Abstract
This group has advocated a return to the notional Palæolithic diet with fruits, vegetables, roots, leaves, seeds, phytochemical antioxidants and proteins, etc. Phytoestrogens, viz. lignans, isoflavonoids and flavonoids are weak oestrogenic constituents of such a diet and may have a considerable impact on human health and disease. The aim of this paper was to conduct a preliminary overview of about 2000 research-led studies from the 1930s to the present time reported in the literature on flavonoids/isoflavonoids/lignans and to assemble evidence for a future strictly formal literature review on the health benefits and risks of flavonoids in a variety of diseases.
Keywords: Antioxidants, cancer, endocrine, epidemiology, epigenetics, flavonoids, imprinting
Introduction
Vital evidence is needed to understand the effect of dietary phytoestrogens and related compounds on human growth regulation and their impact on health and disease. This review brings together strands of evidence which support the view of moderating our diet, and offers potential solutions to the global epidemic of cardiovascular disease and endocrine cancer through selective dietary practice and functional foods. The first of the four sections sets the epigenetic scene and the dietary context. The second section identifies some basic structures that are candidate phytoestrogens likely to influence enzyme systems and influence mechanisms of hormone action in endocrine cancer; and action as antioxidants. The third section describes possible human phytoestrogen interaction with the epigenome before and after transplantation of the fertilized egg (germ layers before differentiation: brain, liver and kidney); the foetal development of maleness; the prenatal endocrinology; and that of puberty and adulthood. Finally, the fourth section provides dietary intervention concepts that provide insight into dietary advice and the development of functional foods against a backdrop of global epidemiological and notional Palæolithic dietary evidence supported by a beneficial lifestyle.
Epigenetics
Gregor Mendel's experiments1 from 1853-1863 on Pisum sativum at the Augustinian Abbey of St Thomas in Brno, Czech Republic led to two generalizations known as the Law of Segregation and the Law of Independent Assortment, which were then collectively known as Mendel's Laws of Inheritance. It was McClintock's techniques2 that allowed her to confirm experimentally the work of Thomas Hunt Morgan3 on crossover viz. that genetic traits were associated with an exchange of genetic material by chromosomes i.e. parts of the genome can jump around and cause mutations or alter gene expression. McClintock4 recognized that the epigenetics was taking place even before the structure of DNA was elucidated5. Over the next 50 years or so theories were developed i.a. on diet or the wider environment and how such factors might bring about beneficial change6,7,8. When Bird defined epigenetics as ‘the structural adaption of chromosomal regions to register, signal or perpetuate altered activity states’9, the strait-jacket of heritability was loosened, because this description focussed on genes and chromosomes and epigenetic marks, that once deposited, adapted to environmental and intrinsic stimuli.
Critical timings for epigenetic change and expression
Flavonoids, food restriction, and omega-3 fatty acids may exert epigenetic changes8 that produce phenotypes for disease such as cancer, cardiovascular, and many other diseases. The regulation of these marks and subsequent transcription may involve the now well-known DNA methylation, post-translational histone modifications10, and ncRNAs (non-coding RNAs)11. The ability of a DNA sequence or transcript to regulate the expression of one or more genes on the same chromosome (cis) or different chromosomes, or mature RNAs in the cytoplasm (trans), is extremely important in the development of the placenta and foetus. These regulatory mechanisms serve to express imprinted (parent-of-origin specific) and non-imprinted genes which lead to placental development in such a way that might adversely affect the health of the foetus, and/or mother, in both short- and long-term. The process is more complex: the imprintor is a protein that exercises control over a region of the genome such that the imprint may give rise to changes in development later in life. Epigenetic marks that are imprints are predominantly in clusters within the genome and wish to preserve their parent-origin monoallelic expression12.
Flavonoid imprinting
This paper focuses on the endocrinology of human development and the potential importance of imprinting in the aetiologies of breast and prostate cancer wherein dietary flavonoids and natural oestrogens may exert influence and potentially offer hope for the development of functional foods. It also draws attention to the notional Palæolithic diet with fruits, vegetables, roots, leaves, seeds, honey, meat, fish and eggs i.a. which have a low w-6/w-3 ratio of fatty acids, high monounsaturated fatty acids, fibre, phytochemical antioxidants and proteins. Phytoestrogens, viz. lignans, isoflavonoids and flavonoids are weak oestrogenic constituents of such a diet and may have a considerable impact on human health and disease at critical stages of human development13. In the ancient Indian systems of Ayurvedic and Siddha medicine, healers treated patients with asthma and other breathing disorders with diet, exercise, and herbs. Although not obviously subscribing to the concept of an immortality drink, mythologically appearing in at least two Indo-European cultures, viz. Greek and Sanskrit, ambrosia and amrita, it is highly probable that much dietary information can be gleaned and applied to enhancing longevity with a good quality of life starting with the epigenetics of foeto-placental development, leading to pivotal expression in perinatal and pubertal stages that perhaps express or inhibit the onset of endocrine and cardiovascular related diseases (Fig. 1).
