Abstract
Choroidal neovascularisation (CNV) is a common vision-threatening complication of myopia and pathological myopia. Despite significant advances in understanding the epidemiology, pathogenesis and natural history of myopic CNV, there is no standard definition of myopic CNV and its relationship to axial length and other myopic degenerative changes. Several treatments are available to ophthalmologists, but with the advent of new therapies there is a need for further consensus and clinical management recommendations. Verteporfin photodynamic therapy has been an established treatment for subfoveal myopic CNV for many years, but this treatment does not restore visual acuity and is associated with long-term chorioretinal atrophy. More recently, clinical trials investigating the efficacy and safety of anti-vascular endothelial growth factor agents in patients with myopic CNV have demonstrated substantial visual acuity gains and quality of life increases compared with photodynamic therapy. These enhanced outcomes provide updated evidence-based clinical management guidelines of myopic CNV, and increase the need for a generally accepted definition for myopic CNV. This review critically summarises the latest myopic CNV literature in the context of clinical experience and recommends a myopic CNV treatment algorithm.
Keywords: Neovascularisation, Treatment Medical, Retina
Introduction
Myopic choroidal neovascularisation (CNV) is a common vision-threatening complication of myopia and pathological myopia (PM).1–4 The clinical definition and terminology surrounding myopic CNV varies, with myopic CNV also commonly being referred to as subretinal neovascularisation in PM, Fuchs’ spot or Forster–Fuchs’ retinal spot in PM, and disciform degeneration in PM. While myopic CNV is historically thought to only occur in eyes with PM, it is now recognised that myopic CNV can occur at any degree of myopia and in eyes without typical myopic degenerative fundus changes.5 6 Therefore, in clinical practice, CNV can be attributed to be ‘myopic’ in aetiology by the refractive status of the eye and the exclusion of other disorders associated with CNV.
There are now effective therapeutic options for myopic CNV, in particular anti-vascular endothelial growth factor (VEGF) therapy. This review summarises current concepts in pathogenesis, epidemiology, natural history, and management options for myopic CNV.
Pathogenesis of myopic CNV
Several theories have been proposed to explain the development of myopic CNV, reviewed in detail elsewhere.4 The mechanical theory is based on the assumption that the progressive and excessive elongation of the anteroposterior axis causes a mechanical stress on the retina, leading to an imbalance between pro-angiogenic and anti-angiogenic factors, resulting in myopic CNV.7 In support, the presence of lacquer cracks has been shown to be a predisposing factor for the development of myopic CNV.8 9
The heredodegenerative theory states that myopic refractive errors are genetically predetermined.4 5 10 In support, studies have shown that single nucleotide polymorphisms in several genes (eg, pigment epithelium-derived factor) are associated with the development and progression of myopic CNV.5 11 12
The haemodynamic theory for the development of myopic CNV relates to perfusion changes in the choroidal circulation of the myopic eye, such as choroidal filling delay and diffuse thinning of the choroid.4 13 However, evidence has shown that myopic CNV can develop in eyes with shallow staphyloma and preserved choroidal circulation, suggesting that haemodynamic factors may not have a strong role in the development of myopic CNV.14
