Abstract
A specific luteinizing hormone receptor (LHR) mRNA binding protein (LRBP) has been identified and purified. This LH receptor mRNA binding protein selectively binds to the polypyrimidine rich bipartite sequence in the coding region of the LHR mRNA and accelerates its degradation. In response to preovulatory LH surge, the LH receptor expression in the ovary undergoes downregulation by accelerated degradation of LH receptor mRNA through the involvement of this RNA binding protein. Here we describe the intracellular mechanism triggered by LH/hCG (human chorionic gonadotropin) that leads to the regulated degradation of LH receptor mRNA. Downregulation of LH receptor mRNA was induced by treatment of cultured human granulosa cells with 10 IU of hCG. Activation of downstream target, extracellular signal-regulated protein kinase 1 and 2 (ERK 1/2) showed an increase within five min and sustained up to 1 h. Confocal analysis showed that ERK1/2 translocates to the nucleus after 15 min of hCG treatment. This leads to an increase in LRBP expression which then causes downregulation of LH receptor mRNA by accelerating its degradation. Treatment with UO126 or transfection with ERK specific siRNA (small interfering RNA) resulted in the abolishment of ERK activation as well as LHR mRNA downregulation. RNA electrophoretic mobility gel shift assay of the cytosolic fractions showed that hCG-induced increase in the LH receptor mRNA binding activity was also abrogated by these treatments. These results show that LH/hCG-induced LH receptor mRNA downregulation is initiated by the activation of ERK1/2 pathway by regulating the expression and activity of LH receptor mRNA binding activity.
Keywords: ERK1/2, Human chorionic gonadotropin, luteinizing hormone receptor, mRNA-binding protein, post transcriptional regulation
Introduction
The LH/hCG (luteinizing hormone/human chorionic gonadotropin) receptor (LHR) expressed in the gonads regulates the ovarian and testicular function1,2,3. Although gonadal tissues are the primary sites of its expression, it is now well-established that LHR is also expressed in many non-gonadal tissues4. LHR along with follicle stimulating hormone (FSH) receptor and thyroid stimulating hormone (TSH) receptor belongs to the family of rhodopsin/β2 adrenergic receptor subfamily of G protein coupled receptors5. Binding of the LH or hCG to the extracellular domain of LHR causes a conformational change leading to guanosine di-and triphosphate (GDP-GTP) exchange on the cognate G protein. This results in the dissociation of the inhibitory beta-gamma subunit from the alpha subunit leading to the activation of adenylatecyclase to increase the intracellular concentration of adenosine cyclic 3’, 5’ cyclic monophosphate (cAMP). Cyclic AMP then activates protein kinase cascade and activates multiple signaling pathways culminating in increased steroidogenesis and stimulation of other cellular functions. It has been shown that at higher concentrations, LH or hCG can also activate phospholipase C resulting in the formation of inositoltriphosphate (IP3) and diacylglycerol (DAG)6,7,9. The physiological significance of this pathway in LH-regulated ovarian function is not clearly understood.
LHR is expressed in the follicles, theca cells and corpus luteum of the ovary and the expression pattern shows considerable changes throughout ovarian cycle10,11,12. In growing follicles, LHR expression is regulated by the combined actions of FSH and estradiol. Consequently, the expression of LHR increases during follicular growth as it acquires the ability to secrete estrogens13. The receptor expression is transiently downregulated in response to pre-ovulatory LH surge, which then recovers from down-regulation after 72 h reaching maximum level by the mid luteal phase10,11,12,14. The LHR level falls off as the corpus luteum undergoes regression. The molecular mechanism underlying the regulation of LHR expression has been investigated in depth in our laboratory. Using rodent and human ovarian models, we have determined that the regulation of LHR occurs through post-transcriptional mechanisms mediated by a specific LHR mRNA binding protein15,16. This article will focus on the signaling pathways involved in the post-transcriptional regulation of LHR expression.
LHR downregulation
The downregulation of LHR occurs in response to preovulatory LH surge or in response to the administration of a pharmacological dose of LH or hCG. The loss of the ligand binding activity is closely coupled to the loss of the steady state levels of LHR mRNA. In human, this phenomenon occurs during ovulation induction by the administration of hCG prior to ovum retrieval for in vitro fertilization16. Granulosa cells isolated from the retrieval fluids showed complete loss of LHR mRNA on the day of retrieval, when examined by Northern blot analysis (Fig. 1)16. The granulosa cells were then cultured for different periods of time and RNA was extracted from the harvested cells for examination of the reappearance of LHR mRNA. The recovery from down regulation began approximately 48 h later and reached maximum level by day 4 in culture (Fig. 1). This phenomenon can be demonstrated in a pseudopregnant rat model by treatment with a single dose of 50 IU of hCG to down regulate LHR. The loss of LHR mRNA transcripts during down regulation is shown in Fig. 2A17. The left hand panel shows the expression of LHR mRNA transcripts in the saline treated control ovaries. The expression of LHR mRNA transcripts in response to the administration of hCG that mimics preovulatory LH surge is shown on the right hand panel. The LHR mRNA expression remains suppressed up to 48 hours and recovers from downregulation by 72 h10,15. The loss of receptor mRNA could be either due to a decrease in the rate of synthesis or due to increased degradation. To examine these possibilities, nuclear run-on assays were performed to determine the transcription rate during hCG-induced LHR downregulation15. The results showed that the transcription rate remained identical in both control and hCG treated groups suggesting that the loss of the steady state levels of LHR mRNA is not due to decreased transcription, but rather resulted from increased degradation15. Furthermore, the half-life of LHR mRNA was significantly reduced in the hCG treated group when compared to the mRNA decay rate of the control group (Fig. 2B). There was approximately a three-fold decrease in mRNA half-life in the down- regulated group.
