | 94.2 | 22.8 | 125 | 122±22.1 | 94.0±24.7 | ||
| 500 | 12.0 | 89.9 | 27.5 | 125 | 81.1±12.1 | 148±14.1 | ||
| CDC | 5 | 11.8 | 88.8 | 11.7 | 100 | 88.4±11.3 | 129±15.5 | |
| 50 | 14.5 | 108 | 3.1 | 94.5 | 79.6±4.2 | 101±8.9 | ||
| 500 | 9.4 | 102 | 9.5 | 95.7 | 110±9.5 | 91.1±17.7 | ||
| TCDC | 5 | 6.4 | 83.7 | 18.3 | 77.6 | 128±21.6 | 141±24.2 | |
| 50 | 5.2 | 98.4 | 11.3 | 88.0 | 143±18.9 | 121±16.6 | ||
| 500 | 3.6 | 100 | 13.4 | 86.9 | 171±25.6 | 116±20.5 | ||
| GCDC | 5 | 8.4 | 90.7 | 14.7 | 82.4 | 88.6±7.1 | 116±10.0 | |
| 50 | 6.1 | 104 | 12.4 | 98.5 | 106±4.9 | 106±6.4 | ||
| 500 | 4.3 | 101 | 7.3 | 96.7 | 101±8.1 | 114±7.6 | ||
| UDC | 5 | 2.7 | 88.5 | 4.6 | 83.1 | 80.0±6.9 | 130±12.0 | |
| 50 | 5.2 | 110 | 6.4 | 94.9 | 89.3±4.6 | 111±7.6 | ||
| 500 | 3.9 | 97.3 | 6.8 | 87.9 | 102±7.4 | 102±6.9 | ||
| TUDC | 5 | 10.2 | 103 | 26.7 | 69.2 | 117±19.9 | 146±32.8 | |
| 50 | 4.1 | 105 | 21.2 | 90.4 | 133±15.7 | 113±9.8 | ||
| 500 | 3.5 | 99.9 | 16.1 | 87.8 | 142±10.8 | 111±14.3 | ||
| GUDC | 5 | 7.0 | 95.3 | 6.2 | 74.8 | 106±9.6 | 117±15.4 | |
| 50 | 4.1 | 106 | 12.5 | 93.3 | 99.4±8.3 | 110±14.1 | ||
| 500 | 4.3 | 108 | 9.0 | 108 | 94.8±19.6 | 140±28.2 | ||
| DC | 5 | 18.7 | 106 | 8.9 | 103 | 85.5±9.5 | 116±31.0 | |
| 50 | 8.9 | 97.2 | 7.5 | 98.6 | 85.1±4.8 | 97.3±11.1 | ||
| 500 | 9.0 | 98.8 | 12.1 | 98.9 | 66.6±6.0 | 121±11.8 | ||
| TDC | 5 | 4.5 | 89.3 | 16.7 | 76.0 | 145±19.8 | 138±24.4 | |
| 50 | 5.8 | 110 | 13.5 | 88.2 | 154±21.2 | 121±18.4 | ||
| 500 | 8.4 | 103 | 13.5 | 95.7 | 189±22.7 | 114±14.4 | ||
| GDC | 5 | 3.6 | 93.5 | 14.0 | 71.6 | 97.2±8.3 | 116±11.0 | |
| 50 | 6.2 | 99.5 | 9.0 | 95.8 | 102±5.0 | 107±9.2 | ||
| 500 | 5.6 | 102 | 8.9 | 99.4 | 103±5.0 | 114±8.1 | ||
