ABSTRACT
Peritoneal metastasis from gastric cancer is often undetectable by routine imaging studies. Even a microscopic metastasis detected only by cytologic examination of the peritoneal washes denotes a dismal prognosis, and surgery is ruled out as futile for patients who turn out to be cytology-positive by staging laparoscopy. On the other hand, recent developments in cancer chemotherapy have improved the outcome of the cytology-positive population to the point where a certain proportion of these patients survive for 5 years through a straightforward strategy of radical surgery followed by chemotherapy. Thus, there is certainly a role for surgeons in patients with minimal peritoneal metastasis, both in clinical practice and in clinical trials where multimodal treatment strategies including surgery are to be explored. Even in this category of patients, surgery in combination with various types of chemotherapy remains the only hope for a cure.
Key Words: Gastric cancer, Peritoneal metastasis, Cytology, Peritoneal carcinomatosis, D2 dissection
INTRODUCTION
In the Far East, the most common pattern of disease failure after curative resection of gastric carcinoma is reported to be peritoneal carcinomatosis.1,2) Cancer cells that are exfoliated from the serosal surface of the stomach and scattered into the peritoneal cavity are considered to be responsible for this fatal condition.3 Detection of free cancer cells in the peritoneal cavity through various methods has therefore been attempted to understand the risk for recurrence.3-5) Moreover, conventional cytologic examination of the peritoneal washes through Papanicolaou staining has become mandatory in Japan as a tool for the prediction of prognosis and, ultimately, one of the key components of the staging system.6) Patients with viable cancer cells in the peritoneal cavity are bound to harbor micrometastases on the peritoneal surface and are at a high risk of suffering from peritoneal carcinomatosis. Such a status is defined as CY1, and cancer diagnosed as CY1 through laparotomy or staging laparoscopy is invariably classified into Stage IV by the Japanese Classification of Gastric Carcinoma.6) Treatment options recommended by the Japanese Treatment Guidelines for Gastric Cancer for Stage IV gastric cancer are chemotherapy, radiotherapy, supportive care, and palliative surgery that includes gastrectomy to circumvent bleeding, and by-pass surgery or ileostomy for those with obstruction of the gastrointestinal tract. However, it does not include gastrectomy with curative intent. These facts point to the notion that a CY1-stage patient is no longer eligible for surgery with curative intent, despite the absence of any macroscopic evidence of disease. This notion could be accepted in countries where morbidity and mortality from gastric cancer surgery cannot be dismissed as negligible,7) but the prognosis of curatively resected gastric cancer in general remains dismal.8) Medical professionals under such circumstances should seek out any useful criteria so as to avoid futile and toxic modes of treatment, and cytologic examination could be useful in that regard. On the contrary, such a notion has been perceived as regrettable in Japan, where gastrectomies have been performed safely, and have generally provided relatively favorable outcomes. This article provides an overview of current treatment strategies for minimal peritoneal disease in gastric cancer in Japan.
OUTCOME OF PATIENTS WITH CY1 STATUS
The dismal prognosis of CY1 disease had been well-documented not only in the West but also in the Far East.3,9) Several researchers have reported that the chance of survival of CY1 patients is equivalent to that of patients with macroscopic peritoneal deposits.10) The accuracy of cytologic examination may be an issue for debate, as it has been questioned by Leake et al. in their review article.11) This study featured several investigators who adopted immunostaining and RT-PCR techniques, in addition to the conventional cytologic examination, to more sensitively detect minimal cancer cells. It is apparent that the weakness in the conventional cytologic examination lies in its relatively low sensitivity, an area where techniques such as RT-PCR could do better.3) Given its high specificity, however, conventional cytologic examination remains useful in identifying patients for whom some fundamental considerations are necessary to establish the treatment strategy, such as delivering highly toxic treatments or making serious decisions such as ruling out surgery.