Fig. 1.
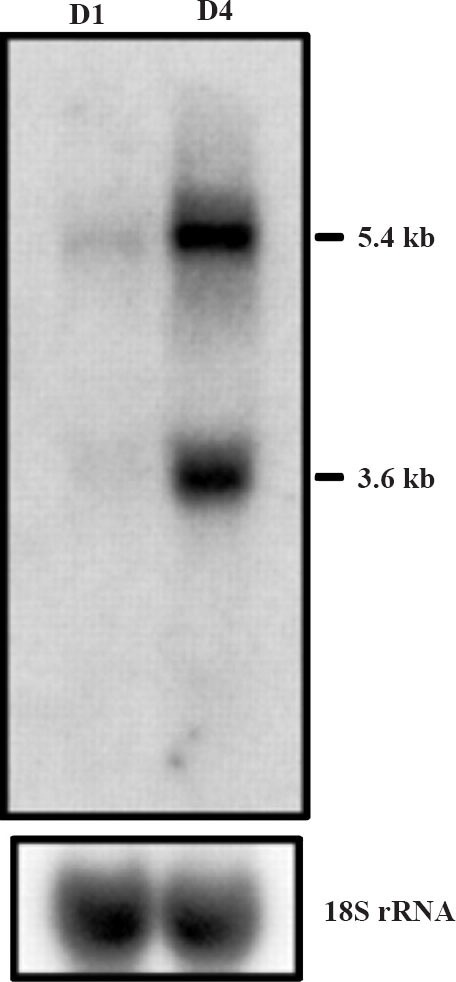
Northern blot analysis of LHR mRNA in human granulosa cells immediately after retrieval (D1) and after 4 days of incubation in serum free media (D4). Total RNA was extracted from granulosa cells from day 1 and four days of incubation (D4) to recover from downregulation. RNA was separated on agarose-formaldehyde gel, transferred to nitrocellulose membranes, hybridized with the 32P-labelled hLHRcDNA, and exposed to x-ray film. To monitor RNA loading, the blot was stripped and rehybridized with radiolabelled cDNA for 18S rRNA. The blot shown is one representative of three experiments with similar results. (Source: Ref. 16, Reproduced with permission).
Fig. 2.
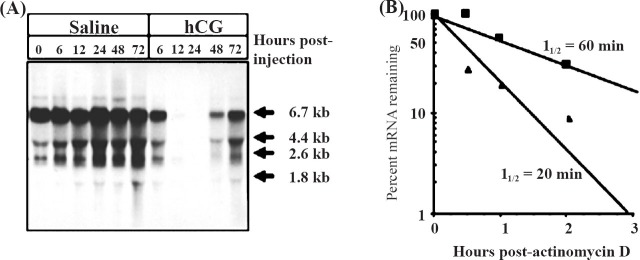
Hormonal control of LHR mRNA expression in the ovary. A. Northern blot hybridization analysis of steady state LHR mRNA levels during hCG-induced downregulation. Autoradiogram of Northern blot hybridization analysis of total RNA isolated at the indicated times from the ovaries of saline-injected (control) (lanes 1-6) or hCG-injected (downregulated) (lanes 7-11) rats. Blots were probed using a labelled cDNA encoding the LHR carboxyl terminus and a portion of the 3’-UTR (nucleotides 1936-2682). B. LHR mRNA half-life determination in control and 12 h downregulated rat ovaries. Cell suspensions were incubated with 10 µg/ml actinomycin D for 2h. Duplicate aliquots of 20 × 106 cells were removed at the indicated times. Total RNA was isolated and assayed for LHR mRNA by solution hybridization. Each data point represents the average of duplicate determinations. ▀ Contol, ▲ downregulated. (Source: Ref. 17, Reproduced with permission).
