Abstract
Contraception is a basic human right for its role on health, quality of life and wellbeing of the woman and of the society as a whole. Since the introduction of female hormonal contraception the responsibility of family planning has always been with women. Currently there are only a few contraceptive methods available for men, but recently, men have become more interested in supporting their partners actively. Over the last few decades different trials have been performed providing important advances in the development of a safe and effective hormonal contraceptive for men. This paper summarizes some of the most recent trials.
Keywords: Fertility, gonadotropins, hormonal, male contraception, spermatogenic suppression, testosterone
Introduction
Unwanted pregnancy is a major global problem and a big burden that increases social costs and health care. To provide men and women with the option of sharing family planning responsibilities satisfies not only fundamental individual rights but also social needs. Currently, the world population is about 6.9 billion, and it is growing at the rate of 80 million per year. It is projected that the global population will exceed 9 billion by 2050 (“U.S. Census Bureau – World POP Clock Projection” July 2012 – July 2013 data).
Many recent surveys suggest that family planning strategies such as pregnancy prevention, promoting healthy sexual practices or using effective contraception, when available, work well1,2,3. Many years of clinical experience with female hormonal contraceptives have shown that the provision of a wider range of choices could improve access and use. At present, male controlled methods include condoms and vasectomy. These are not optimal or generally acceptable because of the high user failure rate with condoms and the difficult reversibility of vasectomy. In spite of the shortcomings, one third of couples using contraception worldwide relies on a male method1. These observations would suggest that if new contraceptives for men were available, many couples worldwide would use them. Therefore, a new hormonal option for men would certainly increase male contraceptive use thus contributing to a more equal sharing of family planning.
Among all the approaches undertaken for the development of new male contraceptives, hormonal methods are potentially closest to a possible clinical application. However, despite significant progress showing the contraceptive efficacy of hormonal regimens for men in comparison to female hormonal methods and their feasibility and acceptability, research in this field has not led to an approved product. Availability of male hormonal contraceptives could provide the male partner a stable relationship with an opportunity to share the family planning responsibility4, and give men in general the opportunity to regain control over their fertility.
Main requirements for an ideal male contraceptive should be (i) acceptability for both partners; (ii) utilization independent of the sexual act; (iii) absence of short or long-term toxic side effects; (iv) no interference with libido, potency, or sexual activity; (v) absence of impact on eventual offspring; (vi) rapid effectiveness and full reversibility; and (vii) effectiveness comparable to female methods5,6,7,8.
Mechanism of action and efficacy studies
The hormonal approach to male contraception is based on the reversible suppression of gonadotropins leading to reversible suppression of the spermatogenetic process. Current strategy focuses on the administration of sex steroid hormones which provide negative feedback on the hypothalamic-pituitary axis (HPA) inhibiting leutenizing and follicle stimulating hormones (LH and FSH) release, suppressing intratesticular androgen production and thus sperm production. Spermatogenesis is dependent on the action of FSH on the Sertoli cells and on high intratesticular testosterone (T) concentration. Both the decrease of T and suppression of FSH lead to a decrease of Sertoli cell function essential for germ cells maturation. The decrease of LH concentration suppresses T production by the Leydig cells; therefore, an add-back androgen therapy is required to maintain physiological levels of T and consequently androgen-dependent physiological functions4,5,7,8.
The validity of this concept was initially proven in two large WHO-supported studies in which weekly injections of testosterone enanthate (TE) at a dose of 200 mg were administered to healthy volunteers9,10. In those studies no pregnancies occurred among men who reached azoospermia, there were two pregnancies among subjects with a sperm count suppressed below 1 million/ml and four pregnancies among those with a sperm count suppressed below 3 million/ml. These results suggest that only azoospermia or severe oligozoospermia represents the ideal goal for contraception9,10.
More recently, other efficacy studies in which different formulations of T alone or in combination with a progestin were used, have confirmed that sperm suppression achieved through hormone based regimens may provide optimal contraceptive protection (Table).
Table.