Diagnosis of myopic CNV
Myopic CNV is typically seen as a small, flat, greyish membrane on slit-lamp biomicroscopy that may have a hyper-pigmented border if chronic or recurrent.1–4 Symptoms of myopic CNV include a decrease in vision, central scotoma and/or metamorphopsia.15 16
The standard tests for diagnosing myopic CNV are fundus biomicroscopy, fluorescein angiography (FA) and optical coherence tomography (OCT). FA and OCT are generally recommended baseline diagnostic tests for myopic CNV in conjunction with colour photos and clinical examination. FA demonstrates the presence, type, area and activity of myopic CNV, and helps exclude other disorders.4 17 The majority of myopic CNV presents as a ‘classic’ pattern on FA,18 with well defined hyperfluorescence in the early phases and leakage of fluorescein dye during the late phases.4 15 17 OCT is usually mandatory for the identification of the fovea, assessment of retinal thickness and presence of extracellular fluid, and for establishing a baseline to judge future treatment response.15 On OCT, myopic CNV presents as a highly reflective area contiguous above the retinal pigment epithelium (sometimes referred to as ‘type 2 CNV’) with minimal subretinal fluid.4 Fundus autofluorescence, which allows the visualisation of accumulated lipofuscin within the retinal pigment epithelium, may be included as part of any basic diagnosis and follow-up examination, as it may aid in the assessment of myopic CNV progression (and associated geographic atrophy).4
There are several differential diagnoses and pathologies that must be excluded from myopic CNV when examining a patient with myopia and vision loss (table 1). Other complications of PM should be identified with OCT/FA, such as myopic traction maculopathy, epiretinal membrane, vitreomacular traction and myopic full-thickness or lamellar macular hole, as these require different treatments from myopic CNV. In particular, retinal haemorrhage due to new lacquer crack formation and macular exudative changes associated with a dome-shaped macula or a staphyloma should be identified and excluded on OCT/FA (figure 1).4 In case of significant haemorrhage, indocyanine green angiography (ICGA) can identify the presence of lacquer cracks and/or CNV. It should be noted that OCT alone cannot differentiate myopic CNV from subretinal bleeding due to new lacquer crack formation, which could lead to unnecessary treatment by anti-VEGF therapy for subretinal bleeding without CNV. In addition, myopic CNV should be differentiated from other causes of CNV (eg, multifocal choroiditis or punctate inner choroidopathy or age-related macular degeneration (AMD)).19–21 Importantly, myopic CNV has different lesion characteristics to AMD-CNV, especially in younger individuals,4 22 but is a predominantly ‘classic’, ‘type 2’ CNV; that is, smaller than that of AMD, with minimal subretinal fluid and an absence of drusen at the typical age of onset.4
Table 1.
Coexisting pathologies and differential diagnosis for myopic CNV
| Other co-existing degenerative changes associated with myopia | Differential diagnosis for CNV |
|---|---|
| Myopic traction maculopathy (foveoschisis) | Neovascular AMD |
| Macular hole | Myopic macular haemorrhage due to lacquer cracks |
| Retinal tear/detachment | Punctate inner choroidopathy (usually coexists with myopia) |
| Dome-shaped macula | Multifocal choroiditis |
| Staphyloma | Idiopathic CNV* |
| Atrophic changes (patchy atrophy, tesselated changes and diffuse atrophy) |
*Idiopathic CNV in a myope is myopic CNV.
AMD, age-related macular degeneration; CNV, choroidal neovascularisation.
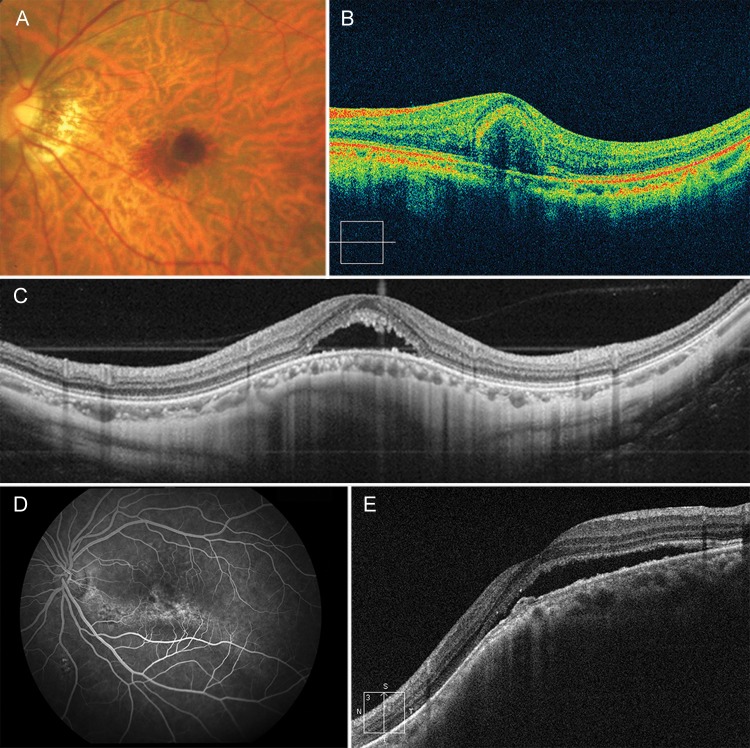
Figure 1.