The steady state level of mRNA, in general, is controlled by the rate of its synthesis and the rate of degradation. It is now clear that highly regulated mRNAs are controlled, at least in part, by modulating their degradation. In the majority of instances of post-transcriptional regulation of mRNA, the changes in the stability of mRNA appear to result from changes in the binding of cytoplasmic proteins, known as trans factors, to defined sequences or structures, known as cis elements, in the target mRNA, forming a ribonucleoprotein complex. These trans factors can either increase or decrease the stability of RNA. The binding of proteins to mRNAs can occur either on 3’ end, 5’ end or on the coding sequence18,19,20,21,22,23,24. On the basis of these facts, the possible existence of an LHR mRNA binding protein was examined in the cytosolic fractions from the ovaries after injection with a dose of hCG that is known to downregulate LHR mRNA expression.
Identification of LHR mRNA binding protein
Our first attempt was to examine whether a specific LHR mRNA binding protein participates in LH/hCG regulated downregulation of LHR mRNA expression. Cytosolic fractions from control and downregulated ovaries were prepared and the ability of these fractions to bind LHR mRNA was examined by performing RNA electrophoretic mobility shift assay (REMSA)17. A 100,000 × g supernatant (S100) fraction of the ovarian homogenate was incubated with [32P] labelled LHR mRNA that was prepared by transcribing the full length cDNA encoding the LHR in the presence of [α32P] UTP. After treatment with RNAses to degrade unreacted [32P] labelled RNA, the ribonucleoprotein complex was subjected to electrophoresis under non-denaturing conditions, and autoradiography. We compared the ability of the S100 fractions from the control and hCG downregulated ovaries to bind radiolabelled LHR mRNA. The results showed that there were two prominent bands designated as LRBP-1 and LRBP-2 (LHR binding protein), corresponding to molecular weight 50 and 45 kDa, respectively, in both the control and down regulated ovaries17. The intensity of LRBP-1 was 3-fold higher in the downregulated group compared to the saline treated control. No significant change in the intensity in LRBP-2 was noticed. Since the hormonal induction of LRBP-1 was significantly higher, we focused our attention only on LRBP-1.
Studies were carried out to determine the nucleotide sequence involved in the interaction of LHR mRNA with proteins in the S100 fraction. Truncated segments of the full length LHR mRNA were incubated with ovarian S-100 fractions and electrophoretic mobility shift assays were performed to localize the region of LHR mRNA that interacted with the protein. The results of these studies coupled with the results from RNA hydroxyl radical foot- printing revealed that the contact site of the interaction of LRBP-1 with LHR mRNA consisted of a bipartite polypyrimidine- rich sequence corresponding to the nucleotides 203-220 located in the region encoding the amino terminus of LHR25.
After establishing the existence of an inducible RNA binding protein that recognizes a specific structure of the LHR mRNA, we examined the LHR mRNA binding activity under conditions that mimic follicle maturation and the LH surge26. Since the expression of the LHR is increased during follicle maturation and, conversely, the LHR expression is decreased during downregulation, these two paradigms were selected to examine whether changes in LHR expression have any bearing on the LHR mRNA binding activity. In response to treatment of 23 day old rats with pregnant mare's serum gonadotropin (PMSG), as expected, there was an increase in the expression of LHR mRNA by 56 h due to the induction of LHR mRNA by the FSH type of activity associated with pregnant mare's serum gonadotropin (PMSG). Fifty six hours after PMSG treatment, administration of 50 IU of hCG caused a transient decrease in LHR mRNA expression during the ensuing 24 h period as a result of downregulation. The LHR mRNA level returned to the hCG pretreatment level by 72 h. Thus, the changes in LHR mRNA were consistent with the expected up- and downregulation following the treatments. The LHR mRNA binding activity showed a strikingly different pattern in response to these treatments. When the expression of LHR mRNA was high in response to PMSG treatment, the LRBP activity showed a decrease. Conversely, an increased expression of LHR mRNA binding activity was seen when LHR mRNA expression was low in response to LHR downregulation26. Thus, there appeared to be an inverse relationship between the expression of LHR mRNA binding activity and LHR mRNA expression. These results have clearly pointed out that LRBP is a regulator of LHR mRNA expression.
Signaling mechanism involved in LRBP-mediated LHR mRNA degradation
The cellular mechanism that triggers the LRBP expression in response to LH/hCG treatment that leads to LHR mRNA downregulation was examined using cultured human granulosa cells. Specifically, the participation of protein kinase A (PKA) and ERK signaling pathways, the two downstream signaling molecules involved in LH action was examined during hCG- induced downregulation of LHR expression. First, we examined the possible participation of PKA in this process by blocking PKA activity by pretreatment with 10 micromolar concentrations of a pharmacological inhibitor, H89 prior to treatment with 10 IU of hCG for 12 h to down regulate LH receptor. The effect of blockade of PKA by H89 on the downregulation of LHR and induction of LRBP were determined by performing real time PCR. The results showed that, as expected, treatment with hCG, resulted in downregulation of LHR mRNA (Fig. 3A)27. This down-regulation was abolished when cells were preincubated with H89 prior to hCG addition. These results showed that the hCG-induced activation of PKA is required for inducing downregulation of LHR27.