Fig. 1.
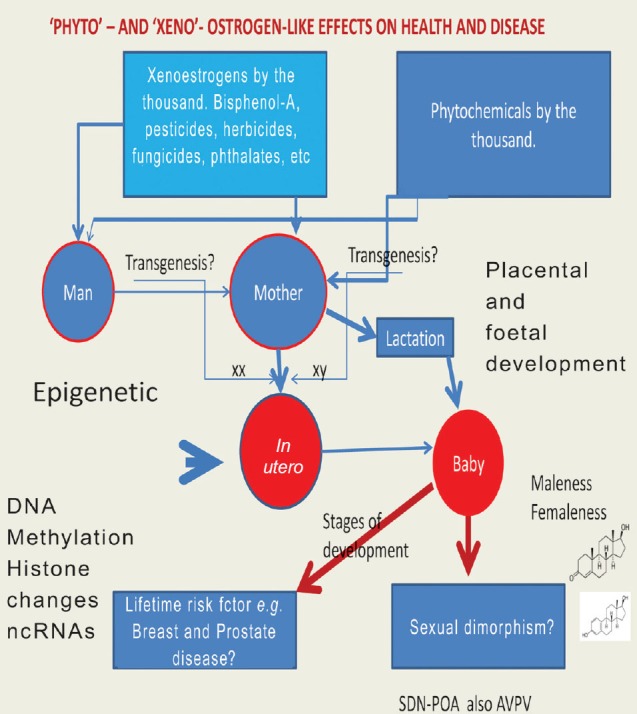
The interrelation between dietary xenoestrogens and phytochemicals in male and female that may introduce an exogenous gene (transgenesis), and evoking the widely accepted theory that an individual animal develops by the gradual differentiation and elaboration of a fertilized egg cell, as indicated, through DNA methylation and other changes, e.g. histone modifications (epigenetics) which imprint on foeto-placental development and lifetime risk of non-communicable diseases. SDN-POA refers to the sexual dimorphic nucleus located in the pre-optic area of the brain which is linked to sexual behaviour; AVPV is the anteroventral periventicular nucleus of the hypothalamus and is particularly implicated in imprinting rats. The chemical structures (lower right) refer to testosterone and oestradiol (maleness/femaleness). The term by the thousand implies a multifarious very large indeterminate number of compounds.
Candidate phytoestrogen molecular prerequisites
These candidates must at least, satisfy certain criteria if these are to be used for healthcare14. These must be present in the human diet although this is not an absolute requirement. These must be present in biological fluids (e.g. urine, blood, saliva, prostatic fluid, breast aspirates). These must be absorbed/metabolized and the uptake substantial enough to elicit a timely demonstrable beneficial effect at cellular/tissue level. It is this latter statement which is critical to understanding risk of disease at different stages of human development; lifetime safety is difficult to measure. Overall, the benefits must outweigh any adverse effects so that these can become components of a functional food, synthesised or otherwise to reflect epidemiological concentrations of bioflavonoids in populations at lower risk of disease, or as a product per se.
Pathogenicity
It is likely that based on chemical structure and receptor mechanisms, phytoestrogens exhibit antagonist or agonist interaction with natural oestradiol in endocrine tissues, principally, brain uterus, breast and prostate. For example, in the mouse uterine growth test for various phytoestrogens, it seems that the spatial spatial distance of 10.3 Å between the C3 and C18 atoms of the oestradiol molecule is a key feature of such oestrogenic activity because this spatial difference is also present between the C3 and C18 of miroestrol and the C7 and C4 configurations of genistein and other similar compounds showing similar activity15. On this and a colossal amount of historical evidence on a mouse bioassay16 subsequently developed over time, with later development of cell lines, there is need to be cautious – research on hormone replacement therapy and oral contraceptives testify to potential problems. However, flavonoid benefits, probably mainly through oestrogen-like activity and antioxidant mechanisms for scavenging free radicals17, seem to have an impact on cardiovascular disease (myocardium, microvasculature. smooth muscle cell. cerebrum); metabolic disorders (type II diabetes, metabolic syndrome, obesity; cancer (breast. prostate, ovary, endometrium, cervix, Erhlich ascites tumour); brain and mental health (Alzheimer's disease, Parkinson's disease, multiple sclerosis, cognition, neurodegeneration), gastrointestinal issues and related cancer (mouth, stomach, oesophagus, colon-rectum, liver, pancreas, gut microflora); and other benefits effected possibly at key times of human development as adjudged from mainly rodent experimentation18.