| LC | 5 | 7.0 | 175 | 17.9 | 186 | 68.0±4.1 | 94.1±10.7 | |
| 50 | 11.9 | 116 | 15.5 | 109 | 62.8±8.2 | 85.5±19.4 | ||
| 500 | 17.2 | 106 | 29.5 | 98.3 | 89.7±17.4 | 88.9±19.7 | ||
| TLC | 5 | 7.1 | 84.1 | 25.5 | 66.6 | 241±53.7 | 131±36.2 | |
| 50 | 6.9 | 104 | 10.1 | 83.9 | 261±23.4 | 110±10.1 | ||
| 500 | 4.4 | 97.6 | 11.3 | 83.9 | 330±30.0 | 100±11.8 | ||
| GLC | 5 | 17.8 | 103 | 17.8 | 107 | 107±6.8 | 97.7±7.6 | |
| 50 | 15.5 | 125 | 14.8 | 139 | 147±17.4 | 96.7±24.2 | ||
| 500 | 7.9 | 111 | 17.5 | 137 | 117±9.2 | 85.6±18.7 | ||
| DHC | 5 | n.d.* | n.d.* | n.d.* | n.d.* | n.a.† | n.a.† | |
| 50 | 7.0 | 90.5 | 22.2 | 112 | 98.6±13.8 | 82.8±16.4 | ||
| 500 | 11.6 | 82.8 | 26.7 | 93.9 | 71.8±5.3 | 116±18.6 | ||
| TDHC | 5 | 15.2 | 106 | 17.2 | 53.4 | 52.9±5.3 | 133±20.2 | |
| 50 | 10.1 | 100 | 17.7 | 53.1 | 58.0±2.3 | 107±9.5 | ||
| 500 | 8.6 | 104 | 15.1 | 59.2 | 65.1±7.5 | 127±22.1 | ||
| GDHC | 5 | 10.2 | 87.8 | 7.7 | 56.8 | 80.0±9.8 | 116±19.4 | |
| 50 | 8.7 | 94.1 | 16.3 | 69.2 | 68.1±5.4 | 105±10.6 | ||
| 500 | 4.7 | 82.8 | 9.5 | 69.5 | 59.5±3.7 | 118±4.6 | ||
| αMC | 5 | 9.0 | 84.6 | 7.0 | 69.4 | 87.2±5.9 | 104±8.0 | |
| 50 | 2.6 | 96.6 | 2.6 | 92.6 | 77.0±13.9 | 113±29.9 | ||
| 500 | 6.3 | 97.9 | 4.5 | 84.4 | 79.9±14.1 | 122±23.0 | ||
| βMC | 5 | 6.4 | 121 | 7.3 | 78.7 | 82.2±8.7 | 132±14.8 | |
| 50 | 3.8 | 104 | 8.8 | 89.0 | 79.2±14.7 | 122±30.7 | ||
| 500 | 3.7 | 99.3 | 6.7 | 88.4 | 93.9±14.5 | 112±20.6 | ||
| HDC | 5 | 9.0 | 115 | 5.8 | 105 | 81.9±9.6 | 123±10.8 | |
| 50 | 5.9 | 112 | 7.7 | 99.5 | 91.4±7.1 | 111±12.0 | ||
| 500 | 2.6 | 97.4 | 7.7 | 101 | 103±9.9 | 104±8.9 | ||
*: Not detected
†: Not applicable
Table 4.2.