Fig. 3.
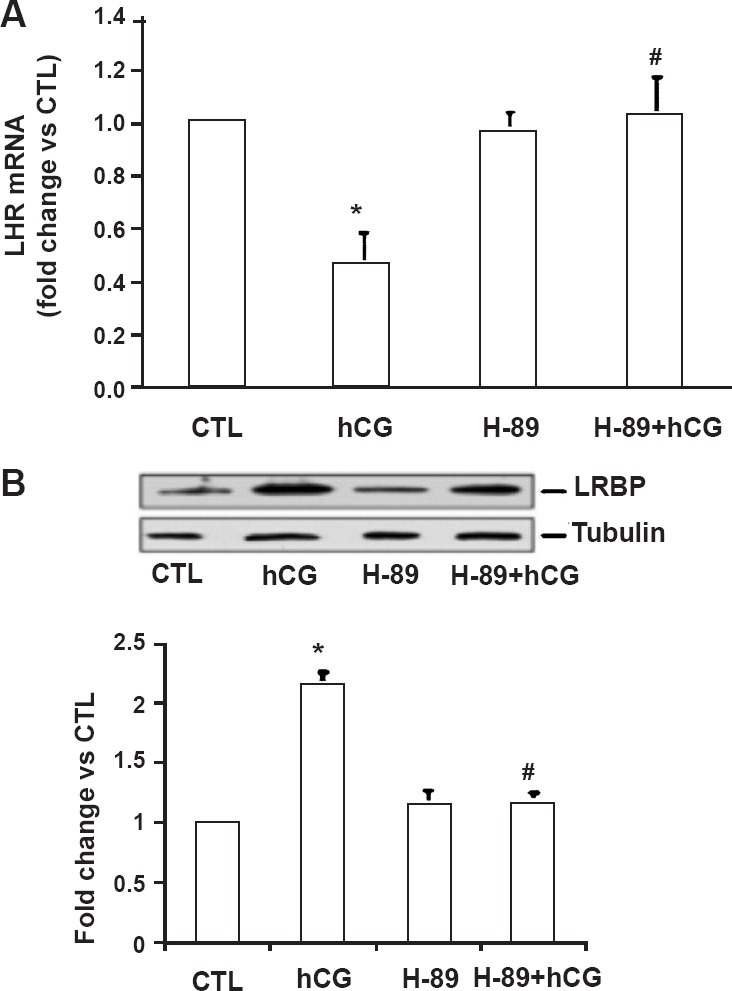
Effect of PKA inhibitor H-89 on the expression of LHR mRNA and LRBP protein in human granulosa cells. Day 3 granulosa cells were serum-starved, treated with hCG (10 IU/ml) with or without the PKA inhibitor H-89 (10 µM; 1 h pretreatment) for a total of 12 h, and were either processed for total RNA isolation or lysed using RIPA buffer. A. Total RNAs were reverse transcribed, and the resulting cDNAs were subjected to real-time PCR quantitation using specific primers and probes for LHR. The graph represents changes in mRNA levels normalized to 18S rRNA, shown as fold change vs. control. Error bars, mean ± SE. *, P < 0.05 vs. control CTL; #, P < 0.05 vs. hCG; n = 4. B. Cell lysates were subjected to Western blot analysis to detect LRBP using LRBP antibody. The membranes were then stripped and reprobed for β-tubulin. The lower panel represents densitometric scanning of the LRBP normalized for tubulin and expressed as fold change vs. CTL. The blot shown is representative of three independent experiments, and the results in the bar graph are average and SE of three experiments. *, P < 0.05 vs. CTL; #, P < 0.05 vs. hCG. (Source: Ref 27, Reproduced with permission).
Since LH/hCG- induced LHR mRNA downregulation is mediated by LRBP16,25,26,28, the effect of preincubation with H89 on the expression of LRBP was examined27. The results showed that while treatment with hCG alone caused an increase in LRBP expression (Fig. 3B), more importantly, treatment with H89 prior to hCG treatment caused a significant reduction in the expression of LRBP. From these results, we concluded that abolition of hCG-induced PKA activation abrogated both LRBP expression and downregulation of LHR mRNA.
