ABSTRACT
Studies that seek to determine the etiology of schizophrenia through pathological images and morphological abnormalities of the brain have been conducted since the era of E. Kraepelin, and pioneers in neuropathology such as A. Alzheimer have also eagerly pursued such studies. However, there have been no disease-specific findings, and there was a brief era in which it was said that "schizophrenia is the graveyard of neuropathologists." However, since the 1980s, neuroimaging studies with CT and MRI etc., have been used in many reports of cases of schizophrenia with abnormal brain morphology, thus generating renewed interest in developments within brain tissue and leading to new neuropathological studies. There are now many reports in which, in addition to morphological observations, cell distribution and the like are image-processed and statistically processed through computers. Due to methodological problems in making progress in the field of cerebral pathology, we have not yet been able to observe disease-specific findings, although there are several findings with high certainty. However, the neurodevelopmental hypothesis has been supported as being able to reasonably explain the accumulated findings of previous studies. At the same time, results of recent molecular-biological studies have revealed the risk genes for this disease, and because many of those genes are associated with functions related to nerve differentiation, development, and plasticity, there is growing interest in their correlations with cerebral pathology. We are now on the verge of uncovering the etiology of this disease by integrating cerebral neuroimaging, molecular genetics, and cerebral neuropathology. In that sense, neuropathological studies of this disease from new viewpoints have become essential.
Key Words: Schizophrenia, Neuropathology, Postmortem brain, Neurodevelopmental hypothesis
INTRODUCTION
More than 100 years ago, E. Kraepelin (1856–1926) hypothesized that organic abnormalities of the brain occurred in a syndrome called precox dementia (i.e., schizophrenia). Since then, the cerebral pathology of schizophrenia has been examined extensively by pioneers in the field of neuropathology, such as A. Alzheimer (1864–1915) and others. From this era, A. Alzheimer’s observation on "Psychotics (Psychosen)" is particularly notable.1) Prior to his landmark studies on dementia patients, he had made detailed observations of the cerebral pathology of psychotic patients. Consequently, he found that patients with psychotic symptoms exhibited no gliosis. This indicated that the brain had not been subjected to a great enough impact to cause the loss of nerve cells after the brain had achieved growth, and it was neuropathologically estimated that prognoses for such cases would be better than those for dementia patients. This was a significant finding regarding the pathological conditions of schizophrenia, which was called "presenile dementia" at the time. This finding has also greatly influenced current studies aiming to reveal the pathological conditions of this illness. Namely, it asserted that the etiology of schizophrenia involves more neurodevelopmental elements than neurodegeneration elements. This result has led to the neurodevelopmental hypothesis of this disorder that was later propounded; however, this was all that could be discovered in the era of A. Alzheimer. Neuropathological research of this disease continued, but historically, compared to the great advances revealing the neuropathological conditions of degenerative diseases such as Alzheimer’s disease, no significant findings other than the absence of gliosis were found. As an expression describing this prolonged era, it was said that "schizophrenia is a graveyard for neuropathologists." At the 1st International Congress of Neuropathology in 1952 (in Rome), the conclusion that "there is no neuropathology of schizophrenia" was announced with no objections. Over the following 30 years, research on the cerebral pathology of schizophrenia declined. In Japan, around 1960, Tatetsu2) proceeded with an extensive study regarding "the contribution of the morphological background of schizophrenia," reporting findings such as changes in the nerve cell’s process; increased thickening and argyrophilic property of axons and dendrites, particularly apical dendrites; sharpened contrast thereof in specimen media; and enlarged neuron nuclei and shrunken neuron-cell circumference. At the 4th International Congress of Neuropathology in 1961 (in München), Tatetsu delivered a lecture on his cerebral pathological findings of schizophrenia, and it is said that he was basked in the audience’s applause. Although similar studies have been conducted subsequently, many have proved fruitless, and there have been very few new reports. This is partially due to a background specific to brain tissue. Namely, the cranial nerve of a brain undergoing neuropathological research is subjected to various effects before the brain is used as a microscopic specimen. It is subjected to effects caused by various elements, such as the nutritional state during the developmental and maturational stages of the brain, history of infection, history of spasmodic disorders, intake of neural stimuli (e.g., alcohol), changes accompanying the aging process, and low oxygen during the agonal stage. Traces of these effects are left on tissues to various extents. Therefore, there have been significant differences among individuals, and this background of having difficulty in identifying which findings were disease-specific created barriers against an approach toward revealing the disease’s etiology, thus leading to a decline in this study approach.
Subsequently, in the 1980s, with progress in brain imaging technology such as CT, morphological abnormalities of schizophrenia were reported, and moreover, with MRI, PET, SPECT, and the like, detailed brain images including the functions of schizophrenia cases were examined. Now, the occurrence of volume changes of the brain (lateral ventricular enlargement and decreases in volume of the medial temporal lobe) in schizophrenia cases has been accepted by many researchers.3) Moreover, in longitudinal studies, the occurrence of progressive volume changes of the brain in the disease’s course has also been accepted in reproducible research reports.4) As an effect of these imaging studies, neuropathological studies were conducted again. At the 11th International Congress of Neuropathology in 1990, a workshop for the "Neuropathology of schizophrenia" was conducted. In addition to conventional observations of specimens, studies now use techniques such as image analysis with a computer, and immunohistological special staining. Moreover, in the 1990s, striking progress was made in genome research and a number of risk genes for schizophrenia were reported. Among these genes, several potential candidate genes are being proven to be associated with the development and differentiation of nerves and the formation of neural networks, and it can be said that neural pathology, image information, and genome research are on the verge of integrating these findings. With the three elements of advances in neuroimaging, brain tissue staining, and molecular psychiatry, it is considered that histological studies are essential not only for revealing etiology, but also for converging various study results.
OVERVIEW OF MORPHOLOGICAL STUDIES
A number of morphological studies on schizophrenia were reported throughout the 1980s and 1990s. Investigations of pathological research studied the entire forebrain. As with reports of imaging studies, many of these focused on the medial temporal cortex, the limbic cortex, and the frontal lobe. They can be classified into: quantitative studies including volume changes of the tissue and decreases in the size, density, and number of cells; and qualitative studies including disordered arrangement of neurons, and cytoarchitectic abnormality or misplaced neurons (Table 1). A problem with this series of morphological studies is that these variations do not indicate visible accumulations or morphological changes of abnormal proteins, but are minute changes that can be confirmed only after image analysis and statistical analysis. There may be insufficient evidence to directly associate these changes observed in brain tissue, on which the effects of various events occurring in the body from birth to death have been left as traces, with the etiology of schizophrenia. Therefore, some study results have poor reproducibility, but among them, a relatively high number of findings that appear strong in terms of certainty have been collected (Table 2). Decreased cortical volume, which is substantially confirmed in these reports, is consistent with details reported regarding atrophy of the parieto-temporal lobe in schizophrenia cases in neuroimaging studies with MRI.24,25) In addition, in examining these findings, it should be noted that the tissue is more flexible than conventionally believed. Various animal experiments have revealed that neurogenesis occurs once in the completed brain and the synapses and neuronal processes are then actively formed.26) This volume change is reported to be suppressed by drug treatment intervention in actual schizophrenic patients.27)
Table 1.