Precision, accuracy, matrix effect, and recovery data of 21 bile acids in rat liver samples
| BAs | concentration | intra-day (n=6) | inter-day (n=6) | matrix effect | recovery | |||
|---|---|---|---|---|---|---|---|---|
| ng/g liver | CV (%) |
accuracy (%) |
CV (%) |
accuracy (%) |
(n=6, %) | (n=6, %) | ||
| C | 50 | 2.6 | 99.1 | 3.3 | 98.8 | 103±5.0 | 98.6±6.5 | |
| 500 | 2.5 | 91.0 | 5.6 | 91.0 | 93.8±5.7 | 106±6.4 | ||
| 5000 | 1.1 | 103 | 1.8 | 102 | 103±12.5 | 97.7±11.4 | ||
| TC | 50 | 8.0 | 104 | 5.6 | 111 | 105±9.0 | 113±9.3 | |
| 500 | 2.2 | 112 | 6.3 | 121 | 123±5.6 | 98.6±6.2 | ||
| 5000 | 2.0 | 114 | 3.5 | 117 | 130±13.8 | 99.2±12.0 | ||
| GC | 50 | 3.1 | 92.1 | 7.0 | 111 | 87.3±9.3 | 91.7±9.2 | |
| 500 | 4.4 | 101 | 8.8 | 116 | 81.0±5.0 | 104±8.4 | ||
| 5000 | 4.0 | 78.5 | 5.4 | 85.7 | 98.7±13.6 | 96.0±11.2 | ||
| CDC | 50 | 14.0 | 100 | 14.2 | 107 | 65.0±11.4 | 123±14.4 | |
| 500 | 4.4 | 78.6 | 9.5 | 87.7 | 111±10.6 | 95.1±13.1 | ||
| 5000 | 3.3 | 94.9 | 4.6 | 90.9 | 94.3±19.3 | 102±16.9 | ||
| TCDC | 50 | 4.0 | 106 | 3.4 | 106 | 172±6.4 | 97.3±4.1 | |
| 500 | 3.3 | 119 | 4.8 | 120 | 150±3.3 | 104±3.7 | ||
| 5000 | 1.0 | 107 | 2.7 | 110 | 144±14.7 | 100±13.1 | ||
| GCDC | 50 | 3.7 | 91.6 | 3.2 | 94.8 | 108±5.9 | 96.1±9.4 | |
| 500 | 2.9 | 102 | 5.9 | 106 | 99.7±4.2 | 105±5.6 | ||
| 5000 | 2.9 | 85.1 | 1.9 | 109 | 104±16.3 | 98.9±13.8 | ||
| UDC | 50 | 9.0 | 102 | 7.6 | 103 | 68.5±8.1 | 134±11.6 | |
| 500 | 2.9 | 111 | 7.1 | 109 | 99.2±6.4 | 101±8.3 | ||
| 5000 | 2.2 | 105 | 3.6 | 105 | 106±9.3 | 93.6±9.7 | ||
| TUDC | 50 | 6.7 | 95.8 | 4.4 | 106 | 114±6.1 | 97.9±8.2 | |
| 500 | 4.1 | 112 | 6.4 | 118 | 125±5.9 | 106±9.7 | ||
| 5000 | 2.6 | 111 | 2.4 | 89.7 | 131±15.2 | 99.5±12.5 | ||
| GUDC | 50 | 2.2 | 91.3 | 7.7 | 98.8 | 81.6±8.7 | 102±7.7 | |
| 500 | 3.5 | 109 | 7.2 | 118 | 85.3±6.9 | 107±8.1 | ||
| 5000 | 4.5 | 101 | 4.0 | 105 | 104±14.3 | 101±11.0 | ||
| DC | 50 | 3.5 | 84.7 | 8.4 | 97.2 | 97.1±16.4 | 86.0±19.8 | |
| 500 | 6.0 | 107 | 14.1 | 108 | 95.4±8.3 | 99.7±5.2 | ||
| 5000 | 6.4 | 83.0 | 13.3 | 107 | 105±6.0 | 83.1±7.6 | ||
| TDC | 50 | 3.7 | 113 | 6.5 | 106 | 170±6.5 | 103±4.2 | |
| 500 | 2.8 | 113 | 5.9 | 120 | 163±3.1 | 101±4.4 | ||
| 5000 | 1.4 | 110 | 2.5 | 113 | 153±15.7 | 102±13.3 | ||