LINITIS PLASTICA-TYPE GASTRIC CANCER AS A PARTICULARLY AGGRESSIVE DISEASE ENTITY
The authors have looked specifically at the outcomes of linitis plastica-type gastric cancer.12) This diffusely invasive type of cancer usually requires total gastrectomy that has a detrimental effect on the quality of life of the patients, while it rarely induces massive hemorrhage or gastric outlet disease until relatively late in the course of the disease. Through the analysis of peritoneal washes from 47 consecutive patients suffering from linitis plastica, the authors found that 43% of patients who were deemed operable by the preoperative imaging technique were CY1 at surgery.12) By using the RT-PCR technique, the likelihood of detecting free cancer cells in the same population rose to 83%. This figure turned out to be even more accurate than what was provided after conventional cytologic examination, since as much as 77% of the same population was found at surgery or during the follow-up to suffer from peritoneal carcinomatosis.12) Fukagawa et al. found that the prognosis of the linitis plastica-type was outstandingly dismal even among patients who were diagnosed as CY1.9) The authors made it a rule at around year 2000 to perform staging laparoscopy for all patients with linitis plastica, and to avoid surgery not only for those with peritoneal deposits but also for those with the CY1 status.13)
EFFECT OF NOVEL ANTICANCER AGENTS AGAINST MINIMAL DISEASE IN THE PERITONEAL CAVITY
The recent introduction of more effective anticancer drugs into the clinic have changed the picture of CY1 disease in the Far East.14-16) Tegafur-gimeuracil-oxo (S-1), an oral fluoropyrimidine, has shown its potential to treat intraperitoneal micrometastasis in a phase III adjuvant trial, ACTS-GC, where the incidence of peritoneal carcinomatosis has declined considerably after curative surgery for Stage II/III gastric cancer through administration of S-1, compared with treatment by surgery alone.17) While this phase III trial was on-going, the authors conducted a phase II trial to test postoperative S-1 after radical surgery for CY1-stage gastric cancer (CY1 cancer is classified as Stage IV and is ineligible for the ACTS-GC trial), with no other signs of incurable disease. Forty-seven patients were accrued, among whom 14 patients had linitis plastica-type cancer. The two-year survival rate, this study’s primary endpoint, was as high as 46%, and was significantly superior to the historical control.18) Further follow-up has shown that 26% of patients survived for 5 years. Five-year survival data in this study cannot be dismissed as trivial, given that patients with gastric cancer who are treated by chemotherapy alone rarely survive for 5 years. Thus, radical surgery could be recommended for patients with CY1 as the sole non-curative factor, provided that adequate chemotherapy is given perioperatively. Patients with linitis plastica did suffer from an inferior prognosis, but there were long-term survivors even among this population (Fig. 1). Patients who had a small number of visible peritoneal deposits in addition to the CY1 status were also eligible for this study, provided that these deposits were resected along with the stomach to leave the CY1 status as the only non-curative factor. Surprisingly, survival of this subset of patients was not inferior to that of the patients who had no visible peritoneal deposits (Fig. 2). Thus, a proposal could be made to define the CY1 status as "optimally debulked gastric cancer." Surgery to downsize the tumors could be meaningful only when chemotherapy has sufficient power to control minimal disease. Arguably, surgery to dissect all tumors >1 cm in diameter is considered to be a useful part of multimodal therapy for ovarian cancer (optimally debulked ovarian cancer), which is generally more sensitive to chemotherapy. In case of gastric cancer, the size of residual disease will have to be microscopic to expect benefits from chemotherapy, but if all macroscopic disease could be removed through surgery, there is some hope.
Fig. 1.