Identification of candidate molecular structures
The flavonoids investigated comprised15, i.a., flavones (leafy spices: apigenin), flavonols (widespread in many foods: kaempferol, quercetin), flavanone (citrus fruits: hesperetin, naringenin), flavanols (grapes/red wine: catechins, epicatechins, and gallocatechins and gallates), and isoflavonoids (Leguminosae: genistein, diadzein, biochanin A, formononetin). Theaflavins and thearubigins19 and other polyphenolic compounds are also found in beverages. Historically, ‘oestrogen-like’ activity has been reported by several groups20,21,22,23,24.
Phytoestrogens in foods: composition
Generally, these data are scattered among several thousand publications and largely pertain to rodents and humans under a variety of experiment conditions but progress on integrating information is being made in several countries, e.g. Finland. Finnish workers25 have extended the Finnish National Food Composition Database (Fineli) for lignans: 180 foods for lignans and 160 foods for isoflavones, etc. The mean lignan intake was 434 μg/day and the isoflavone intake was 788 μg/day. The sources of lignans included seeds, cereals, fruit, berries and vegetables and for isoflavones were processed meat products/sausages containing soya as an ingredient, and legumes. However, values for most compounds are highly dispersed and exhibit wide ranges between and within subjects for particular dietary scenarios and any experimental designs on dietary comparisons should take cognisance of this fact.
Pitfalls and limitations of using cell and tissue culture
The choice of animal or cell line model is of fundamental importance if extrapolation to the human is to be made: for example, the similarities and contrasts in the anatomy, physiology and biochemistry of the human compared to guinea pig, rat and canine species in trying to model benign prostatic hyperplasia (BPH). A cell culture is an artificial system in the sense that cultures developed on hard flat surfaces, colonies, are hardly representative of the tissue milieu in three-dimension but when cells are grown in suspensions, then any intercellular signalling activity or physical or biochemical influences occurring in vivo must be of concern. Often candidate molecules, under test in ‘placebo/control’ situations, represent simply a small part in a very complex system in vivo where physiology, anatomy, and biochemistry and body clocks operate throughout the body with attendant regulatory mechanisms14. Dietary phytoestrogens studies must have a biochemical scientific audit trail from food, digestion, uptake into blood, tissue distribution, and excretion(s) and proper measurements and experimental design are needed to augment screening methods in culture.
Placental-foetal imprinting
It is now over 40 years since Herbst and Scully26 noticed an association of mothers treated with diethylstilboestrol for impending abortion during the first trimester and the occurrence of vaginal adenocarcinomas in their daughters. This occurrence was much earlier than usual and this observation was a landmark discovery of transplacental imprinting. The number of mothers taking stilboestrol in the 1960s on the increase26. The foetal site of biological origin was believed to be in the Müllerian ducts which develop into the fallopian tube, uterus and cervix and the upper two thirds of the vagina. The US Department of Agriculture banned the use of stilboestrol in animal feed for seven days prior to slaughter27. It would seem that other co-factors, other than toxicity, were operating in this transgenerational condition. It would be appropriate for those adolescent girls and women whose mothers were treated with stilboestrol to have vaginal examinations; those landmark cases with adenocarcinomas28 had a delayed diagnosis because vaginal bleeding was mistaken for anovulatory cycles. The molecular processes involved in gene imprinting are complex. Given the correlation of breast and prostate cancers, consideration of some common aetiology was sought, such as diet, having influence on the development of the Wolffian duct signalling risk of prostate cancer initiated and developed in future years. What is the trigger for such tumour development: is it related to mechanisms in adolescence or something more subtle? Perhaps brain-body interaction in the neonatal period of development, or epigenetic deregulation needs to be elucidated but discussion on embryonic mechanisms of Wolffian duct development29 is outside the scope of this paper.