| GDC | 50 | 3.9 | 101 | 2.8 | 97.4 | 112±19.0 | 101±20.5 | |
| 500 | 3.0 | 110 | 4.8 | 87.5 | 106±5.4 | 107±6.3 | ||
| 5000 | 1.9 | 101 | 3.2 | 103 | 114±15.1 | 99.5±12.8 | ||
| LC | 50 | 15.3 | 90.3 | 11.2 | 84.8 | 67.4±10.0 | 108±17.8 | |
| 500 | 11.9 | 91.8 | 16.7 | 96.1 | 102±15.1 | 70.8±14.5 | ||
| 5000 | 6.9 | 100 | 6.9 | 97.9 | 98.6±11.6 | 69.0±16.7 | ||
| TLC | 50 | 5.6 | 116 | 9.3 | 103 | 368±7.6 | 84.2±12.3 | |
| 500 | 5.4 | 103 | 5.7 | 113 | 260±4.9 | 97.1±6.0 | ||
| 5000 | 3.4 | 89.9 | 5.8 | 95.9 | 242±16.5 | 91.8±15.5 | ||
| GLC | 50 | 12.5 | 90.1 | 8.6 | 96.0 | 166±14.0 | 74.3±11.7 | |
| 500 | 8.1 | 109 | 14.2 | 108 | 93.5±17.4 | 104±13.0 | ||
| 5000 | 8.4 | 99.6 | 9.5 | 106 | 114±12.3 | 81.6±9.6 | ||
| DHC | 50 | 19.9 | 91.0 | 25.9 | 180 | 28.5±34.8 | 191±28.3 | |
| 500 | 3.0 | 115 | 4.0 | 117 | 84.4±19.5 | 88.0±21.8 | ||
| 5000 | 7.1 | 130 | 7.0 | 133 | 89.1±3.7 | 76.7±6.9 | ||
| TDHC | 50 | 18.0 | 119 | 9.9 | 114 | 43.7±15.2 | 121±25.5 | |
| 500 | 5.9 | 140 | 7.1 | 140 | 75.1±11.3 | 103±14.9 | ||
| 5000 | 5.4 | 83.2 | 9.0 | 88.3 | 91.7±6.7 | 88.4±8.6 | ||
| GDHC | 50 | 14.3 | 92.2 | 6.2 | 101 | 41.5±13.6 | 94.9±24.9 | |
| 500 | 10.7 | 101 | 7.5 | 109 | 48.4±10.0 | 94.9±18.2 | ||
| 5000 | 11.0 | 110 | 3.0 | 87.8 | 56.8±14.6 | 86.5±15.1 | ||
| αMC | 50 | 4.0 | 94.2 | 3.5 | 94.6 | 77.7±6.3 | 105±9.4 | |
| 500 | 2.4 | 110 | 6.4 | 111 | 85.1±5.5 | 105±6.9 | ||
| 5000 | 3.2 | 94.0 | 2.4 | 93.5 | 89.9±12.8 | 97.8±11.0 | ||
| βMC | 50 | 3.4 | 99.5 | 3.5 | 96.7 | 83.6±6.1 | 114±8.2 | |
| 500 | 2.0 | 111 | 6.4 | 109 | 89.9±7.4 | 104±8.2 | ||
| 5000 | 3.1 | 104 | 2.8 | 102 | 98.6±10.7 | 95.8±9.7 | ||
| HDC | 50 | 8.0 | 108 | 7.6 | 101 | 58.3±13.2 | 150±13.1 | |
| 500 | 1.5 | 112 | 5.0 | 108 | 99.1±8.1 | 98.8±8.6 | ||
| 5000 | 1.9 | 93.0 | 3.2 | 95.7 | 109±9.7 | 93.5±9.1 | ||
*: Not detected
Quantitative analysis of rat serum and liver samples
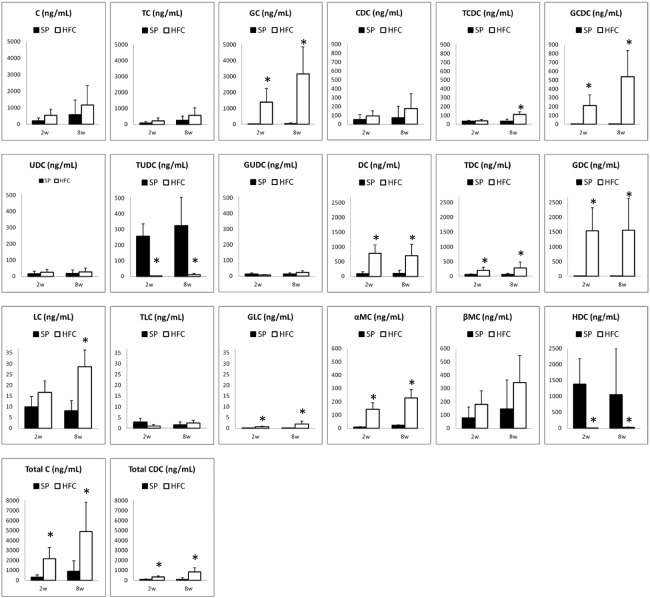
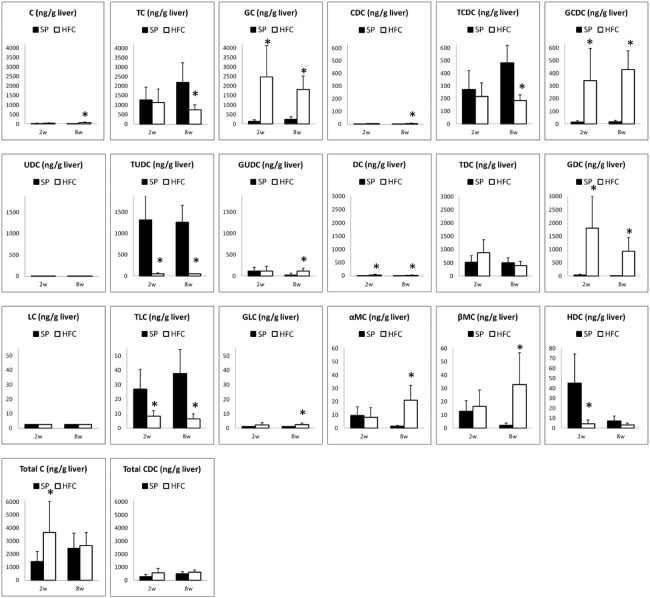