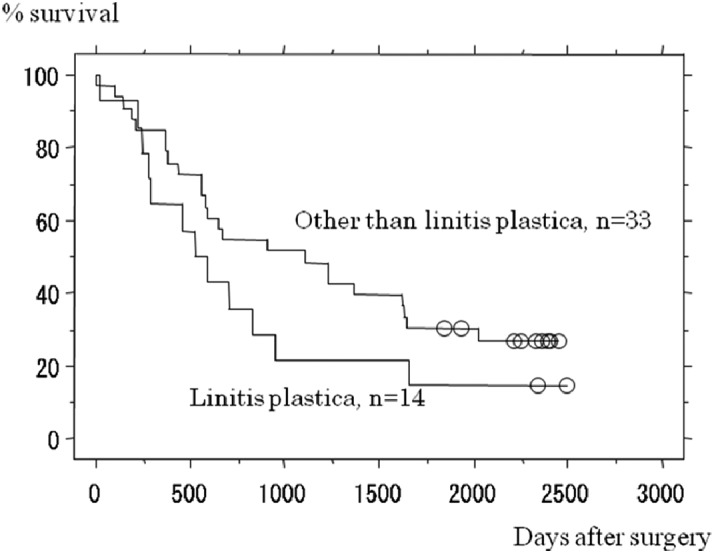
Survival curves for patients entered onto the CCOG0301 trial (a phase II trial testing radical surgery followed by postoperative chemotherapy with S-1 for patients positive for peritoneal washing cytology), stratified by whether or not the primary cancer was linitis plastica-type. Difference between survival curves as assessed by Logrank test was not significant (p=0.1527).
Fig. 2.
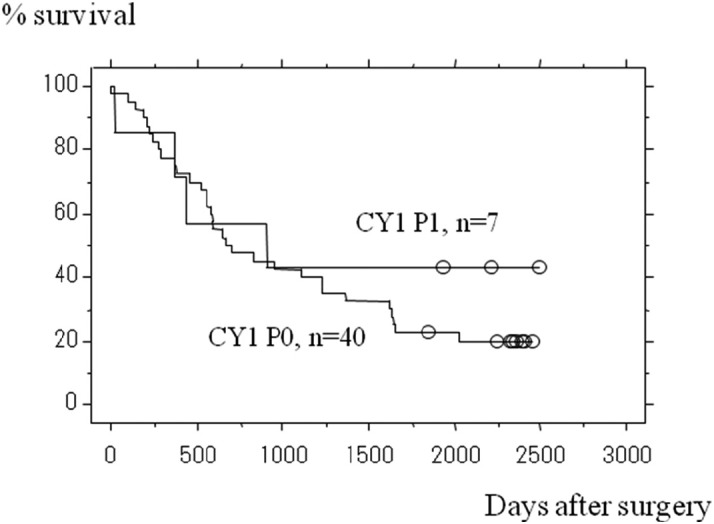
Survival curves for patients entered onto the CCOG0301 trial (a phase II trial testing radical surgery followed by postoperative chemotherapy with S-1 for patients positive for peritoneal washing cytology), stratified by the presence or absence of macroscopic peritoneal deposits. Difference between survival curves as assessed by Logrank test was not significant (p=0.4770).
COMBINATION CHEMOTHERAPY AFTER RADICAL SURGERY FOR GASTRIC CANCER
The combination of S-1 with cisplatin,19) in addition to showing superior overall survival in the advanced/metastatic setting than S-1 monotherapy,20) has shown in a subset analysis of the same phase III trial a remarkable efficacy against peritoneal disease. An attempt to treat Stage IV patients by S-1/CDDP following gastric resection, however, was a failure, showing poor relative drug intensity due to adverse events and patient refusal.21) Adverse reactions such as nausea, anorexia and general fatigue were found to be intolerable during the early postoperative period for patients who underwent gastrectomy. Takahari et al. have shown that compliance could be improved considerably through treating patients with S-1 only in the first cycle, and adding CDDP in the subsequent cycles.22) The difficulty in administering the S-1/CDDP combination shortly after surgery prompted other investigators to perform staging laparoscopy, to detect CY1 and P1 patients and treat them preoperatively.23) Survival of patients whose cytologic examination turned negative after the preoperative S-1/CDDP generally did well, while the prognosis of those with no effect against the CY1 status was poor. Preoperative treatment combined with laparoscopy is therefore a useful option that enables the selection of appropriate patients to be sent for radical surgery, but its prognostic effect in the intention-to-treat population as a whole remains to be elucidated.