Differential diagnosis for myopic choroidal neovascularisation (CNV): (A and B) haemorrhage due to lacquer cracks; (C) dome-shaped macula with serous retinal detachment; and (D and E) macular fluid due to staphyloma.
Epidemiology of myopic CNV
A recent systematic review has indicated that the prevalence of PM is 1–3% in adults, and that 5–11% of patients with PM develop CNV.23 While these data provide some insight into the epidemiology of myopic CNV, the results should be interpreted with caution, since the definitions of myopia, PM and myopic CNV between studies were not uniform.23 Furthermore, the data were based on only a few published studies, indicating the need for further incidence and prevalence studies in different populations.
Natural history of myopic CNV
Several of the phenotypic features of PM are associated with increased risk of myopic CNV—these include lacquer cracks,8 patchy atrophy,8 thinning of the choriocapillaris and choroid,13 and CNV in the fellow eye.6 In a retrospective study of 73 patients with PM, 17 (23%) presented with bilateral myopic CNV.24 Furthermore, one study has shown that after initial presentation of myopic CNV, CNV develops in the fellow eye in 35% of patients within 8 years.8 There appears to be three main stages of myopic CNV, all of which are associated with vision loss (figure 2).4 The initial phase results in direct damage to photoreceptors, causing central visual loss.3 25 26 Then, as the CNV regresses,26 a fibrous pigmented scar forms, sometimes referred to as Fuchs’ spot or Forster–Fuchs’ retinal spot. Finally, atrophy forms around the regressed CNV, which is a major late complication of myopic CNV and a key contributor to the poor long-term visual outcomes associated with the condition.4 25 26 It is important to note that CNV can occur in eyes with no other evident degenerative changes or isolated tesselated fundus.1 27 28
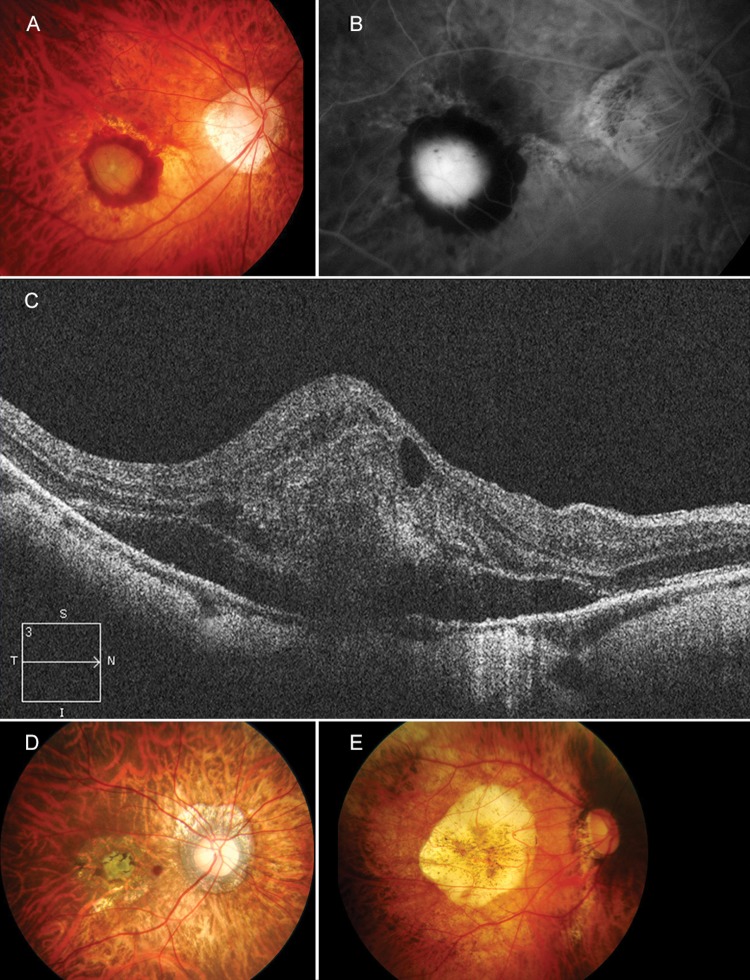
Figure 2.