Using the present method, we have measured and compared bile acid profiles of the rats fed with SP and HFC diet. The comparisons of the rat serum samples among four groups were shown in Fig. 3, those of liver samples, shown in Fig. 4. For the primary bile acids, C and CDC, in both serum and liver samples, no differences were found between SP and HFC diet groups. However, the total amount of C and its conjugates (C group), as well as that of CDC and its conjugates (CDC group), also increased in the serum samples. The increase of C and CDC groups must be due to the increase of cholesterol by HFC diet. Moreover, their glycine conjugates (GC and GCDC) obviously increased by the HFC diet groups, compared to the taurine conjugates. Conjugation of glycine and taurine is catalyzed only by amino acid N-acetyltransferase (BAAT) and BAAT is strictly located in peroxisome.8,9,24) For explaining the discrepancy of both conjugates, a possibility that intracellular taurine could be easily exhausted compared to glycine, may exist; it remains for further investigation. Recently, Kitamori et al. reported that the feeding of HFC diet for 8 weeks induced steathohepatitis and the severe fibrosis progression in a new rat strain SHRSP5/Dmcr, which is a model of non-alcoholic fatty liver disease (NAFLD).22) In our experiments, no fibrosis was observed in SHRSP rats after feeding HFC diet for 8 weeks. Interestingly, the change of bile acid profile in the serum by HFC diet seems similar to that in the liver, except LC and TLC; this finding suggests that the change of bile acids in the serum would reflect the pathological change in the liver. Taken together, the change of some bile acids and the conjugates could be applied to diagnose NAFLD in the early stage.
Fig. 3.
The values of BAs in serum samples of SHRSP rats fed with control (SP) diet (shown in filled bars) and high fat and cholesterol-containing (HFC) diet (shown in open bars). The C or CDC group represents the sums of C, TC and GC, or CDC, TCDC and GCDC, respectively. The values of DHC, TDHC, and GDHC were below the LOQ values (data not shown).
*: significant differences between each of the samples fed with SP and each of those fed with HFC diet (p <0.05).
Fig. 4.
The values of BAs in liver samples of SHRSP rats fed with control (SP) diet (shown in filled bars) and high fat and cholesterol-containing (HFC) diet (shown in open bars). The C or CDC group represents the sums of C, TC and GC, or CDC, TCDC and GCDC, respectively. The values of DHC, TDHC, and GDHC were below the LOQ values (data not shown).