Characteristics and results of efficacy studies with hormonal contraceptive regimens in men
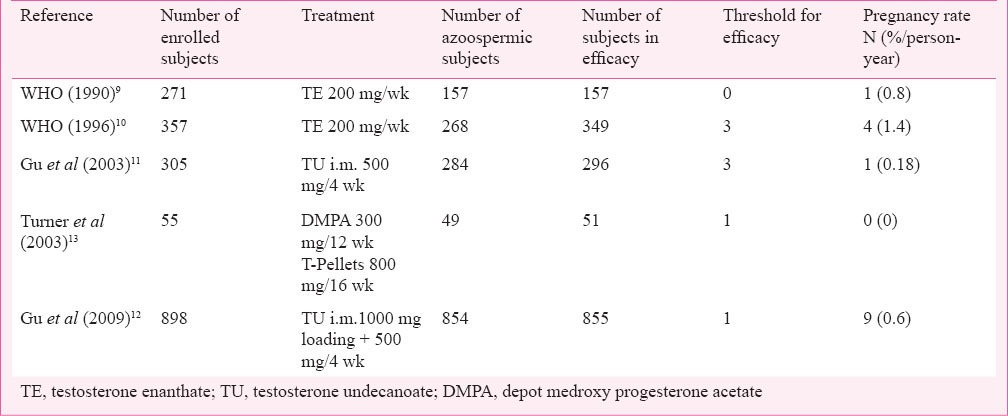
In the first large Chinese efficacy trial, 308 men received monthly injections of 500 mg testosterone undecanoate (TU) after a 1000 mg loading dose11. Forty three per cent men become azoospermic, only nine of them failed to suppress to sperm concentrations below 3 million sperm/ml, the threshold for entering the efficacy phase. During the 12-month efficacy phase, 296 men used the TU injections as a sole mean of contraception. Six of them had a rebound of sperm production, and one pregnancy attributed to this rebound occurred. Overall, this regimen had a 96.7 per cent contraceptive efficacy in Chinese men11 (Table). This study was followed by another efficacy study also performed in China. This is the largest efficacy study on male contraception ever performed. It was supported by the WHO and a total of 1045 couples were enrolled to receive TU injected at a dose of 1000 mg followed by 500 mg every month for 30 months12. A total of 733 couples completed the efficacy phase. Forty three participants (4.8%) did not achieve azoospermia or severe oligozoospermia within the six month suppression phase. There were nine pregnancies in 1554.1 person-years of exposure in the 24-month efficacy phase for a cumulative contraceptive failure rate of 1.1 per 100 men. The combined method failure rate was 6.1 per cent, comprising 4.8 per cent with inadequate suppression and 1.3 per cent with post-suppression sperm rebound. The authors showed that among the couples who completed the efficacy phase, the contraceptive protection provided by these regimens was excellent not only when compared with male condoms (2% for perfect use, 15% for typical use) or withdrawal but also with female oral hormonal contraception. Although the authors concluded that the “monthly injection of 500 mg TU provides safe, effective, reversible, and reliable contraception”12, the monthly injections were considered to be the most inconvenient part of this regimen in another Chinese study14. The Chinese authorities considered this regimen unsuitable for the Chinese contraceptive market and did not approve the use of TU for contraception.
Another important although smaller trial to evaluate the efficacy of a contraceptive regimen was performed using testosterone-pellets (four 200 mg implants, every four or six months) and 300 mg depot medroxyprogesterone acetate (DMPA), injected every three months. No pregnancies occurred in 426 person-months (35.5 person-years; 95% confidence limits for contraceptive failure rate, 0-8%/annum)13.