Principal reports in morphological abnormality in templal cortex, limbic and frontal cortex in schizophrena
| Are | Findings | Reporter | |
|---|---|---|---|
| Temporal lobe Limbic | quantity | Volume loss in hippocampus, amygdala, parahippocampus | Bogerts, 19855, 19906; Altshuler, 19907 |
| Enlargement in left ventlicle | Borgerts, 19855; Heckers, 19908, 19919 | ||
| Volume loss in hippocampal formation | Colter, 198710; Heckers, 19919 | ||
| Neuronal loss in hippocampal formation | Benes, 199811; Casanova, 199012 | ||
| quality | Cytoarchitectic abnormality in hippocampus, cinglate | Arnold, 1991a13; Conrad, 199114 | |
| Distribution abnormality in NADPH-d positive neuron | Akbarian, 1993b15 | ||
| Frontal lobe | quantity | Neuronal loss (number/density) | Benes, 198616; Arnold, 199517 |
| Decreased density of internal neurons | Benes, 199118 | ||
| Increased vertical axsons in anterior cingrate cortex | Benes, 1987b19 | ||
| Narrowing cell body & decreased density in anterior cingrate | Chana, 200320 | ||
| quality | Abnormal array of neurons in layer II | Benes, 198616, 1987a21 | |
| Distribution abnormality in NADPH-d positive neuron | Akbarian, 1993a22 | ||
| (Modified from Harrison, 199923) | |||
Also, some findings were reported in cerebellum, brainstem, nucleus basalis, thalamus, corpus callosum and another area of cerebral cortex.
Table 2.
Certainty in schizophrenia neuropathology
| Macroscopic findings | Strength of evidence |
| Enlarged lateral and third ventricles | shown by meta-analysis |
| Decreased cortical volume | shown by meta-analysis |
| The above changes present in first-episode patients | strong |
| Disproportionate volume loss from temporal lobe (incl. hippocampus) | strong |
| Decreased thalamic volume | good |
| Cortical volume loss affects grey rather than white matter | good |
| Enlarged basal ganglia secondary to antipsychotic medication | moderate |
| Histological findings | |
| Absence of gliosis as an intrinsic feature | good |
| Smaller cortical and hippocampal neurons | good |
| Fewer neurons in dorsal thalamus | good |
| Reduced synaptic and dendritic markers in hippocampus | good |
| Maldistribution of white matter neurons | moderate |
| Miscellaneous | |
| Alzheimer’s disease is not more common in schizophrenia | Shown by meta-analysis |
| (Modified from Harrison, 199923) | |
Among these morphological studies, some interesting reports include a series of studies by Akbarian et al. that focus on cells containing NADPH-d (nicotinamide adenosine dinudeotide phosphate-diaphorase).15,22) NADPH-d is a coenzyme for a NO (nitric oxide) synthesis-related electron transport system. NO itself is involved in the plasticity of synapses in the central nervous system, learning, memory, etc. It has been found that NADPH-d coexists with a synthetase of this NO, NOS (nitric oxide synthetase). Also, NADPH-d is found resistant to nerve cell damage factors including degeneration and ischemia. In addition, the NADPH-d-containing cells are interneurons derived from subplates in the fetal period, which play an important role in differentiation and development of the central nerve, and the majority of these cells have properties for finishing their role in the maturation process and approaching cell death. Anatomically, the NADPH-d-containing cells extend over the layers II to VI of the cerebral cortex and the cerebral white matter. Since NADPH-d-containing cells are resistant to cytopathy in the developmental process of the brain, it is considered that this remaining cell distribution indicates the developmental process of the central nervous system. Based on these concepts, Akbarian et al.15,22)observed distributions of NADPH-d-containing cells in the lateral prefrontal area and the cortex/white matter of the temporal lobe in a postmortem schizophrenic brain. Compared to a control brain, it was found that NADPH-d-containing cells had decreased in the cortex and increased in the deep white matter. This led to the conclusion that in the schizophrenic brain, the nerve cells exhibit abnormal migration from the deep layer toward the upper cortex layer in the developmental process.
Our group has also conducted studies using immunohistological techniques, not only regarding cytoarchitectic images of the nerve cells, but also regarding which functional nerve systems exhibit changes in schizophrenia cases.28,29) Research on both aspects of function and anatomy focusing on these neurotransmitters and neuromodulators are important.
FROM MORPHOLOGY TO THE NEURODEVELOPMENTAL HYPOTHESIS, AND ON TO NEURAL PLASTICITY
In order to uncover the characteristics of cytoarchitectonic abnormalities in these morphological changes, the presence or absence of gliosis on the tissue is noted. In many studies, in addition to classic staining techniques such as Holzer staining, immunohistological staining of the glia with GFAP has often been performed, but no gliosis has been found.30) As described above, the absence of gliosis which was first reported by A. Alzheimer more than 100 years ago, has been rediscovered. From this finding, it is understood that this morphological change without gliosis occurs in the very early period of the formation of the nervous system (i.e., in the fetal period early in gestation). Therefore, the neurodevelopmental hypothesis, which states that developmental dysfunction is greatly involved in the etiology of schizophrenia, has been propounded. This is also supported by many reports indicating that various problems related to learning functions and social adjustment capabilities, as well as abnormal motor functions, are exhibited before schizophrenia is clinically developed31-33). Moreover, it may also be related to the fact that minor physical anomalies including decreased head circumference at birth are commonly observed.34) In addition, the relationship between neurodevelopmental dysfunction and schizophrenia is supported by reports indicating that abnormalities during the perinatal period have an odd ratio of 2.0 for the subsequent development of schizophrenia35) and that low birth weight has an odd ratio of 2.6 for the subsequent development of schizophrenia.36) There have been several reports indicating that effects such as infections during the fetal period directly act upon these developmental problems, and are associated with the development of schizophrenia.
From reports indicating that volume changes of the brain in primary schizophrenia cases are continuously observed in neuroimaging studies, and from the fact that structural abnormalities of the brain are observed over time in the course of schizophrenia,37) it has been assumed that, in addition to the aforementioned neuronal developments, continued neuronal degeneration is also involved. As a very commonly-known clinical fact, at the onset of schizophrenia, noticeable psychotic symptoms such as hallucinations and delusions, which are called positive symptoms, are commonly observed in the foreground, but over the course, so-called negative symptoms, such as decreased motivation and decreased societal participation, become predominant, which makes social adjustment very difficult without sufficient treatment intervention. For these cases, it is estimated that original neurodevelopmental dysfunctions are followed by persistent changes of the brain; in other words, there is impaired adaptability or plasticity of the brain.
On the other hand, based on studies of families, twins, and adopted children, it has been revealed that there is a genetic background for schizophrenia.38) In current molecular genetic studies, no definitive responsible genes for schizophrenia have yet been found, but it is estimated that the cause may be an interaction of multiple genes, and several potential risk candidate genes are being reported.
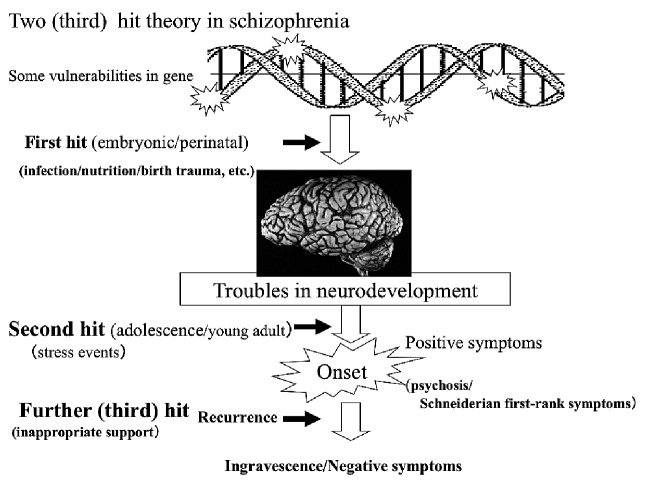
To consider the onset and pathological model of schizophrenia as described above, the two hit theory used in physical disorders has been propounded (Fig. 1). According to this theory, damage such as infection during the perinatal period is added as an original genetic weaknesses39) (so called "first hit"), which impairs neuronal development, thus forming a potential basis for onset. Subsequently, during adolescence and early adulthood, psychological stressors (so called "second hit") cause symptoms to become manifest. Subsequently, exacerbation and recurrence are repeated and negative symptoms become predominant, and it is considered that these lead to the development of neural degeneration as observed in imaging studies. Recently, intervention in the early stages of the disease has become important, and that period is called the critical period. It is believed that the prognosis of the disease will be different if that period is missed. Missing opportunities for appropriate intervention during that period or providing inappropriate responses is called the third hit, which is believed to accelerate the progression of the disease.