*: significant differences between each of the samples fed with SP and each of those fed with HFC diet (p <0.05). not shown).
CONCLUSIONS
We have developed a simple and rapid method to determine the 21 kinds of bile acids in rat serum and liver samples. This method includes a simple solvent extraction procedure followed by UPLC-MS-MS detection. Also, we have shown some bile acids and the conjugates in the serum and liver of SHRSP rats, changed after HFC diet; this method could be utilized for early diagnosis of NAFLD.
ACKNOWLEDGEMENT
This study was supported in part by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology.
Abbreviations
- BA
bile acid
- C
cholic acid
- CDC
chenodeoxycholic acid
- CV
coefficient of variation
- DC
deoxycholic acid
- DHC
dehydrocholic acid
- GC
glycocholic acid
- GCDC
glycochenodeoxycholic acid
- GDC
glycodeoxycholic acid
- GDHC
glycodehydrocholic acid
- GLC
glycolithocholic acid
- GUDC
glycoursodeoxycholic acid
- HDC
hyodeoxycholic acid
- HPLC
high-performance liquid chromatography
- IS
internal standard
- LC
lithocholic acid
- LOD
limit of detection
- LOQ
limit of quantitation
- α-MC
alpha-muricholic acid
- β-MC
beta-muricholic acid
- MRM
multiple reaction monitoring
- MS-MS
tandem mass spectrometry
- NAFLD
non-alcoholic fatty liver disease
- SD
standard deviation of the mean
- SHRSP rat
stroke-prone spontaneously hypertensive rat
- TC
taurocholic acid
- TCDC
taurochenodeoxycholic acid
- TDC
taurodeoxycholic acid
- TDHC
taurodehydrocholic acid
- TLC
taurolithocholic acid
- TUDC
tauroursodeoxycholic acid
- UDC
ursodeoxycholic acid
- UPLC
ultra-performance liquid chromatography
REFERENCES
- 1).Li T, Chiang JYL. Bile acid signaling in liver metabolism and diseases. J Lipid Res, 2012; 2012: 754067. [DOI] [PMC free article] [PubMed]
- 2).Monte MJ, Marin JJG, Antelo A, Varquez-Tato J. Bile acids: Chemistry, physiology, and pathophysiology. World J Gastroenterol, 2009; 15: 804–816. [DOI] [PMC free article] [PubMed]
- 3).Hoffmann AF. The enterohepatic circulation of bile acids in mammals: form and functions. Frontier Biosci, 2009; 14: 2584–2598. [DOI] [PubMed]
- 4).Chiang JYL. Bile acids: regulation of synthesis. J Lipid Res, 2009; 50: 1955–1966. [DOI] [PMC free article] [PubMed]
- 5).Chiang JYL. III. Bile acids and nuclear receptors. Am J Physiol Gastrointest Liver Physiol, 2003; 284: G349–G356. [DOI] [PubMed]
- 6).Hofmann AF. The continuing importance of bile acids in liver and intestinal desease. Arch Intern Med, 1999; 159: 2647–2658. [DOI] [PubMed]
- 7).Ridlon JM, Kang D-J, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res, 2006; 47: 241–259. [DOI] [PubMed]
- 8).Falany CN, Johnson MR, Barnes S, Diasio RB. Glycine and taurin conjugation of bile acids by a single enzyme. J Biol Chem, 1994; 269: 19375–19379. [PubMed]
- 9).Pellicoro A, Faber KN. Review article: the function and regulation of proteins involved in bile salt biosynthesis and transport. Alimentary Pharmacol Therap, 2007; 26: 149–160. [DOI] [PubMed]
- 10).Pauli-Magnus C, Meier PJ. Hepatobiliary transporters and drug-induced cholestasis. Hepatology, 2006; 44: 778–787. [DOI] [PubMed]
- 11).Griffiths WJ, Sjövall J. Bile acids: analysis in biological fluids and tissues. J Lipid Res, 2010; 51: 23–41. [DOI] [PMC free article] [PubMed]