Environment-driven changes in epigenetic regulation occurring in pre- and post- natal development suggest a link to predisposition to cardiovascular disease, diabetes, metabolic syndrome, obesity and mental conditions30. A well-known example is the effect of maternal diet on coat colour and obesity in Avy/a mice offspring31. Other environmental factors like tobacco smoke, alcohol, radiation and chemicals have been shown to influence disease susceptibility and can even induce transgenerational phenotypic effects such as drugs32 and nutrition33,34. One of the best known examples in humans is the effect of diethylstilbestrol, which was given to pregnant women in the sixties, to prevent miscarriages35. This chemical, however, leads to uterus malformations in the offspring, caused by epigenetic deregulation35,36,37. On the one hand, there are several types of oestrogen such as those used for birth control, hormone-replacement therapy and in feed supplements for livestock, etc. There are innumerable xenoestrogens in plastics, pesticides, herbicides, phthalates, and excretion products that find their way into watercourses, and although diluted may re-appear in recycled water. Nature produced salicylic acid from salicin [an extract from the inner bark of white willow (Salix alba)], which exists in plants for the biological defence of the plant but, subject to circadian timing and dose, also aids human well-being. There is now evidence to support the action of phytoestrogens in foetal development, particularly mice fed soy food after fertilization but before germ layer differentiation, where effects persist into adulthood, at least in the mouse38.
Maleness and femaleness
Effective transfer of phytoestrogens probably takes place from mother to foetus39 which may have some influence on the hierarchal mechanisms involved in the sexual differentiation in the brain. The early work of Jost et al40 demonstrated that male gonads were required for male phenotype with regression of Müllerian duct development by the anti-Müllerian hormone secreted from Sertoli cells. Foetal castration produced the female phenotype and unilateral castration produces an ipsilateral Müllerian duct. A similar type of unilateral action of the testis on the growth of the prostate was also discovered in experiments involving the epididymides, vas deferens and deferential vein41. Many experiments were conducted12 which demonstrated that androgens were needed for maleness.
Phytoestrogens in perinatal period
In rats there is a critical perinatal period in which the androgen is responsible for programming higher centres of the brain and soy food given to rats decreases Leydig cell secretion of androsterone and testosterone42. In rats, perinatal but not ‘postnatal’ isoflavone exposure gave improved bone mineral density43.
Phytoestrogens inhibit key enzymes (5α-reductase, 17β-hydroxysteroid dehydrogenase, tyrosine kinase) that are implicated in androgen/oestrogen metabolism and growth regulatory pathways, and in the latter context may inhibit angiogenesis. Sexually dimorphic nucleus of the preoptic area (SDN-POA) volumes in rats were significantly increased by phytoestrogens44, predominantly isoflavones, compared to control and in maze experiments produced a better performance in females and a poorer performance in males. It is possible that phytoestrogens may have an impact on the growth and protection of cholinergic neurones and it remains to be seen whether these are beneficial against neural damage such as Alzheimer's disease.
Intervention concepts-Phytoestrogens in adolescence and adulthood: lifetime risk of breast and prostate cancer
Female: Putting endocrine disruptors aside, e.g. pharmacological, biocidal and herbicidal products, plastics, etc., dietary phytoestrogens probably have an impact on the endocrine status of the individual. In the female, the key stages of life are foeto-placental development (epigenetics), perinatal environment, e.g. milk consumption and hormonal environment, puberty/menarche and adolescence and lifestyle, pregnancy, menopause and post-menopause. Although the oestrogen effects on breast cancer risk are well known (lower risk: later age at menarche, earlier age at first pregnancy, early menopause, etc.), these do not adequately account for the geographical/ethnic differences which undoubtedly involve diet and lifestyle, particularly soy foods in Asiatic populations. For example, a comparison between British and Thai postmenarchal girls45 show that the British girls, who were generally larger and not so lean appear to develop their ovulatory cycles earlier; and leaner athletes have lower breast cancer risk. A vegetarian lifestyle of white American girls was also associated with a later age at menarche46. Although not conclusive, phytoestrogens and a Palæolithic diet may be the reason, because the oestrogen-adsorptive faecal fibre may lead to a fall in plasma oestrogens and this has an impact on the hypothalamus and development of the luteinizing hormone-releasing hormone (LHRH) generator. It would seem that isoflavones do have other possible biological functions. An experiment by Mardon et al43 provides evidence that phytoestrogen exposure may lead to a higher bone mineral density (BMD) later in life. In an earlier study a positive correlation was found between prostatic cancer and female breast cancer incidence in 24 countries suggesting a common aetiology47 and dietary oestrogens have since been strongly implicated as a major factor13. So the underlying thrust of this paperbuilds on the questions posed by Wynder et al47, viz. ‘Is there commonality in breast and prostate cancer’and ‘Could dietary phytoestrogens and related compounds have a role in ameliorating oestrogenic effects to reduce cancer? Fig. 2 shows windows of risk/exposure for the prostate and it may well be that, in principle, the model for female oestrogen risk for breast caner is not too dissimilar.