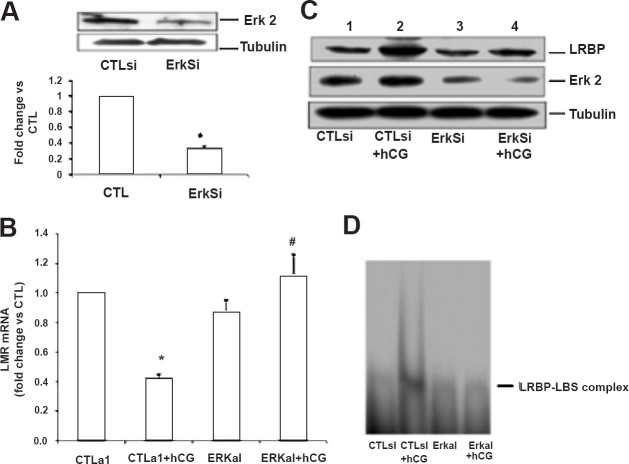
It has been shown that ERK is downstream of PKA in G protein-coupled receptor (GPCR) mediated signaling pathways29,30,31,32. To demonstrate that this is also true in human granulosa cells, the sensitivity of H89 on ERK1/2 activation in response to hCG was examined. Treatment of granulosa cells with hCG markedly increased the phosphorylation of ERK1/2 in a time dependent manner, starting as early as 5 min and lasting upto 30 min, but decreased to control levels by 1 h (Fig. 4A)27. Pretreatment with the PKA inhibitor, H89 blocked this increase clearly showing that ERK1/2 is downstream of PKA in human granulosa cells (Fig 4B). Therefore, the involvement of ERK1/2 in hCG-mediated downregulation of LHR mRNA, the effect of blocking ERK activation on LHR downregulation and LRBP expression were examined by depleting ERK using siRNA technique. First, the effectiveness of ERK specific siRNA to suppress ERK expression was assessed by incubating human granulosa cells with ERK-specific siRNA for 48 h. The results showed that ERK specific siRNA was effective in significantly reducing ERK expression while a non-specific siRNA produced no change in ERK level (Fig. 5A)27. After establishing the effectiveness of ERK specific siRNA to block ERK expression, the effect of ERK depletion on LHR mRNA downregulation was examined. Human granulosa cells were exposed to ERK-specific siRNA for 48 h to knockdown ERK expression and the ability of hCG to downregulate LH receptor was tested by incubating with 10 IU of hCG for 12 h. The results showed that while hCG alone caused downregulation of LHR, this downregulation by hCG was not seen in cells pretreated with ERK specific siRNA (Fig. 5B). Furthermore, hCG-induced LRBP expression and activity were also significantly reduced in cells where ERK expression was knocked down by siRNA compared to controls treated with a non-specific siRNA (Fig. 5C and D). These results clearly demonstrated that LH-mediated ERK activation is required for LHR downregulation and that this response is mediated by LRBP27.
Fig. 4.
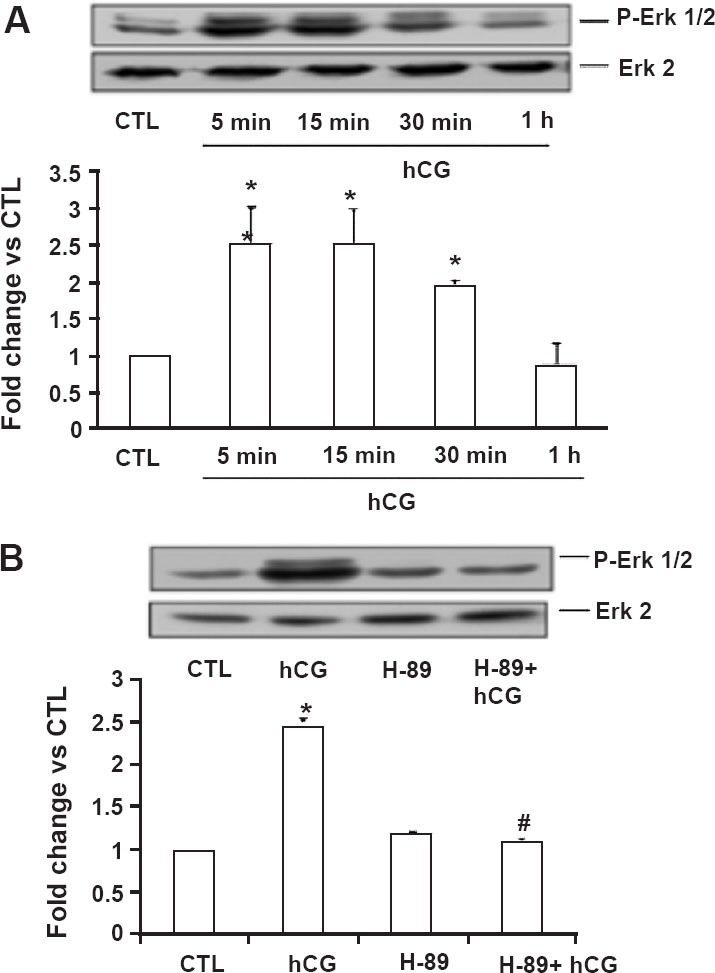
Activation of ERK1/2 in human granulosa cells. Day 4 granulosa cells were serum-starved, treated with hCG (10 IU/ml) alone for different time intervals (5 min, 15 min, 30 min, and 1 h; (A) or in the presence of H-89 (10 µM; 1 h pretreatment) for 15 min (B) and were lysed using RIPA buffer. The cell lysates were subjected to Western blot analysis to detect p-ERK1/2. The membranes were then stripped and reprobed for total ERK2. Lower panels represent densitometric scanning of the p-ERK1/2 signals normalized with ERK2 and expressed as fold change vs. CTL. The blots shown are representative of three independent experiments, and the results in the bar graphs are average and SE of three experiments. *, P < 0.05 vs. CTL; #, P < 0.05 vs. hCG. (Source: Ref. 27, Reproduced with permission).