Fig. 1 .
The two (third) hits theory of schizophrenia’s illness course. Some vurnerabilities exist in genes. Some impacts, including infection or birth trauma etc. in the embryonic/perinatal period on the brain are the first hit. These factors may later induce neurodevelopmental dysfunction. Psychological stressors will trigger the onset of schizophrenic symptoms as a second hit. Lack of appropriate support will lead to progression of the pathology of the illness as a third hit.
In considering the factors for onset after the first hit, a series of studies by Benes et al.16,18,19,21,40) can be cited. In the cingulate gyrus of a postmortem schizophrenic brain, Benes et al. found a narrowed functional column (smallest functional cell group) in the cortex, increased vertical neuronal fibers (mainly excitatory fibers), and decreased inhibitory inter-neurons in the cortex. According to these observations, in the cingulate gyrus, vertical axons and pyramidal cells form excessive excitatory synapses, and moreover, inhibitory inter-neurons are decreased and the pyramidal cells are susceptible to excessive firing. This is a very interesting model because abnormalities of neural circuits are estimated based on these morphological abnormalities. These studies are related to the hypothesis that thinking impairments and cognitive impairments in schizophrenia are related to abnormalities of the neural network composed of axons, dendrites, and synapses.
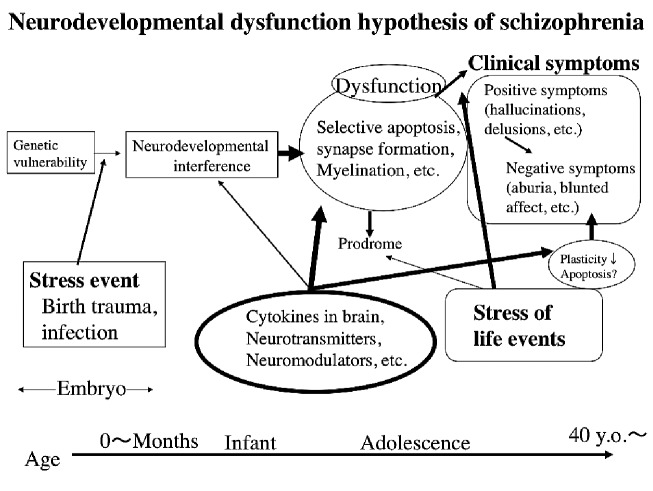
When the neurodevelopmental hypothesis is considered from this viewpoint in combination with subsequent progressive pathological conditions, it can be represented in a diagram (Fig. 2). This shows the two-hit theory described earlier in further functional detail. It shows that neurotransmitters, neuromodulators, and neurotrophic factors are involved in nerve development, maturation, and the differentiation and formation of networks, indicating that functional maintenance actions of the nerve systems over an entire lifetime, such as plasticity and retention of synapses, are very important. In relation to this hypothesis, there have been many findings regarding, for example, BDNF (Brain Derived Neurotrophic Factor), which is one of the important cytokines involved in neuronal development, in schizophrenia cases.27,41,42)
Fig. 2.
Conceptual diagram of the neurodevelopmental dysfunction hypothesis of schizophrenia. Some genetic vulnerabilities may induce interference in neurodevelopment during growth period via some stressful events. Neural dysfunction, including selective apoptosis, synaptic formation and/or myelination may lead to inapporopriate neurotransmisson and clinical symptoms. Individual circumstances and life events of each patient may affect characteristics of clinical symptoms to varying degrees. In every stage, many cytokines, neurotransmitters or neuromodulators support neuronal development. Failure of these supportive mechanisms is closely related to the etiology of schizophrenia. Recent studies indicate that negative symptoms induce reduced synaptic plasticity due to insufficient neurotrophic action.
CHANGES IN BRAIN MORPHOLOGY, SYNAPSES, AND NERVE FIBERS
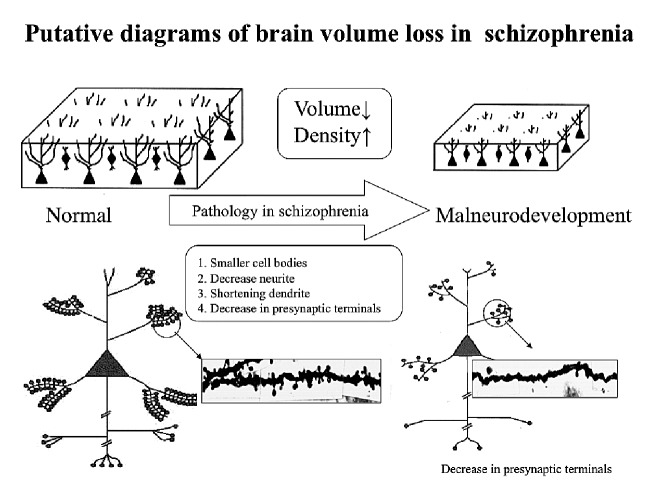
Now, how should volume changes of schizophrenic brains observed in brain images be understood? Selemon et al.43) estimated that volume changes of schizophrenic brains are due to insufficient development of cell bodies and the neuronal processes of schizophrenic nerve cells, which lead to reductions in the thickness of the cortex. They propounded this as the "reduced neuropil hypothesis." The neuropil (neuronal process) in the cortex is basically composed of axons, dendrites, and pre- and post-synaptic organs. Decreased dendritic spines of pyramidal cell neuronal processes and decreased lengths of the dendrites in the cortex of the frontal and temporal lobes in schizophrenia cases have been reported (see Fig. 3). In addition, decreases in the markers for synapses, synaptophysin, SNAP-25, MAP-2, synapsin, synataxin, complexin I & II, GAP43, etc., have also been reported (summarized in Table 3). Electrophysiological and neuropsychological studies have also associated these phenomena with failures in the dynamic interactions within the brain in schizophrenia cases. In addition, in electron-microscopic observations, abnormalities of synaptic density and accumulation, as well as morphological abnormalities of the dendritic spines, have been reported.74) Common cerebral sites in which these abnormalities are reported include the hippocampal region, the dorsolateral prefrontal area, and the anterior cingulate gyrus cortex, and it has been reported that presynaptic markers are decreased in these sites.
Fig. 3.
Decrease of brain volume may induce an increase of neuron density. Pathological factors including: 1. smaller cell bodies, 2. decrease neurites, 3. shortening of dendrites, and 4. A decrease in presynaptic terminals, is considered to induce volume decrease (modified from Selemon et al., 1995,43 Glantz et al., 2000,44 and Glantz et al., 200645).
Table 3.