INTRAPERITONEAL ADMINISTRATION OF CISPLATIN
Before the advent of S-1, several investigators looked at intraperitoneal (IP) administration of anticancer drugs for the treatment and prevention of peritoneal carcinomatosis. Taxanes have been found to not readily absorb into the blood stream when given IP,24) and the ratio of drug concentration between the peritoneal cavity versus peripheral blood is thus kept outstandingly high for a prolonged period. On the contrary, cisplatin was found to be absorbed into the blood stream quite promptly upon IP. As this finding implicates, the attempt to incorporate IP cisplatin, given on the day of surgery with one postoperative course of intravenous 5FU/CDDP and followed by UFT in T4-stage cancer (CY0), was an utter failure, and the survival curve was identical to that of the surgery alone group.25) Although compliance with the lengthy oral UFT treatment was reportedly poor, almost all patients should have had IP CDDP, since it had to be administered during surgery. Another Korean group has shown in their phase III trial that an adjuvant "treatment A" containing IP CDDP showed a significantly longer survival than the "treatment B," which contained no IP CDDP. However, since the patients in the treatment A group were also given intravenous mitomycin C on the day of surgery, followed by a longer postoperative course of the capecitabine/CDDP combination instead of a shorter treatment with capecitabine alone in the treatment B, IP CDDP could not be interpreted as the only reason for improvement of survival in this population. We concluded from these and other studies that a single dose of IP CDDP is insufficient to show efficacy, although it is not possible to discard at this stage the theory that repeated administration of CDDP through IP reservoir is effective.
INTRAPERITONEAL ADMINISTRATION OF PACLITAXEL AND ONGOING RANDOMIZED TRIAL
More recently, several investigators turned to paclitaxel and made sporadic efforts claiming that this drug is effective when given as IP.26) However, these attempts did not follow the general rules, i.e., a dose-finding study should be conducted first, followed by a phase II study to evaluate its efficacy (since most patients suffer from cancer with non-measurable lesions, the primary endpoint needs to be survival) and safety. Moreover, IP paclitaxel has not been formally approved by the Ministry of Health, Labor and Welfare in Japan, and its use, along with other government-approved modes of treatment, will result in either the investigator or the patient paying all medical expenses for the treatments given, outside of the social insurance system. To overcome this critical situation, the authors used the "kodo iryo (meaning "high standard medical practice")" system, whereby the details and current status of the unapproved treatment, including the known safety profile and the advantage and disadvantage of providing such a drug or technique, need to be fully reported to the Ministry. When the Ministry of Health, Labor and Welfare grants the "kodo iryo," the cost of treatment other than the new technique will be covered by the insurance, while expenses needed for the new treatment could either be paid by the patients or the investigators. Medical practice performed using the "kodo iryo" will need to be scientifically designed, and generate evidence aimed at the future incorporation of the new treatment into general practice. Moreover, IP paclitaxel against peritoneal disease, with no validated surrogate endpoint to show efficacy, must take a form of randomized trial to be regarded as "evidence-generating."
Motivated further by the result of a phase III study in ovarian cancer with peritoneal metastases, where the IP group had a significantly prolonged survival over the intravenous administration (IV) group,27) the authors consulted the government and were eventually permitted to use the "kodo iryo" system to conduct a multi-institutional randomized phase II trial to test the efficacy of IP paclitaxel following gastrectomy (the INPACT trial). In this trial, IP paclitaxel was compared to postoperative IV paclitaxel, given by the same schedule to eradicate micrometastasis in patients with particularly high risks for developing peritoneal carcinomatosis, such as linitis plastica-type, those with a minimal number of peritoneal deposits resected, and those with a CY1 status.28) In both treatment arms, a choice of either the S-1/CDDP combination or S-1 monotherapy was to be given after 7 doses of either the IP or IV paclitaxel, depending on the patients’ condition. A sequential use of IV paclitaxel with S-1 or S-1/CDDP merits further explanation. The sequential IV paclitaxel/S-1 combination is the new test arm being explored in a pivotal phase III trial, testing new modes of systemic adjuvant chemotherapy for patients with serosa-positive gastric cancer.29) The final results of this trial are expected to be publicized in the early 2013.