Fig. 5.
ERK1/2 silencing inhibits hCG-induced decrease in LHR mRNA levels and increases in LRBP protein expression and binding activity. Granulosa cells were transfected with either control siRNA (CTLsi) or ERK 1/2 siRNA (ERKsi) and cultured for 48 h. After serum-starving for another 24 h, cells were treated with hCG (10 IU/ml) for 12 h and processed for total RNA isolation, for Western blot analysis, or for REMSA. A. ERK1/2 silencing was confirmed by the Western Blot analysis of cell lysates using total ERK2 antibody. B. Total RNAs were reverse transcribed, and the resulting cDNAs were subjected to real-time PCR quantitation using LHR-specific primers and probes. The graph represents changes in mRNA levels normalized to 18S rRNA and are shown as fold change vs. control. Error bars, mean ± SE.*, P < 0.05 vs. CTL; #, P < 0.05 vs. hCG; n = 3. C. Cell lysates were subjected to Western blot analysis to detect LRBP using specific antibody. The same membranes were then stripped and reprobed for ERK2 and β-tubulin. The blot shown is a representative of three independent experiments. D. G el mobility shift analysis was performed with [32P]-labelled rat LBS (1.5 × 105 cpm) and S100 fractions containing equal amounts of total protein extracted from the different treatment groups. The autoradiogram shown is representative of three independent experiments. (Source: Ref. 27, Reproduced with permission).
Conclusion
In summary, based on the present and ongoing studies, we propose a model for the mechanism for LH/hCG-mediated downregulation of LHR mRNA expression, as depicted in Fig. 627. In response to preovulatory LH surge or in response to hCG administration to induce ovulation, the initial interaction of LH with its receptor results in increased cyclic AMP production. This increase leads to activation of PKA and ERK signaling cascade to increase LRBP expression. LRBP in turn binds LHR mRNA and forms an untranslatable ribonucleoprotein complex and targets LHR mRNA for degradation in P bodies. Thus, accelerated degradation results in the transient loss of LH receptor. This loss of LH receptor causes a temporary halt in the LH/hCG-mediated signaling during the differentiation of granulosa cells to luteal cells. Thus, LH signaling through PKA and ERK1/2 pathway is required for LHR down regulation in response to preovulatory LH surge or in response to hCG treatment for ovulation induction.
Fig. 6.
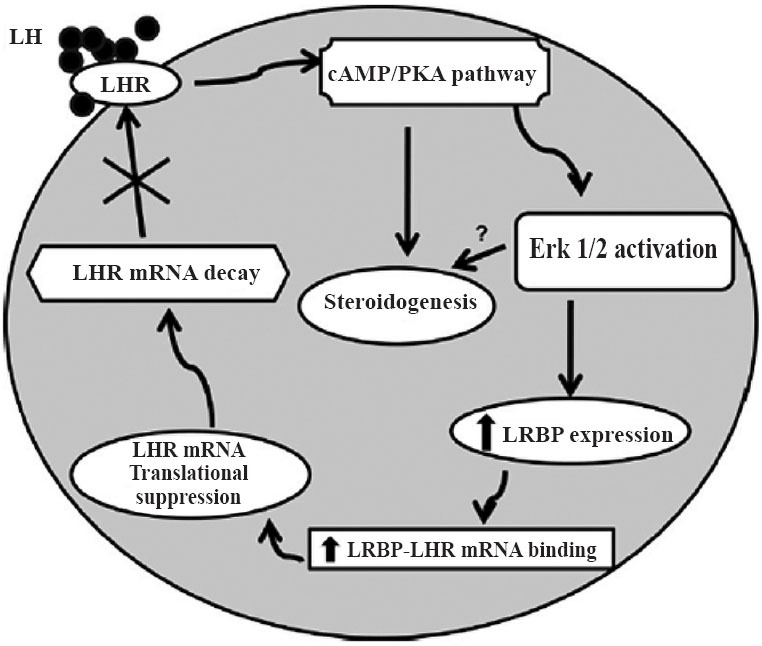
Schematic model depicting the proposed signaling pathway in LH/hCG-induced LHR mRNA down regulation. Binding of ligand to LH receptor induces activation of ERK1/2 through the cAMP/PKA pathway. This leads to an increase in the expression of LRBP and thereby its LHR mRNA binding activity, which ultimately results in LHR mRNA degradation. (Source: Ref. 27, Reproduced with permission).