Reports of abnormality in synaptic associate protein/neurite in schizophrenic brain
| Area | Fimdings | Reporter |
|---|---|---|
| Hippocampal temporolimbic | Decrease of MAP2/5 in hippocampal dendrite | Arnold, 1991b46 |
| Decrease of Synapsin in hippocampus | Browning, 199347 | |
| Decrease of Synaptophysinin hippocampus | Eastwood, 199548 | |
| Decrease of Complexin I and II | Harisson/Eastwood, 199849 | |
| Decrease of SNAP | Young, 199850 | |
| Decrease of Complexin I and II | Eastwood/Harrison, 200051 | |
| Decrease of Dysbindin1in subiculm | Talbot, 200452 | |
| Decrease of VGLUT1mRNAin hippocampus | Eastwood/Harrison, 200553 | |
| Decrease of the ratio of ComplexinI/ComplexinII | Sawada, 200554 | |
| Decrease of spine in CA3 of schizophrenia (electron microscopic) | Kolomeets, 200555 | |
| Frontal lobe | Decrease of NE positive-vertical fiber | Benes, 1987b19 |
| Decrease of density of synapse | Aganova/Uranova, 199256 | |
| Decrease of Synaptphysin in BA9,10,20 | Perrone-Bizzozero, 199657 | |
| Changes of distribution of TH-positive fiber | Benes, 199758 | |
| Increase of Synaptophysin, SNAP-25, Syntaxin in BA24 | Gabriel, 199759 | |
| Increase of Syntaxin in BA24 | Honer, 199760 | |
| Decrease of Synaptophysin in BA9,46 | Glanz/Lewis, 199761 | |
| Decrease of neurites of Pyramydal neuron | Garey, 199862 | |
| Decrease of SNAP-25in BA10,20/Increase in BA9 | Thompson, 199863 | |
| Selective decrease of GABAnergic nerve ending GABA | Woo, 199864 | |
| Decrease of Synaptophysin, SNAP-25 in BA10 | Karson, 199965 | |
| Decrease of Synaptophysin, Myelin basic protein in prefrontal cortex | Honer, 199966 | |
| Decrease density of GAT-1-positive neuron in Layer II-III of coretx | Pierri, 199967 | |
| Decrease expression of PSD95 in BA9 | Ohnuma, 200068 | |
| Increase of GAD65-immunoreactivity in Layer II-III of cortex | Benes, 200069 | |
| Increase of GABA(A)-receptor in BA46 | Volk, 200270 | |
| Decrease of SAP97 | Toyooka, 200271 | |
| Decrease of VAMP | Halim, 200372 | |
| Decrease of GABA membrane transporter1in BA46 | Konopaske, 200673 |
MAP: microtubule associated protein, SNAP: Synaptosoal associated protein, BA: Brodmann Area, NF: neurofilament, TH: Tyrosin Hydroxilase, GAT1: GABA membrane transporter1, PSD: Postsynaptic density protein95, GAD: Glutamate decarboxylase, VAMP: Vesicle associated Membrane protein
It is widely known that NMDA receptors are related to the plasticity of synapses for higher cognitive function via LPT (long-term potentiation). The activation of NMDA receptors is considered to cause calcium ions to flow into the cells, activate enzymes such as CaMKII, and synthesize proteins necessary for altering synapses via the CREB signaling pathway. From studies using genetically altered animals, CREB was found to be essential for the composition of memory via LTP.75) Moreover, the expression of NMDA receptors in postsynapses, the abnormal expression of subunits of relevant receptors such as NR1, NR2A, and NR28, and the relevant binding proteins PSD95, SAP97, and SAP98, have been reported.76)
FROM NEUROPATHOLOGY TO NEUROTRANSMITTER
It has been revealed that plasticity, not only of synapses but also serotonin and GABA itself, is related to neuronal development and nerve plasticity. Also, both of these are involved in the proliferation, migration, differentiation, synaptogenesis, and death of the nerve cells.77)
One of the classic hypotheses related to schizophrenia is the dopamine hypothesis, which is widely accepted because classic antipsychotic drugs have actions to block dopamine receptors. It has been collaterally proven that dopamine-releasing drugs such as methamphetamine cause schizophrenia-like symptoms. It has also been verified through meta-analyses that dopamine content and D2 receptor density are increased in schizophrenic brains.78) However, it is not clear whether this increase indicates an original pathological condition of the disease or is due to antipsychotic drugs.
Because LSD, which has a high affinity with serotonin receptors, causes psychotic symptoms, it has been believed that serotonin (5-TH) is also greatly involved in the pathological conditions of schizophrenia.79) In studies of postmortem brains, decreases in 5-HT2A receptors and increases in 5-HT1A have been reported.80) The serotonin nervous system and the dopamine nervous system have been found to have dense interactions within the brain, but pathological conditions cannot be explained based only on changes in the neurotransmitters.
In addition, because phencyclidine, an NMDA antagonist, exhibits schizophrenia-like psychotic symptoms, the impaired glutamic-acid hypothesis has also been proposed.81) From the hippocampus of the diseased brain, decreased expression of non-NMDA receptors, increases in NMDA receptors, increased reuptake of glutamate in the frontal lobe, and other findings have been reported.82,83)
There arises a question of whether these abnormalities and changes of neurotransmitters constitute impairment of the actual nerve cell functions or are related to morphological variations of the nerve cells, and whether they are caused by impaired plasticity of the synapses or malfunctions of the respective neural networks. One of the pressing issues in the future will likely be to see whether these questions, along with research methodologies, have been adequately addressed.
MOLECULAR BIOLOGY AND NEUROPTHOLOGY
Harrison and Weinberger have pointed out that studies of "genetic cytoarchitecture" would enable a better understanding of the etiology of schizophrenia.84) From recent results in the field of molecular psychiatry, there have been a series of reports supporting their neuropathological findings. Several risk genes for schizophrenia and items associated with brain morphology and cerebral pathology will now be discussed. Major reports available at the time of writing are shown in Table 4.
Table 4.
Susceptibility gene and related neuropathological/morphological/brain tissue findings
| Susceptibility gene for schizophrenia | Reported findings (reporter) |
|---|---|
| DISC1 | Reduces neurite extension in DISC1 mutation (Ozeki, 200385) Impairs neurite outgrowth in DISC1 mutation (Kamiya, 200586) |
| Dysbindin | Presynaptic dysbindin-1 reductions in hippocampus of schizophrenia (Talbot, 200452) Dysbindin might influence exocytotic glutamate release (Numakawa, 200487) |
| NRG1 (neureglin1) | Type 1 isoform was significantly increased in schizophrenia DLPFC (Hashimoto, 200488) |
| COMT | Polymorphism of COMT gene might contribute to morphological abnormalities in schizophrenia (Ohnishi, 200689) |
| RGS4 (regulator of G protein signaling 4) | Decreases of RGS4 expression in schizophrenia (Mirnics, 200190) |
| BDNF | BDNF gene variation may influence brain morphology (Agarts, 200642) Polymorphisms in the BDNF gene may be associated with variation in frontal lobe morphology (Varnäs, 200891) |
| NOTCH4 | NOTCH4 allelic variability was correlated with frontal lobe brain tissue volumes (Wassink, 200392) |
DISC1 (Disrupted-in schizophrenia 1) is currently the most credible disease candidate gene for schizophrenia and mood disorders, and has been found to be involved in the adjustments of neuronal migration and elongation of neuronal processes during the neurodevelopmental period. This candidate gene (suspectable gene) was discovered through genetic research on the Scottish multiplex psychiatric disease family. Gene mutations of this DISC1 reportedly inhibit elongation of neuronal processes and damage development of the cerebral cortex. The functions of dysbindin in the brain are not well understood, but because its addition to cultured nerve cells increases secretion of glutamic acid,87) it is considered to be greatly involved in the glutamic nerve system.