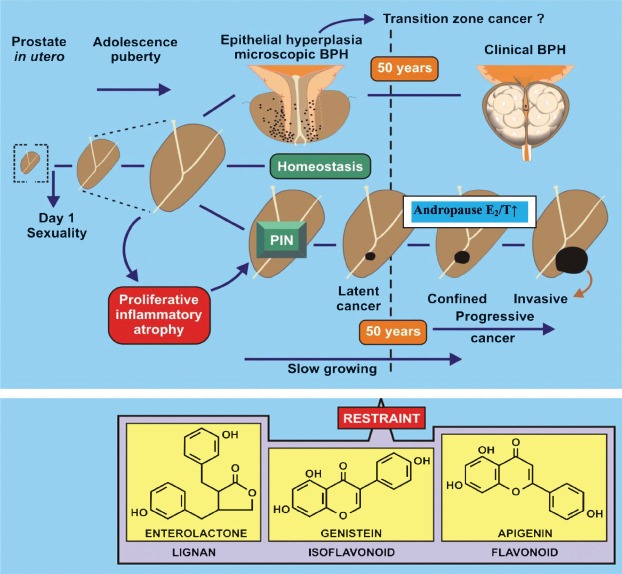
Fig. 2.
Hypothetical model of stages in the development of benign prostatic hyperplasia (BPH) and cancer. The influence of phytoestrogens on sexual dimorphism, and later in life, the increasing oestrogen status in the male may be redressed by phytoestrogens at mid-life and hence reduces the incidence of clinical BPH and cancer. Prostatic intraepithelial neoplasia (PIN), a possible premalignant lesion may lead to cancer unless restrained by phytoestrogens. E2/T ↑ refers to the relative rise in oestradiol/testosterone ratio of the ageing male.
Male: Fig. 2 shows a schema for the possible development of malignant and benign prostatic tumours. The importance of in utero and perinatal timing has been discussed, vide supra, and it is the period around puberty and early sexual activity that an imbalance in growth regulation might occur48. Contrary to perhaps uninformed expectation, oestrogen imbalance over androgens has been inferred/stated in relation to the development of benign enlargement of the prostate48,49,50. As (BPH) and cancer occur in the older man, it is possible that it is the increasing oestrogen status in the male which influences the regulatory growth of the prostate. This suggests that androgen replacement therapy or phytoestrogens, acting through receptor or signalling mechanisms, can redress the androgen/oestrogen imbalance at mid-life and reduce the incidence of clinical BPH and cancer.
Gastrointestinal disease: Gastrointestinal activities play a crucial role in phytoestrogen metabolism. Black tea has a prokinetic effect which may have some importance in health: also some dietary phytoestrogens are believed to have antioxidant, antiviral, anti-inflammatory, antibacterial, and anticarcinogenic properties. One encouraging role for green tea polyphenols and some spices in inflammatory diseases such as atherosclerosis, myocardial infarction, diabetes, arthritis, Crohn's disease, etc. is the interruption/modulation of the activation of nuclear transcription factor-kappaB51. There is growing evidence to suggest “reasoning for seasoning”. Though it has to be said not all responses are related to phytoestrogens but certainly more research is needed in this promising field.
In conclusion, epidemiological evidence suggests that differences in diet are responsible for much of the global differences in non communicable diseases (NCDs) observed; and phytoestrogens appear to have some commonality with the Palæolithic or Mediterranean diet. The literature abounds with inference about the potential benefits of phytoestrogen from the work of Doll and Peto52 some years ago which suggested 35 per cent of cancer deaths, range 10-70 per cent, could be attributed to diet. The celebrated Japanese epidemiologist, Takeshi Hirayama53, provided evidence that intake of green-yellow vegetable (GYV) soup, often with soybean soup, presumably rich in phytoestrogens, was associated with a reduction in mortality from breast and prostate cancer. Later studies by Adlercreutz (and his group)54, demonstrated, i.a., that daidzein and genistein were higher in Japanese and Chinese, who are large consumers of soy products. It is pertinent to point out that a number of epidemiological studies have not demonstrated an association between prostatic cancer risk and phytoestrogens55, but given the multifarious aspects of such dietary investigation it is of crucial importance to have good experiment design such as the ‘setting’, the sampling framework, the instruments used, the analytical techniques, national food-phytoestrogen databases, and contributions from endocrinologists, nutritionists, and those from the food production and manufacturing industry of which tea production is a good example. New phytochemicals are being found almost every day from wild Yichun blue honeysuckle (Lonicera caerulea L.) berries containing high level of anthocyanins which has strong antioxidant activity to quercetin inhibition of alpha-glucosidases of importance in glucose control in type 2 diabetes56. It is possible, given proper research evaluations and outcomes, that safe functional food products could be manufactured based on epidemiological information.