Active myopic choroidal neovascularisation (CNV) imaged via (A) colour fundus photography; (B) fluorescein angiography; and (C) optical coherence tomography. (D) A fibrous pigmented scar (Fuchs’ spot). (E) Chorioretinal atrophy following regression of active myopic CNV.
Factors generally associated with poor visual prognosis include subfoveal (rather than juxtafoveal or extrafoveal) location (determined via OCT),3 29 age >40 years,27 28 size of the CNV lesion (>400 µm)3 and lower baseline best-corrected visual acuity (BCVA).27 29 Long-term studies show almost all patients lose significant vision.3 26–31 In a 10-year follow-up of 25 patients with myopic CNV, visual acuity was <20/200 in 89% and 96% of patients 5 and 10 years after onset of CNV, respectively.26
Treatment options for myopic CNV
Before the introduction of intravitreal anti-VEGF therapy for CNV, laser photocoagulation, verteporfin photodynamic therapy (vPDT) and surgical excision or macular translocation were performed to treat CNV. These were recently reviewed elsewhere4; the most common treatments are summarised below.
Laser photocoagulation
Laser photocoagulation was used widely to treat extrafoveal myopic CNV,32–34 although evidence supporting its use is limited.4 Laser causes retinal tissue damage with laser scar expansion or atrophy, does not maintain long-term visual acuity and is associated with a high rate of recurrence.33–35
Verteporfin photodynamic therapy
vPDT is an established, approved treatment for subfoveal myopic CNV. In the Verteporfin Photodynamic Therapy (VIP trial), while vPDT was well tolerated and more efficacious than placebo over 12 months of treatment, vPDT generally stabilised but did not improve visual acuity.18 These findings are supported by 12 short-term (<12 months)36–47 and 6 long-term (≥36 months)40 45 47–50 studies. However, a 2-year follow-up of the VIP trial showed no statistically significant benefit in visual outcome with vPDT compared with placebo, although the trial may be underpowered to detect differences.51
The most important limitation of vPDT is long-term chorioretinal atrophy which may develop in some patients, contributing to vision loss.40 49 However, since atrophy is a prominent feature of myopic CNV, further studies are required to determine whether this atrophy is accelerated by vPDT or is part of the disease's natural history.8
Anti-VEGF therapy
Ranibizumab
Currently, ranibizumab (Lucentis) is the only licensed anti-VEGF therapy for treatment of myopic CNV. Its use is supported by data from phase II (REPAIR) and phase III trials (RADIANCE).52–54 In addition, the 12-month efficacy and safety of ranibizumab for myopic CNV have been demonstrated in several small prospective and retrospective studies with lower levels of evidence55 (table 2).56–63 Further small studies have demonstrated that BCVA gains are maintained up to 36 months after initiation of treatment.56–58 61
Table 2.