- 12).Perwaiz S, Tuchweber B, Mignault D, Gilat T, Yousef IM. Determination of bile acids in biological fluids by liquid chromatography-electrospray mass spectrometry. J Lipid Res, 2001; 42: 114–119. [PubMed]
- 13).Burkard I, von Eckardstein A, Rentsch. Differentiated quantitation of human bile acids in serum by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B, 2005; 826: 147–159. [DOI] [PubMed]
- 14).Ando M, Kaneko T, Watanabe R, Kikuchi S, Goto T, Iida T, Hishinuma T, Mano N, Goto J. High sensitive analysis of rat serum bile acids by liquid chromatography/electrospray ionization tandem mass spectrometry. J Phamaceut Biomed Anal, 2006; 40: 1179–1186. [DOI] [PubMed]
- 15).Alnouti Y, Csanaky IL, Klaassen CD. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B, 2008; 873: 209–217. [DOI] [PMC free article] [PubMed]
- 16).Yang L, Xiong A, He Y, Wang Z, Wang C, Wang Z, Li W, Yang L, Hu Z. Bile acids metabonomic study on the CCl4- and α-Naphthylisothiocyanate- induced animal models: quantitative analysis of 22 bile acids by ultraperformance liquid chromatography-mass spectrometry. Chem Res Toxicol, 2008; 21: 2280–2288. [DOI] [PubMed]
- 17).Bobeldijk I, Maarten H, de Vries-van der Weij J, Coulier L, Ramaker R, Kleemann R, Kooistra T, Rubingh C, Freidig A, Verheij E. Quantitative profiling of bile acids in biofluids and tissues based on accurate mass high resolution LC-FT-MS: Compound class targeting in a metabolomics workflow. J Chromatogr B, 2008; 871: 306–313. [DOI] [PubMed]
- 18).Xiang X, Han Y, Neuvonen M, Laitila J, Neuvonen PJ, Niemi M. High performance liquid chromatography-tandem mass spectrometry for the determination of bile acid concentrations in human plasma. J Chromatogr B, 2010; 878: 51–60. [DOI] [PubMed]
- 19).Want EJ, Coen M, Masson P, Keun HC, Pearce JTM, Reily MD, Robertson DG, Rohde CM, Holmes E, Lindon JC, Plumb RS, Nicholson JK. Ultra performance liquid chromatography-mass spectrometry profiling of bile acid metabolites in biofluids: application to experimental toxicology studies. Anal Chem, 2010; 82: 5282–5289. [DOI] [PubMed]
- 20).Huang J, Bathena SPR, Csanaky IL, Alnouti Y. Simultaneous characterization of bile acids and their sulfate metabolites in mouse liver, plasma, bile, and urine using LC-MS/MS. J Pharmaceut Biomed Anal, 2010; 55: 1111–1119. [DOI] [PubMed]
- 21).International Conference on Harmonisation of Technical Requirements for Registartion of Pharmaceutical for Human Use. Varidation of Analytical Procedures: Text and Methodology Q2 (R1) 2005.
- 22).Kitamori K, Naito H, Tamada H, Kobayashi M, Miyazawa D, Yasui Y, Sonoda K, Tsuchikura S, Yasui N, Ikeda K, T Moriya, Yamori Y, Nakajima T. Development of novel rat model for high-fat and high-cholesterol diet-induced steatohepatitis and severe fibrosis progression in SHRSP5/Dmcr. Environ Health Prev Med, 2012; 17: 173–182. [DOI] [PMC free article] [PubMed]
- 23).Nomura M, Suzuki Y, Kaneko R, Ogawa T, Hattori H, Seno H, Ishii A. Simple and rapid analysis of amatoxins using UPLC-MS-MS. Forensic Toxicol, 2012; 30: 185–192.
- 24).Pellicolo A, van den Heuvel FAJ, Geuken M, Moshage H, Jansen PLM, Faber KN. Human and rat bile acid-CoA: Amino acid N-acetyltransferase are liver specific peroxisomal enzymes: Implications for intracellular bile salt transport. Hepatology, 2007; 45: 340–348. [DOI] [PubMed]