In the meantime, Ishigami et al. conducted a phase I/II study with a combination of S-1, IV paclitaxel and IP paclitaxel to control peritoneal carcinomatosis from gastric cancer. The combination was successful with a one-year survival rate of 78% and a median survival time of 23.6 months, for a group of previously untreated patients with extensive peritoneal metastases.30) They also were able to use the "kodo iryo" system to conduct a randomized phase III trial, and compared this combination in the first-line setting with S-1/CDDP, the current standard, in patients with previously untreated gastric cancer and gross peritoneal metastases (the PHOENIX-GC trial). Patients with minimal peritoneal metastases were also eligible for the PHOENIX-GC trial, and this intraperitoneal and systemic treatment delivered in combination with surgery could be extremely promising for eradicating minimal peritoneal disease.
HYPERTHERMIC INTRAPERITONEAL CHEMOTHERAPY
The most aggressive treatment that could be proposed for selected cancer patients with peritoneal disease would be hyperthermic intraperitoneal chemotherapy (HIPEC), in which patients receive intraperitoneal perfusion of a heated solution containing anticancer drugs.31) This technique was attempted in the 1980’s by several Japanese groups to treat gastric cancer with peritoneal metastasis, alone and in combination with surgery ranging from gastrectomy to total peritonectomy. Unfortunately, variations in the drugs and devices used, the quality of surgery, and other factors evidenced in various case series reported at the time prevented a majority of surgeons from being convinced that this modality was efficacious. In addition, the toxicity and complexity of the treatment did not allow HIPEC to be recognized as a candidate for standard treatment. More recently, this modality was used to treat tumors of the peritoneal origin by specialists in peritoneal surface malignancy, and some of these specialists began to expand the indication of HIPEC to include peritoneal metastases from cancers of the colorectal and ovarian origin. Gastric cancer, with its tendency to aggressively metastasize throughout hematogenous and lymphatic pathways in addition to peritoneal dissemination, may be the last cancer to benefit from this modality. Nevertheless, addition of HIPEC after surgery for CY1-stage gastric cancer could lead to the elimination of minimal residual disease in the peritoneal cavity.
CONCLUSIONS
Prognosis of gastric cancer patients with free cancer cells in the peritoneal cavity had been extremely poor. However, around 25% of these patients can live for 5 years following radical gastrectomy plus chemotherapy, available as of 2012. Further trials are now being conducted to explore the potential of repeated intraperitoneal chemotherapy for this population in Japan.
REFERENCES
- 1).Maehara Y, Hasuda S, Koga T, Tokunaga E, Kakeji Y, Sugimachi K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg 2000; 87: 353–7. [DOI] [PubMed]
- 2).Yoo C, Noh S, Shin D, Choi S, Min J. Recurrence following curative resection for gastric carcinoma. Br J Surg 2000; 87: 236–42. [DOI] [PubMed]
- 3).Kodera Y, Nakanishi H, Ito S, Mochizuki Y, Ohashi N, Yamamura Y, Fujiwara M, Koike M, Tatematsu M, Nakao A. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: analysis of real time reverse transcriptase-polymerase chain reaction after 5 years of followup. J Am Coll Surg 2006; 202: 231–6. [DOI] [PubMed]