Most promising studies on male hormonal contraception
In order to provide optimal and highly acceptable formulations, lower doses of T than those used when T was administered alone, were administered at long intervals with the addition of a progestin. The rationale for combining androgens with progestin to suppress fertility in men is based on the synergic and additive effects that the two steroids have at the hypothalamus-pituitary level resulting in more rapid and profound gonadotropins and sperm suppression compared to each compound administered alone. Moreover, the combination that uses lower dose of T allows for avoiding side effects derived from supra-physiological serum androgen levels and improving the safety of the regimen15. Based on this rationale, the long-acting T formulation TU has been combined with different progestins such as medroxyprogesterone acetate (MPA), cyproterone acetate (CPA), levonorgestrel (LNG), desogestrel (DSG) and norethisterone enanthate (NETE)15. The addition of the injectable depot preparation NETE at the dose of 200 mg to the TU at the dose of 1000 mg every six weeks resulted in a profound suppression of spermatogenesis with 13 of 14 subjects becoming azoospermic16. When the same regimen was used with an interval of injections extended to eight weeks, 90 per cent of subjects still achieved azoospermia and all volunteers were severely oligozoospermic (<1 million/ml) by the end of the study17. Due to this promising preliminary data, the WHO and Contraceptive Research and Development (CONRAD) planned a large multinational phase IIb efficacy trial in which this combination was administered every eight weeks16,17. The design of the study included two screening/baseline/control visits followed by a six-month suppression period. Couples whose male partner had achieved a sperm concentration of ≤1 million/ml entered the 12-month efficacy period. All men were followed for recovery up to 12 months after stopping hormone administration. The primary outcomes of the study were contraceptive efficacy, degree and timing of suppression of spermatogenesis, while secondary outcomes were maintenance of spermatogenic suppression, reversibility, changes in circulating hormone concentrations safety parameters, and acceptability to men and women. Screening began in July 2008 and recruitment ended on September 30, 2010. In total 487 volunteer couples consented and attended at least one screening visit. Of the 321 couples enrolled, 260 entered the efficacy phase, six failed to suppress and 55 discontinued before suppressing for various reasons. Most frequent adverse events (AEs) related to study products were those expected such as acne, increased libido, injection site pain, myalgia and emotional disorders. These events were reported as mostly “mild” in severity and occurred more frequently in some centers than in others. No systematic or clinically relevant changes in biomedical safety parameters were recorded. However, two serious adverse events, both mood related, occurred in March 2011 which were considered to be possibly or probably related to study products. These events prompted an external peer-review committee serving the WHO Department of Reproductive Health and Research to subsequently recommend stopping the injections18. Recovery of sperm counts has just completed and analysis of the results, both hormonal and clinical data, are eagerly awaited to plan future strategies in the development of hormonal contraceptives for men.
In other studies TU was also combined with DSG or implants of its active metabolite etonogestrel (ENG). Lastly, it was used in an important trial in which 354 men were randomized to receive one of six active treatments consisting of ENG subcutaneous implant (low of high release) and injections of TU at the dose of 750 or 1000 mg every 10 or 12 wk or a placebo. The study showed that spermatogenesis was suppressed to 1 million/ml or less up to the end of the treatment period in 91 per cent of men19. The combination of an ENG implant with TU injections was well-tolerated, providing effective and reversible suppression of spermatogenesis.
In a recent trial, transdermal T gel has been combined with nonandrogenic progestin nestorone (NES). Both hormones were administered daily as transdermal gel. A total of 56 subjects were randomized to receive one of the three treatments consisting of T gel at the dose of 10 g plus NES at the dose of 0,8 or 12 mg, respectively. The gel-gel combination suppressed spermatogenesis to 1 million/ml in 88.5 per cent of the subjects and no serious adverse effects were reported20. This gel-gel combination may represent a more acceptable regimen to some men for long-term use than regimens requiring injections21.
Acceptability
Over the last decades some large studies have been performed in different countries to evaluate the level of acceptability of possible hormonal methods for male contraception22,23,24,25,26. In these studies, between 44-83 per cent of the participants interviewed welcomed new hormonal methods. In two studies performed in Italy and China men participating in clinical trials on potential hormonal injectable contraceptive found the method acceptable14,27. In both studies they agreed that men and women should share responsibility for contraception, even though this form of contraception is often indicated as being most appropriate for use in stable relationships because of its characteristics such as the time required to become effective (12-16 wk or longer), the absence of protection from sexually transmitted infections and the need for partner communication. In the Italian trial27 about one-third of the men who volunteered to participate in this study were young with no children, who were interested in finding an alternative to condoms. This opens an interesting possibility that male contraception may not only be for couples who decide to share the responsibility for family planning but also for young men who want to maintain control over their fertility and avoid fathering children. This population of men may not be willing to give up control of fertility. Seventy nine per cent of the study population indicated that they would use this contraceptive method if it was available, and 74 per cent thought that their partner would appreciate it. These results indicate a high degree of acceptance for this new form of male contraception27.