Acknowledgment
The authors thank the Wolfson Research Institute for Health and Wellbeing, Durham University; and the Cheminformatics Team, ChemSpider, (http://www.chemspider.com), Royal Society of Chemistry Burlington House, Piccadilly, London; for support in various studies, and David Griffiths, Castleton, Gwent, UK, for his graphics skills with respect to Figure 5.
References
- 1.Mendel JG. Experiments on plant hybrids. Debates of the Nature-searching association in Brno. Abhandlungen. 1865;4:3–47. [Google Scholar]
- 2.McClintock The order of the genes C, Sh and Wx in Zea mays with reference to a cytologically known point in the chromosome. Proc Natl Acad Sci USA. 1931;17:485–91. doi: 10.1073/pnas.17.8.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan TH, Sturtevant AH, Muller HJ, Bridges CB. New York: Henry Holt; 1915. The mechanism of mendelian heredity. [Google Scholar]
- 4.McClintock B. Carnegie institution of Washington yearbook. Vol. 50. Washington DC, USA: Carnegie Institution for Science; 1951. Mutable loci in maize; pp. 174–81. [Google Scholar]
- 5.Watson JD, Crick FH. Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature. 1953;171:737–8. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Diet and physical activity: a public health priority. [accessed on December 19, 2014]. Available from: http:/www.who. int/diet physical activity/en .
- 7.Geneva: WHO; 2011. World Health Organization (WHO). Global status report of NCD 2010. [Google Scholar]
- 8.Alam SE, Singh RB, Gupta S, Dherange P, De Meester F, Wilczynska A, et al. Nutritional aspects of epigenetic inheritance. Can J Physiol Pharmacol. 2012;90:989–4. doi: 10.1139/y2012-105. [DOI] [PubMed] [Google Scholar]
- 9.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–8. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 10.Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci USA. 1964;51:786–94. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelissen ECM, van Montfoort APA, Dumoulin JCM, Evers JLH. Epigenetics and the placenta. Hum Reprod Update. 2011;17:397–17. doi: 10.1093/humupd/dmq052. [DOI] [PubMed] [Google Scholar]
- 12.Ishida M, Moore GE. The role of imprinted genes in humans. Mol Aspects Med. 2013;34:826–40. doi: 10.1016/j.mam.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths K, Denis LJ, Turkes A. London: Martin Dunitz; 2002. Oestrogens, phytoestrogens and pathogenesis of prostatic disease; pp. 1–420. [Google Scholar]
- 14.Jargin SV, Wilson DW. Some pitfalls in testing anti-atherogenic agents in cell cultures. World Heart J. 2012;6:149–55. [Google Scholar]
- 15.Griffiths K, Adlercretz H, Boyle P, Denis L, Nicholson RI, Morton MS. Oxford: ISIS Medical Media Ltd; 1996. Nutrition and cancer. [Google Scholar]
- 16.Allen E, Doisy EA. An ovarian hormone; preliminary report on its localization, extraction and partial purification, and action in test animals. J Am Med Assoc. 1923;81:819–21. doi: 10.1001/jama.250.19.2681. [DOI] [PubMed] [Google Scholar]
- 17.Olajuyigbe OO, Afolayan AJ. Phenolic content and antioxidant property of the bark extracts of Ziziphus mucronata Willd. subsp. mucronata Willd. [accessed on January 12, 2015];BMC Complementary and Alternative Medicine. 2011 11:130. doi: 10.1186/1472-6882-11-130. Available from: http://www.biomedcentral.com/1472-6882/11/130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–8. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts EAH. Economic importance of flavonoid substances; tea fermentation. In: Geissman TA, editor. The chemistry of flavonoid compounds. New York: MacMillan Co; 1962. pp. 468–512. [Google Scholar]
- 20.Kerr AJ. The reputed rejuvenator. Siam Soc Natl Hist. 1932;8:336–8. [Google Scholar]
- 21.Schoeller W, Dohrn M, Hohlweg W. About an estrogenic substance : on the tuber of Thai creeper Butea superb. Natural Sci. 1940;28:532–3. [Google Scholar]