Acknowledgment
The studies were supported by the United States Public Health Service grant, NIH HD R37 06656 to the first author (KMJM). Authors thank the Endocrine Society and the American Society of Biological Chemistry and Molecular Biology for granting us permission to reproduce figures from our previous publications.
References
- 1.Menon KMJ, Menon B. Structure, function and regulation of gonadotropin receptors - a perspective. Mol Cell Endocrinol. 2012;356:88–97. doi: 10.1016/j.mce.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–74. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- 3.Menon KMJ, Menon B, Wang L, Gulappa T, Harada M. Molecular regulation of gonadotropin receptor expression: relationship to sterol metabolism. Mol Cell Endocrinol. 2010;329:26–32. doi: 10.1016/j.mce.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao CV. An overview of the past, present, and future of nongonadal LH/hCG actions in reproductive biology and medicine. Semin Reprod Med. 2001;19:7–17. doi: 10.1055/s-2001-13906. [DOI] [PubMed] [Google Scholar]
- 5.McFarland KC, Sprengel R, Phillips HS, Kohler M, Rosemblit N, Nikolics K, et al. Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science. 1989;245:494–9. doi: 10.1126/science.2502842. [DOI] [PubMed] [Google Scholar]
- 6.Davis JS, West LA, Farese RV. Effects of luteinizing hormone on phosphoinositide metabolism in rat granulosa cells. J Biol Chem. 1984;259:15028–34. [PubMed] [Google Scholar]
- 7.Gudermann T, Birnbaumer M, Birnbaumer L. Evidence for dual coupling of the murine luteinizing hormone receptor to adenylyl cyclase and phosphoinositide breakdown and Ca2+ mobilization. Studies with the cloned murine luteinizing hormone receptor expressed in L cells. J Biol Chem. 1992;267:4479–88. [PubMed] [Google Scholar]
- 8.Fu Z, Wang M, Potter D, Miziorko HM, Kim JJ. The structure of a binary complex between a mammalian mevalonate kinase and ATP: insights into the reaction mechanism and human inherited disease. J Biol Chem. 2002;277:18134–42. doi: 10.1074/jbc.M200912200. [DOI] [PubMed] [Google Scholar]
- 9.Munshi UM, Peegel H, Menon KMJ. Palmitoylation of the luteinizing hormone/human chorionic gonadotropin receptor regulates receptor interaction with the arrestin-mediated internalization pathway. Eur J Biochem. 2001;268:1631–9. doi: 10.1046/j.1432-1327.2001.02032.x. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman YM, Peegel H, Sprock MJ, Zhang QY, Menon KMJ. Evidence that human chorionic gonadotropin/luteinizing hormone receptor down-regulation involves decreased levels of receptor messenger ribonucleic acid. Endocrinology. 1991;128:388–93. doi: 10.1210/endo-128-1-388. [DOI] [PubMed] [Google Scholar]
- 11.LaPolt PS, Oikawa M, Jia XC, Dargan C, Hsueh AJ. Gonadotropin-induced up- and down-regulation of rat ovarian LH receptor message levels during follicular growth, ovulation and luteinization. Endocrinology. 1990;126:3277–9. doi: 10.1210/endo-126-6-3277. [DOI] [PubMed] [Google Scholar]
- 12.Segaloff DL, Wang HY, Richards JS. Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular development and luteinization. Mol Endocrinol. 1990;4:1856–65. doi: 10.1210/mend-4-12-1856. [DOI] [PubMed] [Google Scholar]
- 13.Zeleznik AJ. The physiology of follicle selection. Reprod Biol Endocrinol. 2004;2:31. doi: 10.1186/1477-7827-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peegel H, Randolph J, Jr, Midgley AR, Menon KMJ. In situ hybridization of luteinizing hormone/human chorionic gonadotropin receptor messenger ribonucleic acid during hormone-induced down-regulation and the subsequent recovery in rat corpus luteum. Endocrinology. 1994;135:1044–51. doi: 10.1210/endo.135.3.8070346. [DOI] [PubMed] [Google Scholar]
- 15.Lu DL, Peegel H, Mosier SM, Menon KMJ. Loss of lutropin/human choriogonadotropin receptor messenger ribonucleic acid during ligand-induced down-regulation occurs post transcriptionally. Endocrinology. 1993;132:235–40. doi: 10.1210/endo.132.1.8419125. [DOI] [PubMed] [Google Scholar]
- 16.Nair AK, Peegel H, Menon KMJ. The role of luteinizing hormone/human chorionic gonadotropin receptor-specific mRNA binding protein in regulating receptor expression in human ovarian granulosa cells. J Clin Endocrinol Metab. 2006;91:2239–43. doi: 10.1210/jc.2005-2739. [DOI] [PubMed] [Google Scholar]