Conclusion
Studies performed over the last decades have shown that hormonal regimens which induce profound suppression of spermatogenesis in men can be developed. With the best combinations about 95 per cent of men can have their spermatogenesis suppressed to the concentration of 1 million/ml or less, threshold that has been demonstrated to provide an optimal contraceptive protection, similar to the female hormonal contraceptives. However, despite the important advances reached in this field, due to the support of public agencies such as WHO, CONRAD or National Institute of Child Health and Human Development (NHICID) and strongly advocated by dedicated researchers, the development of a marketable hormonal product for male contraception remains an unreachable goal. Market surveys have indicated that a hormonal contraceptive with the characteristics that have been described, would be acceptable to many men and women who are in long-term stable relationships28 and to many single men who want regain control over their fertility. However, no major drug company has shown interest in leading this project to a market that does not seem profitable nor free from possible litigation. So far, no concert action between the scientific community and governments has been undertaken to change this situation. Until this happens, it is not possible to foresee when and whether a male hormonal contraceptive will become available.
References
- 1.Darney PD. Family planning and future. Am J Obstet Gynecol. 2011;205(Suppl):S26–8. doi: 10.1016/j.ajog.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 2.Jensen JT. Why family planning matters. Rev Endocr Metab Disord. 2011;12:55–62. doi: 10.1007/s11154-011-9179-z. [DOI] [PubMed] [Google Scholar]
- 3.Kaiser J. Does family planning bring down fertility? Science. 2011;333:548–9. doi: 10.1126/science.333.6042.548. [DOI] [PubMed] [Google Scholar]
- 4.Ilani N, Swerdloff RS, Wang C. Male hormonal contraception: potential risks and benefits. Rev Endocr Metab Disord. 2011;12:107–17. doi: 10.1007/s11154-011-9183-3. [DOI] [PubMed] [Google Scholar]
- 5.Nieschlag E. Clinicals trials in male hormonal contraception. Contraception. 2010;82:457–70. doi: 10.1016/j.contraception.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Nieschlag E. The struggle for male hormonal contraception. Best Pract Res Clin Endocrinol Metab. 2011;25:369–75. doi: 10.1016/j.beem.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Amory JK. Progress and prospects in male hormonal contraception. Curr Opin Endocrinol Diabetes Obes. 2008;15:255–60. doi: 10.1097/MED.0b013e3282fcc30d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Swerdloff RS. Hormonal approaches to male contraception. Curr Opin Urol. 2010;20:520–4. doi: 10.1097/MOU.0b013e32833f1b4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia in normal men. Lancet. 1990;336:955–9. [PubMed] [Google Scholar]
- 10.World Health Organization Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril. 1996;65:821–9. [PubMed] [Google Scholar]
- 11.Gu YQ, Wang XH, Xu D, Peng L, Cheng L-F, Huang M-K, et al. A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy Chinese men. J Clin Endocrinol Metab. 2003;88:562–8. doi: 10.1210/jc.2002-020447. [DOI] [PubMed] [Google Scholar]
- 12.Gu Y, Liang X, Wu W, Liu M, Song S, Cheng L, et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab. 2009;94:1910–5. doi: 10.1210/jc.2008-1846. [DOI] [PubMed] [Google Scholar]