- 4).Kodera Y, Nakanishi H, Ito S, Yamamura Y, Fujiwara M, Koike M, Hibi K, Ito K, Tatematsu M, Nakao A. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: detection of cytokeratin 20 mRNA in peritoneal washes, in addition to detection of carcinoembryonic antigen. Gastric Cancer 2005; 8: 142–8. [DOI] [PubMed]
- 5).Mori K, Suzuki T, Uozaki H, Nakanishi H, Ueda T, Matsuno Y, Kodera Y, Sakamoto H, Yamamoto N, Sasako M, Kaminishi M, Sasaki H. Detection of minimal gastric cancer cells in peritoneal washings by focused microarray analysis with multiple markers: clinical implications. Ann Surg Oncol 2007; 14: 1694–702. [DOI] [PubMed]
- 6).Aiko T, Sasako M. The new Japanese Classification of Gastric Carcinoma: Points to be revised. Gastric Cancer 1998; 1: 25–30. [DOI] [PubMed]
- 7).Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, Cook P. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet 1996; 347: 995–9. [DOI] [PubMed]
- 8).Mezhir JJ, Shah MA, Jacks LM, Brennan MF, Coit DG, Strong VE. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol 2010; 17: 3173–80. [DOI] [PubMed]
- 9).Fukagawa T, Katai H, Saka M, Morita S, Sasajima Y, Taniguchi H, Sano T, Sasako M. Significance of lavage cytology in advanced gastric cancer patients. World J Surg 2010; 34: 563–8. [DOI] [PubMed]
- 10).Badgwell B, Cormier J, Krishnan S, Yao J, Staerkel G, Lupo PJ, Pisters P, Feig B, Mansfield P. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol 2008; 15: 2684–91. [DOI] [PubMed]
- 11).Leake PA, Cardoso R, Seevaratnam R, Lourenco L, Helyer L, Mahar A, Rowsell C, Coburn NG. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer 2011; 15 Suppl 1: 27–37. [DOI] [PubMed]
- 12).Kodera Y, Nakanishi H, Ito S, Mochizuki Y, Yamamura Y, Fujiwara M, Hibi K, Ito K, Akiyama S, Tatematsu M, Nakao A. Detection of disseminated cancer cells in linitis plastica-type gastric carcinoma. Jpn J Clin Oncol 2004; 34: 525–31. [DOI] [PubMed]
- 13).Kodera Y, Yamamura Y, Ito S, Kanemitsu Y, Shimizu Y, Hirai T, Yasui K, Kato T. Is Borrmann type IV gastric carcinoma a surgical disease? An old question revisited with reference to the result of peritoneal washing cytology. J Surg Oncol 2011; 78: 175–81. [DOI] [PubMed]
- 14).Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 1998; 34: 1715–20. [DOI] [PubMed]
- 15).Sugimachi K, Maehara Y, Horikoshi N, Shimada Y, Sakata Y, Mitachi Y, Taguchi T. An early phase II study of oral S-1, a newly developed 5-fluorouracil derivative for advanced and recurrent gastrointestinal cancers. The S-1 Gastrointestinal Cancer Study Group. Oncology 1999; 57: 202–10. [DOI] [PubMed]
- 16).Yamaguchi K, Tada M, Horikoshi N, Otani T, Takiuchi H, Saitoh S, Kanamaru R, Kasai Y, Koizumi W, Sakata Y, Taguchi T; Paclitaxel Gastric Cancer Study Group in Japan. Phase II study of paclitaxel with 3-h infusion in patients with advanced gastric cancer. Gastric Cancer 2002; 5: 90–5. [DOI] [PubMed]
- 17).Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K; ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007; 357: 1810–20. [DOI] [PubMed]
- 18).Kodera Y, Ito S, Mochizuki Y, Kondo K, Koshikawa K, Suzuki N, Kojima H, Kojima T, Matsui T, Takase T, Tsuboi K, Fujiwara M, Nakao A; Chubu Clinical Oncology Group. A phase II study of radical surgery followed by postoperative chemotherapy with S-1 for gastric carcinoma with free cancer cells in the peritoneal cavity (CCOG0301 study). Eur J Surg Oncol 2009; 35: 1158–63. [DOI] [PubMed]
- 19).Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, Shirao K, Matsumura Y, Gotoh M. Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer 2003; 89: 2207–12. [DOI] [PMC free article] [PubMed]