It has been reported that the expression of dysbindin was decreased in the hippocampus of postmortem schizophrenic brains,52) and it is thought that gene mutations of dysbindin can diminish glutamate nerve function. Neureglin1 is a neurotrophic factor and is a candidate gene for the development of nerves and schizophrenia. Reportedly, it is significantly expressed in the dorsolateral prefrontal cortex of postmortem schizophrenic brains.88) It is found to perform an important function regarding neuronal development for neureglin function.93) In addition, it has recently been revealed that neureglin1 plays an important role in the formation of myelin.94) This is interesting to consider along with reports that myelin hypoplasia is intimately related to the pathological conditions of schizophrenia.95)
COMT is a metabolic enzyme of catecholamines such as dopamine, and the correlation of the gene polymorphism thereof with schizophrenia has been known since a relatively early period.36) The volume of the cerebral cortex reportedly decreases due to this gene polymorphism in patients with chronic schizophrenia.89)
RGS4 is a protein that gained the interest of Mirnics et al.90) because its expression was decreased in the prefrontal area in schizophrenia cases, and subsequently, Chowdari et al.96) discovered a correlation between the polymorphism thereof with schizophrenia. RGS4 is called a regulator of G protein signaling 4, and 28 types of RGS proteins have been discovered so far. RGS proteins serve to terminate the actions of G protein receptors. Because many actions of the neurotransmitters utilize signals via G proteins, it is believed to be involved in the expression of a variety of transmitters. It is estimated that it influences neuronal development through the expression of neuromodulators via G proteins rather than by directly influencing brain morphology.
BDNF is a neurotrophic factor involved in nerve development and differentiation, the plasticity of synapses, etc., and moreover, plays an important role in the synthesis, metabolism, and release of neurotransmitters.97) From these facts, it is thought to be closely associated with the development of schizophrenia related to neurodevelopmental dysfunction and subsequent changes in the nervous system. Studies with meta-analysis have indicated that gene mutations of BDNF can be risk factors for schizophrenia.98) It has been reported that in healthy subjects, gene mutations of BDNF indicate decreases in the volume of hippocampal gray matter, and increases the volume of several types of gray matter such as the dorsolateral frontal area.99) Even in schizophrenic patients, some BDNF gene polymorphisms are reported to involve differences in volume among the caudate nucleus, putamen, and gray matter of the frontal lobe.42) Our group also conducted immunohistological searches for expressions of BDNF and its receptors in postmortem schizophrenic brains, and made several findings.100)
NOTCH 4 genes are known to be involved in the proliferation, differentiation, and migration of neural stem cells, but there are reportedly many mutations of this gene accompanied by decreases in the volume of the prefrontal area in schizophrenia.92)
In addition to the above, many other risk factors related to nerve development have been reported. Molecular biological studies related to such morphologies strongly support the neurodevelopmental hypothesis of schizophrenia, and in the future, it will be important to comprehend the etiology in a multilayered manner while making connections between morphological studies from the neuropathological field and the results of imaging studies.
With advances in molecular biology, a variety of genetically-modified model mice have been created. Within this context, model mice that can be assumed to be schizophrenic have been developed in recent years. One such model is the 14-3-3ε knockout mouse.101) 14-3-3ε protein is a DISC1-related protein that has been found to form combinations with proteins such as NUDEL, LIS1, and GRB2, and to be greatly involved in neuronal elongation, nerve cell development, and neural network formation.102) Recently, our group conducted neuropathological research on the brains of these mice and found developmental dysfunctions in TH (tyrosine hydroxylase) fibers.103) It is necessary to further verify whether such changes actually occur in the brains of schizophrenic patients, but it will likely provide a reasonable explanation of the morphological changes that have been commonly discussed.
COGNITIVE FUNCTIONS IN SCHIZOPHRENIA
It is known that schizophrenia involves impairments of various cognitive functions, and recently, the improvement of those cognitive functions has been one of the goals of treatment.104,105) In addition, mainly in the United States and other developed countries in Europe, the number of elderly schizophrenic patients has increased due to the aging of the population. In routine clinical practice, there are many opportunities to encounter cases in which an elderly schizophrenic patient expresses symptoms of dementia. In cases of the kind, it is necessary to distinguish whether the dementia is to be regarded as an extended symptom of schizophrenia or as a complication of degeneration such as Alzheimer’s dementia. This is because a diagnosis of dementia results in different methods of support and therapeutic regimens in the future from those for schizophrenia, and also results in different uses of social resources and psychological approaches. On the other hand, as described above, if there are any neurodevelopmental problems in the schizophrenic brain, the impact of aging is considered to have a great effect. Definitive diagnoses of dementia are made based on neuropathological research.
Is schizophrenia more likely to involve complications of dementia compared to the general population? Jellinger and Gabriel106) closely studied the postmortem brains of 99 schizophrenic patients aged 55 years or over who satisfied the DSM-III and ICD-10 diagnosis criteria by using the CERAD pathological diagnosis criteria107) and Braak’s neuropathological staging.108) As a result, it was estimated that the risk rate for onset that satisfied the pathological diagnosis criteria for probable AD (Alzheimer disease) or above was 7.1% for patients aged 55 years or over and 8.7% for those aged 65 years or over. From these results, it was concluded that the frequency of Alzheimer-type degeneration observed in elderly schizophrenic patients is equal to or slightly less than that in the general population. Both before and after this study, there were similar neuropathological studies, and among them, even studies on 100 postmortem brains109,110) yielded the result that "the occurrence frequency of Alzheimer’s disease in schizophrenia cases exhibits no significant difference from that of the general population." In other words, even in schizophrenia cases, the risk for dementia is assumed to be equal to that for the general population, and it is necessary to examine the aging of schizophrenic patients in a biological-psychological-sociological manner in terms of cognitive functions.
When considering this problem, one must consider the effects of long-term administration of antipsychotic drugs. Wisniewski et al.111) investigated postmortem brains autopsied from year 1932 to 1952. Because antipsychotic drugs did not exist in the clinical settings in year 1932–1952, those autopsied brains during that period were not affected by drug influences. They concluded that cases treated with antipsychotic drugs had a significantly high frequency of NFT (neurofibrillary tangle), which indicated that the anticholinergic action of antipsychotic drugs could promote the formation of NFT. It has also been reported that phenothiazine drugs promote the formation of NFT112) or conversely inhibit it,113) and that haloperidol, which is a typical anti-psychotic drug, dose-dependently inhibits the production of amyloid beta protein114). However, it is not an exaggeration to say that the neuropathological effects of long-term administration of antipsychotic drugs on the brain have not been examined at all. Furthermore, because so-called atypical anti-psychotic drugs with many neuroleptic actions have been used in recent years, the neuropathological effects of long-term administration of these drugs on the brain remain an issue for close examination.
ISSUES FOR THE FUTURE
Neuroimaging studies have reported that in schizophrenic brains, the volume of the brain changes over time even after onset, a finding now widely accepted. Now, it is necessary to reveal the types of changes occurring in the brain tissue itself during this change in brain volume. Stimulated by advances in neuroimaging studies, neuropathological studies on schizophrenia were actively conducted mainly in the 1990s, and recently, a number of candidate genes related to the neurodevelopmental hypothesis have been verified from the results of molecular genetic studies. Although there are various problems in studying cerebral pathology (Table 5), it is the nerve cells that are responsible for psychoneurotic activity that occurs in "locations" of brain tissue such as synapses, axons, glial cells, and neurotransmitters. In seeking to determine the pathological conditions and etiology of schizophrenia, whether through image information or genome information, it is necessary to understand these factors as neuropathological changes in "location." As clarifying the relationship between genetics and the neuropathological findings in schizophrenia and speculating on their functional convergence, the tendency to integrate molecular biological results in neuropathological and visible forms has entered a new phase.
Table 5.
| Problems in neuropathological study of schizophrenia |
| 1. Small brain samples with precise clinical information |
| 2. Inhomogeneous brain tissue situation (brain tissue influenced by agonal factors (anoxic, infection etc.), post-mortem interval time, or differences of tissue fixation etc.) |
| 3. Difficulty of definding normal brain structure for schizophrenia |
| 4. Difficulty in interpreting abnormal findings (Is it a primary change or secondary/tertiary change?) |
| 5. Discordance between brain histopathology and symptomatological clinical diagnosis |
In addition, mainly in developed countries, the problem of caring for elderly schizophrenic patients has increased due to aging of the population. In considering aging in schizophrenia cases, new approaches for pathological conditions that include drug therapy are required based on accumulations of conventional neuropathological findings regarding the aging of the brain.