- 13.Turner L, Conway AJ, Jimenez M, Liu PY, Forbes E, McLachlan RI, et al. Contraceptive efficacy of a depot progestin and androgen combination in men. J Clin Endocrinol Metab. 2003;88:4659–67. doi: 10.1210/jc.2003-030107. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Shahb HI, Liu Y, Vogelsong KM, Zhang L. The acceptability of an injectable, once-a-month male contraceptive in China. Contraception. 2006;73:548–53. doi: 10.1016/j.contraception.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Meriggiola MC, Farley TMM, Mbizvo MT. A review of androgen-progestin regimens for male contraception. J Androl. 2003;24:466–83. doi: 10.1002/j.1939-4640.2003.tb02695.x. [DOI] [PubMed] [Google Scholar]
- 16.Kamischke A, Venherm S, Ploger D, von Eckardstein S, Nieschlag E. Intramuscular testosterone undecenoate and norethisterone enanthate in a clinical trial for male contraception. J Clin Endocrinol Metab. 2001;86:303–9. doi: 10.1210/jcem.86.1.7057. [DOI] [PubMed] [Google Scholar]
- 17.Meriggiola MC, Costantino A, Saad F, D’Emidio L, Morselli Labate AM, Bertaccini A, et al. Norethisterone enanthate plus testosterone undecanoate for male contraception: Effects of various injection intervals on spermatogenesis, reproductive hormones, testis, and prostate. J Clin Endocrinol Metab. 2005;90:2005–14. doi: 10.1210/jc.2004-1852. [DOI] [PubMed] [Google Scholar]
- 18. [accessed on September 21, 2012]. Available from: http://conrad.org/contraception-trials.html .
- 19.Mommers E, Kersemaekers WM, Elliesen J, Kepers M, Apter D, Behre HM, et al. Male hormonal contraception: A double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:2572–80. doi: 10.1210/jc.2008-0265. [DOI] [PubMed] [Google Scholar]
- 20.Ilani N, Roth MY, Amory JK, Swerdloff RS, Dart C, Page ST, et al. A new combination of testosterone and nestorone transdermal gels for male hormonal contraception. J Clin Endocrinol Metab. 2012;97:1–11. doi: 10.1210/jc.2012-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth MY, Ilani N, Wang C, Page ST, Bremner WJ, Swerdloff RS, et al. Characteristics associated with suppression of spermatogenesis in a male hormonal contraceptive trial using testosterone and Nestorone(®) gels. Andrology. 2013;1:899–905. doi: 10.1111/j.2047-2927.2013.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin CW, Anderson RA, Cheng L, Ho PC, van der Spuy Z, Smith KB, et al. Potential impact of hormonal male contraception: cross-cultural implications for development of novel preparations. Hum Reprod. 2000;15:637–45. doi: 10.1093/humrep/15.3.637. [DOI] [PubMed] [Google Scholar]
- 23.Heinemann K, Saad F, Wiesemes M, White S, Heinemann L. Attitudes toward male fertility control: results of a multinational survey on four continents. Hum Reprod. 2005;20:549–56. doi: 10.1093/humrep/deh574. [DOI] [PubMed] [Google Scholar]
- 24.Heinemann K, Saad F, Wiesemes M, Heinemann L. Expectations toward a novel male fertility control method and potential user types: results of a multinational survey. J Androl. 2005;26:155–62. doi: 10.1002/j.1939-4640.2005.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 25.Ringheim K. Evidence for the acceptability of an injectable hormonal method for men. Fam Plann Perspect. 1995;27:123–8. [PubMed] [Google Scholar]
- 26.Glasier AF, Anakwe R, Everington D, Martin CW, van der Spuy Z, Cheng L, et al. Would women trust their partners to use a male pill? Hum Reprod. 2000;15:646–9. doi: 10.1093/humrep/15.3.646. [DOI] [PubMed] [Google Scholar]
- 27.Meriggiola MC, Cerpolini S, Bremner WJ, Mbizvo MT, Vogelsong KM, Martorana G, et al. Acceptability of an injectable male contraceptive regimen of norethisterone enanthate and testosterone undecanoate for men. Hum Reprod. 2006;21:2033–40. doi: 10.1093/humrep/del094. [DOI] [PubMed] [Google Scholar]
- 28.Liu PY, Mclachlan RI. Male hormonal contraception: so near and yet so far. J Clin Endocrinol Metab. 2008;93:2474–6. doi: 10.1210/jc.2008-1007. [DOI] [PubMed] [Google Scholar]