- 20).Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008; 9: 215–21. [DOI] [PubMed]
- 21).Kodera Y, Ishiyama A, Yoshikawa T, Kinoshita T, Ito S, Yokoyama H, Mochizuki Y, Ito H, Tsuburaya A, Sakamoto J, Nakao A; Chubu Clinical Cancer Group. A feasibility study of postoperative chemotherapy with S-1 and cisplatin (CDDP) for gastric carcinoma (CCOG0703). Gastric Cancer 2010; 13: 197–203. [DOI] [PubMed]
- 22).Takahari D, Hamaguchi T, Yoshimura K, Katai H, Ito S, Fuse N, Kinoshita T, Yasui H, Terashima M, Goto M, Tanigawa N, Shirao K, Sano T, Sasako M. Feasibility study of adjuvant chemotherapy with S-1 plus cisplatin for gastric cancer. Cancer Chemother Pharmacol 2011; 67: 1423–8. [DOI] [PubMed]
- 23).Okabe H, Ueda S, Obama K, Hosogi H, Sakai Y. Induction chemotherapy with S-1 plus cisplatin followed by surgery for treatment of gastric cancer with peritoneal dissemination. Ann Surg Oncol 2009; 16: 3227–36. [DOI] [PubMed]
- 24).Kodera Y, Ito Y, Ito S, Ohashi N, Mochizuki Y, Yamamura Y, Koike M, Fujiwara M, Nakanishi H, Nakao A. Intraperitoneal paclitaxel: a possible impact of regional delivery for prevention of peritoneal carcinomatosis in patients with gastric carcinoma. Hepatogastroenterology 2007; 54: 960–3. [PubMed]
- 25).Miyashiro I, Furukawa H, Sasako M, Yamamoto S, Nashimoto A, Nakajima T, Kinoshita T, Kobayashi O, Arai K; Gastric Cancer Surgical Study Group in the Japan Clinical Oncology Group. Randomized clinical trial of adjuvant chemotherapy with intraperitoneal and intravenous cisplatin followed by oral fluorouracil (UFT) in serosa-positive gastric cancer versus curative resection alone: final results of the Japan Clinical Oncology Group trial JCOG9206-2. Gastric Cancer 2011; 14: 212–8. [DOI] [PubMed]
- 26).Tamura S, Miki H, Okada K, Miyake T, Yoshimura M, Suzuki R, Nakahira S, Nakata K, Okamura S, Sugimoto K, Takatsuka Y. Pilot study of intraperitoneal administration of paclitaxel and oral S-1 for patients with peritoneal metastasis due to advanced gastric cancer. Int J Clin Oncol 2008; 13: 536–40. [DOI] [PubMed]
- 27).Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA; Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006; 354: 34–43. [DOI] [PubMed]
- 28).Kodera Y, Imano M, Yoshikawa T, Takahashi N, Tsuburaya A, Miyashita Y, Morita S, Nakao A, Sakamoto J, Sasako M. A randomized phase II trial to test the efficacy of intra-peritoneal paclitaxel for gastric cancer with high risk for the peritoneal metastasis (INPACT trial). Jpn J Clin Oncol 2011; 41: 283–6. [DOI] [PubMed]
- 29).Tsuburaya A, Sakamoto J, Morita S, Kodera Y, Kobayashi M, Miyashita Y, Macdonald JS. A randomized phase III trial of post-operative adjuvant oral fluoropyrimidine versus sequential paclitaxel/oral fluoropyrimidine; and UFT versus S1 for T3/T4 gastric carcinoma: the Stomach Cancer Adjuvant Multi-institutional Trial Group (Samit) Trial. Jpn J Clin Oncol 2005; 35: 672–5. [DOI] [PubMed]
- 30).Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, Otani K, Kamei T, Soma D, Miyato H, Yamashita H, Nagawa H. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol 2010; 21: 67–70. [DOI] [PubMed]
- 31).Bucher BL, Piso P, Verwaal V, Esquivel J, Derraco M, Yonemura Y, Gonzalez-Moreno S, Pelz J, Konigsrainer A, Strohlein M, Levine EA, Morris D, Bartlett D, Glehen O, Garofalo A, Nissan A. Peritoneal carcinomatosis: cytoreductive surgery and HIPEC--overview and basics. Cancer Invest 2012; 30: 209–24. [DOI] [PubMed]