After the era in which "schizophrenia is the graveyard of neuropathologists," we have now entered an era when we should review information regarding research approaches in a neuropathological manner from other new aspects, and seek to understand abnormalities in brain structure together with other study results in a multilayered manner.
ACKNOWLEDGMENTS
This study was supported by Grants-in-Aid for Scientific Research ((20591400) and (23591701)) from the Japan Society for the Promotion of Science ((year 2008–2010) and (year 2011–2013)), respectively.
REFERENCES
- 1).Alzheimer A. Beiträge zur Pathologischen Anatomie der Hirnrinde und zur anatomiscen Grundlage der Psychozen. Mschr Psychiat Neurol 1897; 2: 82–120.
- 2).Tatetsu S. A contribution of the morphological Background of Schizophrenia, with special reference to the findings in the telencephalon. Acta Neuropathologica 1960; 3: 558–71.
- 3).Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res 2001; 49: 1–52. [DOI] [PMC free article] [PubMed]
- 4).Dickey CC, Salisbury DF, Nagy AI, et al. Follow-up MRI study of prefrontal volumes in first-episode psychotic patients. Schizophr Res 2004; 71: 349–51. [DOI] [PMC free article] [PubMed]
- 5).Bogerts B, Meertz E, Schonfeldt-Bausch R. Basal ganglia and limbic system pathology in schizophrenia. A morphometric study of brain volume and shrinkage. Arch Gen Psychiatry 1985; 42: 784–91. [DOI] [PubMed]
- 6).Bogerts B, Falkai P, Haupts M, et al. Post-mortem volume measurements of limbic system and basal ganglia structures in chronic schizophrenics. Initial results from a new brain collection. Schizophr Res 1990; 3: 295–301. [DOI] [PubMed]
- 7).Altshuler LL, Casanova MF, Goldberg TE, Kleinman JE. The hippocampus and parahippocampus in schizophrenia, suicide, and control brains. Arch Gen Psychiatry 1990; 47: 1029–34. [DOI] [PubMed]
- 8).Heckers S, Heinsen H, Heinsen YC, Beckmann H. Limbic structures and lateral ventricle in schizophrenia. A quantitative postmortem study. Arch Gen Psychiatry 1990; 47: 1016–22. [DOI] [PubMed]
- 9).Heckers S, Heinsen H, Heinsen Y, Beckmann H. Cortex, white matter, and basal ganglia in schizophrenia: a volumetric postmortem study. Biol Psychiatry 1991; 29: 556–66. [DOI] [PubMed]
- 10).Colter N, Battal S, Crow TJ, et al. White matter reduction in the parahippocampal gyrus of patients with schizophrenia. Arch Gen Psychiatry 1987; 44: 1023. [DOI] [PubMed]
- 11).Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry 1998; 44: 88–97. [DOI] [PubMed]
- 12).Casanova MF, Carosella N, Kleinman JE. Neuropathological findings in a suspected case of childhood schizophrenia. J Neuropsychiatry Clin Neurosci 1990; 2: 313–9. [DOI] [PubMed]
- 13).Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR. Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry 1991a; 48: 625–32. [DOI] [PubMed]
- 14).Conrad AH, Clark WA, Conrad GW. Subcellular compartmentalization of myosin isoforms in embryonic chick heart ventricle myocytes during cytokinesis. Cell Motil Cytoskeleton 1991; 19: 189–206. [DOI] [PubMed]
- 15).Akbarian S, Vinuela A, Kim JJ, et al. Distorted distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Arch Gen Psychiatry 1993b; 50: 178–87. [DOI] [PubMed]
- 16).Benes FM, Davidson J, Bird ED. Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch Gen Psychiatry 1986; 43: 31–5. [DOI] [PubMed]
- 17).Arnold SE, Franz BR, Gur RC, et al. Smaller neuron size in schizophrenia in hippocampal subfields that mediate cortical-hippocampal interactions. Am J Psychiatry 1995; 152: 738–48. [DOI] [PubMed]
- 18).Benes FM, McSparren J, Bird ED, et al. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry 1991; 48: 996–1001. [DOI] [PubMed]
- 19).Benes FM, Majocha R, Bird ED, Marotta CA. Increased vertical axon numbers in cingulate cortex of schizophrenics. Arch Gen Psychiatry 1987b; 44: 1017–21. [DOI] [PubMed]
- 20).Chana G, Landau S, Beasley C, et al. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry 2003; 53: 1086–98. [DOI] [PubMed]
- 21).Benes FM, Bird ED. An analysis of the arrangement of neurons in the cingulate cortex of schizophrenic patients. Arch Gen Psychiatry 1987a; 44: 608–16. [DOI] [PubMed]
- 22).Akbarian S, Bunney WE, Jr., Potkin SG, et al. Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry 1993a; 50: 169–77. [DOI] [PubMed]
- 23).Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain 1999; 122 (Pt 4): 593–624. [DOI] [PubMed]
- 24).Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry 2001; 58: 148–57. [DOI] [PubMed]
- 25).Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry 2005; 162: 2233–45. [DOI] [PubMed]
- 26).Keilhoff G, Bernstein HG, Becker A, et al. Increased neurogenesis in a rat ketamine model of schizophrenia. Biol Psychiatry 2004; 56: 317–22. [DOI] [PubMed]
- 27).van Haren NE, Hulshoff Pol HE, Schnack HG, et al. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology 2007; 32: 2057–66. [DOI] [PubMed]
- 28).Iritani S, Kuroki N, Ikeda K, Kazamatsuri H. Calbindin immunoreactivity in the hippocampal formation and neocortex of schizophrenics. Prog Neuropsychopharmacol Biol Psychiatry 1999; 23: 409–21. [DOI] [PubMed]
- 29).Iritani S, Kuroki N, Niizato K, Ikeda K. Morphological changes in neuropeptide Y-positive fiber in the hippocampal formation of schizophrenics. Prog Neuropsychopharmacol Biol Psychiatry 2000; 24: 241–9. [DOI] [PubMed]
- 30).Arnold SE, Trojanowski JQ. Recent advances in defining the neuropathology of schizophrenia. Acta Neuropathol 1996; 92: 217–31. [DOI] [PubMed]
- 31).Olin SC, Mednick SA, Cannon T, et al. School teacher ratings predictive of psychiatric outcome 25 years later. Br J Psychiatry Suppl 1998; 172: 7–13. [PubMed]
- 32).Davidson M, Chen W, Wilce PA. Behavioral analysis of PTZ-kindled rats after acute and chronic ethanol treatments. Pharmacol Biochem Behav 1999; 64: 7–13. [DOI] [PubMed]
- 33).Niemi LT, Suvisaari JM, Tuulio-Henriksson A, Lonnqvist JK. Childhood developmental abnormalities in schizophrenia: evidence from high-risk studies. Schizophr Res 2003; 60: 239–58. [DOI] [PubMed]
- 34).Gourion D, Goldberger C, Bourdel MC, et al. Minor physical anomalies in patients with schizophrenia and their parents: prevalence and pattern of craniofacial abnormalities. Psychiatry Res 2004; 125: 21–8. [DOI] [PubMed]
- 35).Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 2002; 25: 409–32. [DOI] [PubMed]
- 36).Kunugi H, Vallada HP, Sham PC, et al. Catechol-O-methyltransferase polymorphisms and schizophrenia: a transmission disequilibrium study in multiply affected families. Psychiatr Genet 1997; 7: 97–101. [DOI] [PubMed]