- 22.Bennetts HW, Underwood EJ, Sheir FI. A specific breeding problem of sheep on subterranean clover pastures in western Australia. Aust Vet J. 1946;22:2–12. doi: 10.1111/j.1751-0813.1946.tb15473.x. [DOI] [PubMed] [Google Scholar]
- 23.Pope GS, Elcoate PV, Simpson SA, Andrew DG. Isolation of an oestrogenic isoflavone (Biochanin A) from red clover. Chem Ind (Lond) 1953:1092. [Google Scholar]
- 24.Bradbury RB, White DC. Oestrogens and related substances in plants. Vitam Horm. 1954;12:207–33. doi: 10.1016/s0083-6729(08)61013-4. [DOI] [PubMed] [Google Scholar]
- 25.Valsta M, Kilkkinen A, Mazur W, Nurmi T, Lampi AM, Ovaskainen ML, et al. Phyto-oestrogen database of foods and average intake in Finland. Br J Nutr. 2003;89(Suppl 1):S31–8. doi: 10.1079/BJN2002794. [DOI] [PubMed] [Google Scholar]
- 26.Herbst AL, Scully RE. Adenocarcinoma of the vagina in adolescence. A report of 7 cases including 6 clear-cell carcinomas (so-called mesonephromas) Cancer. 1970;25:745–57. doi: 10.1002/1097-0142(197004)25:4<745::aid-cncr2820250402>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration. Update on Bisphenol A for use in food contact applications: 15 January. 2010. [accessed on August 31, 2012]. Available from: http://www.fda.gov/NewsEvents/PublicHealthFocus/ucm197739.htm .
- 28.Herbst AL, Anderson S, Hubby MM, Haenszel WM, Kaufman RH, Noller KL. Risk factors for the development of diethylstilbestrol-associated clear cell adenocarcinoma: a case-control study. Am J Obstet Gynecol. 1986;154:814–22. doi: 10.1016/0002-9378(86)90464-3. [DOI] [PubMed] [Google Scholar]
- 29.Hannema SE, Hughes IA. Regulation of Wolffian duct development. Horm Res. 2007;67:142–51. doi: 10.1159/000096644. [DOI] [PubMed] [Google Scholar]
- 30.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–62. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh SM, Murphy B, O’Reilly RL. Involvement of gene-diet/drug interaction in DNA methylation and its contribution to complex diseases: from cancer to schizophrenia. Clin Genet. 2003;64:451–60. doi: 10.1046/j.1399-0004.2003.00190.x. [DOI] [PubMed] [Google Scholar]
- 33.Feil R. Environmental and nutritional effects on the epigenetic regulation of genes. Mutat Res. 2006;600:46–57. doi: 10.1016/j.mrfmmm.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 34.Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Opin Pediatr. 2009;21:243–51. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, Hursting SD, Davis BJ, McLaclan JA, Barrett JC. Environmental exposure. DNA, methylation, and gene regulation: lessons from diethylstilbestrol induced cancers. Ann N Y Acad Sci. 2003;983:161–9. doi: 10.1111/j.1749-6632.2003.tb05971.x. [DOI] [PubMed] [Google Scholar]
- 36.Bromer JG, Wu J, Zhou Y, Taylor HS. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altering developmental programming. Endocrinology. 2009;150:3376–82. doi: 10.1210/en.2009-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato K, Fukata H, Kogo Y, Ohgane J, Shiota K, Mori C. Neonatal exposure to diethylstilbestrol alters expression of DNA in the mouse uterus. Endocr J. 2009;56:131–9. doi: 10.1507/endocrj.k08e-239. [DOI] [PubMed] [Google Scholar]
- 38.Sato N, Yamakawa N, Masuda M, Sudo K, Hatada I, Muramatsu M. Genome-wide DNA methylation analysis reveals phytoestrogen modification of promoter methylation patterns during embryonic stem cell differentiation. PLoS One. 2011;6:e19278. doi: 10.1371/journal.pone.0019278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Todaka E, Sakurai K, Fukata H, Miyagawa H, Uzuki M, Omori M, et al. Fetal exposure to phytoestrogens - the difference in phytoestrogen status between mother and fetus. Environ Res. 2005;99:195–203. doi: 10.1016/j.envres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Jost A, Vigier B, Prepin J, Perchellet J. Studies on sex differentiation in mammals. Rec Prog Horm Res. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- 41.Pierrepoint CG, Davies P, Wilson DW. The role of the epididymis and ductus deferens in the direct and unilateral control of the prostate and seminal vesicles of the rat. J Reprod Fertil. 1974;41:413–23. doi: 10.1530/jrf.0.0410413. [DOI] [PubMed] [Google Scholar]