- 17.Kash JC, Menon KMJ. Identification of a hormonally regulated luteinizing hormone/human chorionic gonadotropin receptor mRNA binding protein. Increased mRNA binding during receptor down-regulation. J Biol Chem. 1998;273:10658–64. doi: 10.1074/jbc.273.17.10658. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein PL, Herrick DJ, Prokipcak RD, Ross J. Control of c-myc mRNA half-life in vitro by a protein capable of binding to a coding region stability determinant. Genes Dev. 1992;6:642–54. doi: 10.1101/gad.6.4.642. [DOI] [PubMed] [Google Scholar]
- 19.Shyu AB, Belasco JG, Greenberg ME. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991;5:221–31. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- 20.Tai N, Schmitz JC, Chen TM, Chu E. Characterization of a cis-acting regulatory element in the protein-coding region of human dihydrofolate reductase mRNA. Biochem J. 2004;378:999–1006. doi: 10.1042/BJ20031396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin X, Parsels LA, Voeller DM, Allegra CJ, Maley GF, Maley F, et al. Characterization of a cis-acting regulatory element in the protein coding region of thymidylate synthase mRNA. Nucleic Acids Res. 2000;28:1381–9. doi: 10.1093/nar/28.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leibold EA, Munro HN. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5’ untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988;85:2171–5. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwai Y, Bickel M, Pluznik DH, Cohen RB. Identification of sequences within the murine granulocyte-macrophage colony-stimulating factor mRNA 3’-untranslated region that mediate mRNA stabilization induced by mitogen treatment of EL-4 thymoma cells. J Biol Chem. 1991;266:17959–65. [PubMed] [Google Scholar]
- 24.Tholanikunnel BG, Malbon CC. A 20-nucleotide (A + U)-rich element of beta2-adrenergic receptor (beta2AR) mRNA mediates binding to beta2AR-binding protein and is obligate for agonist-induced destabilization of receptor mRNA. J Biol Chem. 1997;272:11471–8. doi: 10.1074/jbc.272.17.11471. [DOI] [PubMed] [Google Scholar]
- 25.Kash JC, Menon KMJ. Sequence-specific binding of a hormonally regulated mRNA binding protein to cytidine-rich sequences in the lutropin receptor open reading frame. Biochemistry. 1999;38:16889–97. doi: 10.1021/bi9915770. [DOI] [PubMed] [Google Scholar]
- 26.Nair AK, Kash JC, Peegel H, Menon KMJ. Post-transcriptional regulation of luteinizing hormone receptor mRNA in the ovary by a novel mRNA-binding protein. J Biol Chem. 2002;277:21468–73. doi: 10.1074/jbc.M111653200. [DOI] [PubMed] [Google Scholar]
- 27.Menon B, Franzo-Romain M, Damanpour S, Menon KM. Luteinizing hormone receptor mRNA down-regulation is mediated through ERK-dependent induction of RNA binding protein. Mol Endocrinol. 2011;25:282–90. doi: 10.1210/me.2010-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nair AK, Menon KMJ. Isolation and characterization of a novel trans-factor for luteinizing hormone receptor mRNA from ovary. J Biol Chem. 2004;279:14937–44. doi: 10.1074/jbc.M309484200. [DOI] [PubMed] [Google Scholar]
- 29.Seger R, Hanoch T, Rosenberg R, Dantes A, Merz WE, Strauss JF, 3rd, et al. The ERK signaling cascade inhibits gonadotropin-stimulated steroidogenesis. J Biol Chem. 2001;276:13957–64. doi: 10.1074/jbc.M006852200. [DOI] [PubMed] [Google Scholar]
- 30.Cameron MR, Foster JS, Bukovsky A, Wimalasena J. Activation of mitogen-activated protein kinases by gonadotropins and cyclic adenosine 5’-monophosphates in porcine granulosa cells. Biol Reprod. 1996;55:111–9. doi: 10.1095/biolreprod55.1.111. [DOI] [PubMed] [Google Scholar]
- 31.Das S, Maizels ET, DeManno D, St Clair E, Adam SA, Hunzicker-Dunn M. A stimulatory role of cyclic adenosine 3’, 5’-monophosphate in follicle-stimulating hormone-activated mitogen-activated protein kinase signaling pathway in rat ovarian granulosa cells. Endocrinology. 1996;137:967–74. doi: 10.1210/endo.137.3.8603610. [DOI] [PubMed] [Google Scholar]
- 32.Salvador LM, Maizels E, Hales DB, Miyamoto E, Yamamoto H, Hunzicker-Dunn M. Acute signaling by the LH receptor is independent of protein kinase C activation. Endocrinology. 2002;143:2986–94. doi: 10.1210/endo.143.8.8976. [DOI] [PubMed] [Google Scholar]