- 37).Price G, Cercignani M, Bagary MS, et al. A volumetric MRI and magnetization transfer imaging follow-up study of patients with first-episode schizophrenia. Schizophr Res 2006; 87: 100–8. [DOI] [PMC free article] [PubMed]
- 38).Tsuang DW, Faraone SV, Tsuang MT. Genetic counseling for psychiatric disorders. Curr Psychiatry Rep 2001; 3: 138–43. [DOI] [PubMed]
- 39).Huttunen MO, Machon RA, Mednick SA. Prenatal factors in the pathogenesis of schizophrenia. Br J Psychiatry Suppl 199415–9. [PubMed]
- 40).Benes FM. Neurobiological investigations in cingulate cortex of schizophrenic brain. Schizophr Bull 1993; 19: 537–49. [DOI] [PubMed]
- 41).Hong CJ, Yu YW, Lin CH, Tsai SJ. An association study of a brain-derived neurotrophic factor Val66Met polymorphism and clozapine response of schizophrenic patients. Neurosci Lett 2003; 349: 206–8. [DOI] [PubMed]
- 42).Agartz I, Sedvall GC, Terenius L, et al. BDNF gene variants and brain morphology in schizophrenia. Am J Med Genet B Neuropsychiatr Genet 2006; 141B: 513–23. [DOI] [PubMed]
- 43).Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry 1995; 52: 805–18; discussion 19–20. [DOI] [PubMed]
- 44).Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 2000; 57: 65–73. [DOI] [PubMed]
- 45).Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr Res 2006; 81: 47–63. [DOI] [PubMed]
- 46).Arnold SE, Lee VM, Gur RE, Trojanowski JQ. Abnormal expression of two microtubule-associated proteins (MAP2 and MAP5) in specific subfields of the hippocampal formation in schizophrenia. Proc Natl Acad Sci U S A 1991b; 88: 10850–4. [DOI] [PMC free article] [PubMed]
- 47).Browning MD, Dudek EM, Rapier JL, et al. Significant reductions in synapsin but not synaptophysin specific activity in the brains of some schizophrenics. Biol Psychiatry 1993; 34: 529–35. [DOI] [PubMed]
- 48).Eastwood SL, Harrison PJ. Decreased synaptophysin in the medial temporal lobe in schizophrenia demonstrated using immunoautoradiography. Neuroscience 1995; 69: 339–43. [DOI] [PubMed]
- 49).Harrison PJ, Eastwood SL. Preferential involvement of excitatory neurons in medial temporal lobe in schizophrenia. Lancet 1998; 352: 1669–73. [DOI] [PubMed]
- 50).Young CE, Arima K, Xie J, et al. SNAP-25 deficit and hippocampal connectivity in schizophrenia. Cereb Cortex 1998; 8: 261–8. [DOI] [PubMed]
- 51).Eastwood SL, Harrison PJ. Hippocampal synaptic pathology in schizophrenia, bipolar disorder and major depression: a study of complexin mRNAs. Mol Psychiatry 2000; 5: 425–32. [DOI] [PubMed]
- 52).Talbot K, Eidem WL, Tinsley CL, et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest 2004; 113: 1353–63. [DOI] [PMC free article] [PubMed]
- 53).Eastwood SL, Harrison PJ. Decreased expression of vesicular glutamate transporter 1 and complexin II mRNAs in schizophrenia: further evidence for a synaptic pathology affecting glutamate neurons. Schizophr Res 2005; 73: 159–72. [DOI] [PubMed]
- 54).Sawada K, Barr AM, Nakamura M, et al. Hippocampal complexin proteins and cognitive dysfunction in schizophrenia. Arch Gen Psychiatry 2005; 62: 263–72. [DOI] [PubMed]
- 55).Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: a postmortem morphometric study. Synapse 2005; 57: 47–55. [DOI] [PubMed]
- 56).Aganova EA, Uranova NA. Morphometric analysis of synaptic contacts in the anterior limbic cortex in the endogenous psychoses. Neurosci Behav Physiol 1992; 22: 59–65. [DOI] [PubMed]
- 57).Perrone-Bizzozero NI, Sower AC, Bird ED, et al. Levels of the growth-associated protein GAP-43 are selectively increased in association cortices in schizophrenia. Proc Natl Acad Sci U S A 1996; 93: 14182–7. [DOI] [PMC free article] [PubMed]
- 58).Benes FM, Todtenkopf MS, Taylor JB. Differential distribution of tyrosine hydroxylase fibers on small and large neurons in layer II of anterior cingulate cortex of schizophrenic brain. Synapse 1997; 25: 80–92. [DOI] [PubMed]
- 59).Gabriel SM, Haroutunian V, Powchik P, et al. Increased concentrations of presynaptic proteins in the cingulate cortex of subjects with schizophrenia. Arch Gen Psychiatry 1997; 54: 559–66. [DOI] [PubMed]
- 60).Honer WG, Falkai P, Young C, et al. Cingulate cortex synaptic terminal proteins and neural cell adhesion molecule in schizophrenia. Neuroscience 1997; 78: 99–110. [DOI] [PubMed]
- 61).Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry 1997; 54: 660–9. [DOI] [PubMed]
- 62).Garey LJ, Ong WY, Patel TS, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry 1998; 65: 446–53. [DOI] [PMC free article] [PubMed]
- 63).Thompson PM, Sower AC, Perrone-Bizzozero NI. Altered levels of the synaptosomal associated protein SNAP-25 in schizophrenia. Biol Psychiatry 1998; 43: 239–43. [DOI] [PubMed]
- 64).Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci U S A 1998; 95: 5341–6. [DOI] [PMC free article] [PubMed]
- 65).Karson CN, Mrak RE, Schluterman KO, et al. Alterations in synaptic proteins and their encoding mRNAs in prefrontal cortex in schizophrenia: a possible neurochemical basis for ‘hypofrontality’. Mol Psychiatry 1999; 4: 39–45. [DOI] [PubMed]
- 66).Honer WG, Falkai P, Chen C, et al. Synaptic and plasticity-associated proteins in anterior frontal cortex in severe mental illness. Neuroscience 1999; 91: 1247–55. [DOI] [PubMed]
- 67).Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry 1999; 156: 1709–19. [DOI] [PubMed]
- 68).Ohnuma T, Kato H, Arai H, et al. Gene expression of PSD95 in prefrontal cortex and hippocampus in schizophrenia. Neuroreport 2000; 11: 3133–7. [DOI] [PubMed]
- 69).Benes FM, Todtenkopf MS, Logiotatos P, Williams M. Glutamate decarboxylase(65)-immunoreactive terminals in cingulate and prefrontal cortices of schizophrenic and bipolar brain. J Chem Neuroanat 2000; 20: 259–69. [DOI] [PubMed]
- 70).Volk DW, Pierri JN, Fritschy JM, et al. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex 2002; 12: 1063–70. [DOI] [PubMed]
- 71).Toyooka K, Iritani S, Makifuchi T, et al. Selective reduction of a PDZ protein, SAP-97, in the prefrontal cortex of patients with chronic schizophrenia. J Neurochem 2002; 83: 797–806. [DOI] [PubMed]
- 72).Halim ND, Weickert CS, McClintock BW, et al. Presynaptic proteins in the prefrontal cortex of patients with schizophrenia and rats with abnormal prefrontal development. Mol Psychiatry 2003; 8: 797–810. [DOI] [PubMed]
- 73).Konopaske GT, Sweet RA, Wu Q, et al. Regional specificity of chandelier neuron axon terminal alterations in schizophrenia. Neuroscience 2006; 138: 189–96. [DOI] [PubMed]
- 74).Roberts RC, Roche JK, Conley RR. Synaptic differences in the patch matrix compartments of subjects with schizophrenia: a postmortem ultrastructural study of the striatum. Neurobiol Dis 2005; 20: 324–35. [DOI] [PubMed]