- 42.Akingbemi BT, Braden TD, Kemppainen BW, Hancock KD, Sherrill JD, Cook SJ, et al. Exposure to phytoestrogens in the perinatal period affects androgen secretion by testicular Leydig cells in the adult rat. Endocrinology. 2007;148:4475–88. doi: 10.1210/en.2007-0327. [DOI] [PubMed] [Google Scholar]
- 43.Mardon J, Mathey J, Kati-Coulibaly S, Puel C, Davicco M-J, Lebecque P, et al. Influence of lifelong soy isoflavones consumption on bone mass in the rat. Exp Biol Med. 2008;233:229–37. doi: 10.3181/0707-RM-202. [DOI] [PubMed] [Google Scholar]
- 44.Lephart ED, West TW, Weber KS, Rhees RW, Setchell KDR, Adlercreutz H, et al. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 45.Wilson DW, Turkes A, Jones R, Danutra V, Read GF, Griffiths K. A comparison of menstrual cycle profiles of salivary progesterone in British and Thai adolescent girls. Eur J Cancer. 1992;28:1162–7. doi: 10.1016/0959-8049(92)90478-k. [DOI] [PubMed] [Google Scholar]
- 46.Kissinger DG, Sanchez A. The association of dietary factors with age at menarche. Nutr Res. 1987;7:471–9. [Google Scholar]
- 47.Wynder EL, Mabuchi K, Whitmore WF. Epidemiology of cancer of the prostate. Cancer. 1971;28:344–60. doi: 10.1002/1097-0142(197108)28:2<344::aid-cncr2820280214>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 48.Sakr WA, Grignon DJ, Haas GP, Heilbrun LK, Pontes JE, Crissman JD. Age and racial distribution of prostatic intraepithelial neoplasia. Eur Urol. 1996;30:138–44. doi: 10.1159/000474163. [DOI] [PubMed] [Google Scholar]
- 49.De Jong SE. The influence of sex hormones on the prostate and its surroundings in the mouse. Acta Brev Neerl Physiol Pharmacol Microbiol. 1935;5:28–32. [Google Scholar]
- 50.Murphy G, Denis LJ, Chatelain C, Griffiths K, Khoury S, Cockett ATK, editors. Paris: SCI; 1997. First International consultation on prostate cancer; pp. 1–375. [Google Scholar]
- 51.Aggarwal BB, Shishodia S. Suppression of the nuclear factor-kappa B activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann NY Acad Sci. 2004;1030:434–41. doi: 10.1196/annals.1329.054. [DOI] [PubMed] [Google Scholar]
- 52.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer. J Nat Cancer Inst. 1981;66:1191–308. [PubMed] [Google Scholar]
- 53.Hirayama T. A large scale cohort study on cancer risks by diet - with special reference to the risk reducing effects of green-yellow vegetable consumption. In: Hayashi Y, Nagano M, Sugimura T, editors. Diet, nutrition and cancer. Tokyo: Japanese Scientific Society Press,; Utrecht: VNU Scientific Press; 1986. pp. 41–53. [PubMed] [Google Scholar]
- 54.Adlercreutz H. The epidemiology of phytoestrogens. In: Adlercreutz H, editor. Bailliere's clinical endocrinology and metabolism. Vol. 12. London: Bailliere and Tindall; 1998. pp. 605–23. [DOI] [PubMed] [Google Scholar]
- 55.Ward HA, Gunter GCK, Mulligan AA, Lentjes MAH, Luben RN, Khaw K-T. Breast, colorectal, and prostate cancer risk in the European prospective investigation into cancer and nutrition – Norfolk in relation to phytoestrogen intake derived from an improved database. Am J Clin Nutr. 2010;91:440–8. doi: 10.3945/ajcn.2009.28282. [DOI] [PubMed] [Google Scholar]
- 56.Jo S-H, Ka E-H, Lee H-S, Apostolidis E, Jang H-D, Kwon Y-I. Comparison of antioxidant potential and rat intestinal α-glucosidases inhibitory activities of quercetin, rutin, and isoquercetin. Int J Appl Res Nat Prod. 2009;2:52–60. [Google Scholar]