- 75).Okabe S, Collin C, Auerbach JM, et al. Hippocampal synaptic plasticity in mice overexpressing an embryonic subunit of the NMDA receptor. J Neurosci 1998; 18: 4177–88. [DOI] [PMC free article] [PubMed]
- 76).Grant SG. Synapse signalling complexes and networks: machines underlying cognition. Bioessays 2003; 25: 1229–35. [DOI] [PubMed]
- 77).Owens DF, Kriegstein AR. Developmental neurotransmitters? Neuron 2002; 36: 989–91. [DOI] [PubMed]
- 78).Zakzanis KK, Hansen KT. Dopamine D2 densities and the schizophrenic brain. Schizophr Res 1998; 32: 201–6. [DOI] [PubMed]
- 79).Harrison PJ, Burnet PW. The 5-HT2A (serotonin2A) receptor gene in the aetiology, pathophysiology and pharmacotherapy of schizophrenia. J Psychopharmacol 1997; 11: 18–20. [DOI] [PubMed]
- 80).Burnet PW, Eastwood SL, Harrison PJ. 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology 1996; 15: 442–55. [DOI] [PubMed]
- 81).Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 1991; 148: 1301–8. [DOI] [PubMed]
- 82).Kerwin R, Patel S, Meldrum B. Quantitative autoradiographic analysis of glutamate binding sites in the hippocampal formation in normal and schizophrenic brain post mortem. Neuroscience 1990; 39: 25–32. [DOI] [PubMed]
- 83).Porter RH, Eastwood SL, Harrison PJ. Distribution of kainate receptor subunit mRNAs in human hippocampus, neocortex and cerebellum, and bilateral reduction of hippocampal GluR6 and KA2 transcripts in schizophrenia. Brain Res 1997; 751: 217–31. [DOI] [PubMed]
- 84).Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 2005; 10: 40–68; image 5. [DOI] [PubMed]
- 85).Ozeki Y, Tomoda T, Kleiderlein J, et al. Disrupted-in-Schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci U S A 2003; 100: 289–94. [DOI] [PMC free article] [PubMed]
- 86).Kamiya A, Kubo K, Tomoda T, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol 2005; 7: 1167–78. [DOI] [PubMed]
- 87).Numakawa T, Yagasaki Y, Ishimoto T, et al. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet 2004; 13: 2699–708. [DOI] [PubMed]
- 88).Hashimoto R, Straub RE, Weickert CS, et al. Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry 2004; 9: 299–307. [DOI] [PubMed]
- 89).Ohnishi T, Hashimoto R, Mori T, et al. The association between the Val158Met polymorphism of the catechol-O-methyl transferase gene and morphological abnormalities of the brain in chronic schizophrenia. Brain 2006; 129: 399–410. [DOI] [PubMed]
- 90).Mirnics K, Middleton FA, Stanwood GD, et al. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol Psychiatry 2001; 6: 293–301. [DOI] [PubMed]
- 91).Varnas K, Lawyer G, Jonsson EG, et al. Brain-derived neurotrophic factor polymorphisms and frontal cortex morphology in schizophrenia. Psychiatr Genet 2008; 18: 177–83. [DOI] [PubMed]
- 92).Wassink TH, Nopoulos P, Pietila J, et al. NOTCH4 and the frontal lobe in schizophrenia. Am J Med Genet B Neuropsychiatr Genet 2003; 118B: 1–7. [DOI] [PubMed]
- 93).Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci 2004; 7: 575–80. [DOI] [PubMed]
- 94).Lemke G. Neuregulin-1 and myelination. Sci STKE 2006; 2006: pe11. [DOI] [PubMed]
- 95).McInnes LA, Lauriat TL. RNA metabolism and dysmyelination in schizophrenia. Neurosci Biobehav Rev 2006; 30: 551–61. [DOI] [PubMed]
- 96).Chowdari KV, Mirnics K, Semwal P, et al. Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Hum Mol Genet 2002; 11: 1373–80. [DOI] [PubMed]
- 97).Ashe PC, Berry MD, Boulton AA. Schizophrenia, a neurodegenerative disorder with neurodevelopmental antecedents. Prog Neuropsychopharmacol Biol Psychiatry 2001; 25: 691–707. [DOI] [PubMed]
- 98).Jonsson EG, Edman-Ahlbom B, Sillen A, et al. Brain-derived neurotrophic factor gene (BDNF) variants and schizophrenia: an association study. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30: 924–33. [DOI] [PubMed]
- 99).Pezawas L, Verchinski BA, Mattay VS, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci 2004; 24: 10099–102. [DOI] [PMC free article] [PubMed]
- 100).Iritani S, Niizato K, Nawa H, et al. Immunohistochemical study of brain-derived neurotrophic factor and its receptor, TrkB, in the hippocampal formation of schizophrenic brains. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27: 801–7. [DOI] [PubMed]
- 101).Ikeda M, Hikita T, Taya S, et al. Identification of YWHAE, a gene encoding 14-3-3epsilon, as a possible susceptibility gene for schizophrenia. Hum Mol Genet 2008; 17: 3212–22. [DOI] [PubMed]
- 102).Taya S, Shinoda T, Tsuboi D, et al. DISC1 regulates the transport of the NUDEL/LIS1/14-3-3epsilon complex through kinesin-1. J Neurosci 2007; 27: 15–26. [DOI] [PMC free article] [PubMed]
- 103).Sekiguchi H, Iritani S, Habuchi C, et al. Impairment of the tyrosine hydroxylase neuronal network in the orbitofrontal cortex of a genetically modified mouse model of schizophrenia. Brain Res 2011; 1392: 47–53. [DOI] [PubMed]
- 104).Carter CS, Barch DM, Buchanan RW, et al. Identifying cognitive mechanisms targeted for treatment development in schizophrenia: an overview of the first meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia Initiative. Biol Psychiatry 2008; 64: 4–10. [DOI] [PMC free article] [PubMed]
- 105).Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biol Psychiatry 2008; 64: 26–33. [DOI] [PubMed]
- 106).Jellinger KA, Gabriel E. No increased incidence of Alzheimer’s disease in elderly schizophrenics. Acta Neuropathol 1999; 97: 165–9. [DOI] [PubMed]
- 107).Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991; 41: 479–86. [DOI] [PubMed]
- 108).Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239–59. [DOI] [PubMed]
- 109).Powchik P, Davidson M, Haroutunian V, et al. Postmortem studies in schizophrenia. Schizophr Bull 1998; 24: 325–41. [DOI] [PubMed]
- 110).Purohit DP, Perl DP, Haroutunian V, et al. Alzheimer disease and related neurodegenerative diseases in elderly patients with schizophrenia: a postmortem neuropathologic study of 100 cases. Arch Gen Psychiatry 1998; 55: 205–11. [DOI] [PubMed]
- 111).Wisniewski HM, Constantinidis J, Wegiel J, et al. Neurofibrillary pathology in brains of elderly schizophrenics treated with neuroleptics. Alzheimer Dis Assoc Disord 1994; 8: 211–27. [DOI] [PubMed]
- 112).Gong CX, Shaikh S, Grundke-Iqbal I, Iqbal K. Inhibition of protein phosphatase-2B (calcineurin) activity towards Alzheimer abnormally phosphorylated tau by neuroleptics. Brain Res 1996; 741: 95–102. [DOI] [PubMed]
- 113).Wischik CM, Edwards PC, Lai RY, et al. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci U S A 1996; 93: 11213–8. [DOI] [PMC free article] [PubMed]
- 114).Higaki J, Murphy GM, Jr., Cordell B. Inhibition of beta-amyloid formation by haloperidol: a possible mechanism for reduced frequency of Alzheimer’s disease pathology in schizophrenia. J Neurochem 1997; 68: 333–6. [DOI] [PubMed]