Mean change in BCVA after 12 months’ treatment with anti-VEGF therapy
| Drug | Study | Design | Total sample size (patients) | Patients receiving anti-VEGF treatment | Mean change in BCVA (ETDRS letters) | Injection number over 12 months (mean) | OCEBM level of evidence55 |
|---|---|---|---|---|---|---|---|
| Ranibizumab | RADIANCE52 | Phase III, randomised, double masked, active controlled, multicentre | 277 | 106* | 13.8 | 4.6 | 2 |
| 116† | 14.4 | 3.5 | |||||
| REPAIR53 | Phase II, prospective, open label, multicentre | 65 | 65 | 13.8 | 3.6 | 4 | |
| Franqueira et al 201257 | Retrospective case series | 39 | 39 | 4.3 | 4.1 | 4 | |
| Monés et al 200959 | Prospective case series | 23 | 23 | 9.5 | 1.5 | 4 | |
| Silva et al 201060 | Prospective case series, multicentre | 32 | 32 | 8 | 3.6 | 4 | |
| Lai et al 200962 | Retrospective case series | 16 | 16 | 15‡ | 3.8 | 4 | |
| Bevacizumab | Ikuno et al 200964 | Retrospective case series | 63 | 63 | 11.5‡ | 2.4 | 4 |
| Chan et al 200965 | Prospective case series | 29 | 29 | 12‡ | 3.6 | 4 | |
| Gharbiya et al 200966 | Prospective case series | 20 | 20 | 18.2 | 4.0 | 4 | |
| Ruiz-Moreno et al 201167 | Prospective, comparative, non-randomised multicentre | 38 | 18§ | 6.3 | 3.2 | 4 | |
| 20¶ | 7.2 | 1.7 | |||||
| Ruiz-Moreno et al 201068 | Retrospective case series, multicentre | 107 | 107 | 8.7 | 1†† | 4 | |
| Ruiz-Moreno et al 2011, 201369 70 | Prospective, randomised, multicentre | 55 | 25 | 11.2 | 3.5 | 3 | |
| Iacono et al 201171 | Prospective case series | 30 | 30 | 3.8 | 4.7 | 4 | |
| Gharbiya et al 201272 | Prospective case series | 30 | 30 | 16.4 | 4.1 | 4 | |
| Hayashi et al 201273 | Prospective case series | 69 | 69 | 10.5‡ | NR‡‡ | 4 | |
| Hayashi et al 200974 | Prospective case series | 156 | 43 | 11.5‡ | 1.6 | 4 |
OCEBM levels of evidence grades are as follows: 1: systematic review of randomised trials; 2: randomised trial; 3: non-randomised controlled cohort/follow-up study; 4: case series, case control, or historical controlled study; 5: mechanism-based reasoning.
*Retreatment according to visual acuity stabilisation criteria.
†Retreatment according to disease activity criteria.
‡Approximate ETDRS changes, based on reported logMAR values.
§Patients received three monthly loading doses.
¶Patients received one loading dose.
††For 60% of patients.
‡‡1.8 injections over 2 years.
BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; logMAR, logarithm of the minimum angle of resolution; OCEBM, Oxford Centre for Evidence-Based Medicine; NR, not reported; VEGF, vascular endothelial growth factor.
The 12-month, randomised RADIANCE trial (N=277) assessed the efficacy and safety of ranibizumab, administered under two different pro re nata (PRN) schedules for myopic CNV compared with vPDT.52 Patients receiving PRN ranibizumab were treated according to two criteria: visual acuity stabilisation criteria (no treatment if no change in BCVA compared with two preceding monthly visits) or disease activity criteria (treatment if there is vision impairment attributable to intraretinal or subretinal fluid, or active leakage secondary to PM as assessed by OCT and/or FA).
RADIANCE showed that both PRN regimens of ranibizumab induced significantly greater gains in BCVA than vPDT (10.5 (visual acuity stabilisation criteria) and 10.6 (disease activity criteria) vs 2.2 letter change (vPDT)) at month 3.52 By month 12, the mean changes in BCVA were 13.8 (visual acuity stabilisation criteria) and 14.4 (disease activity criteria) letters for the two ranibizumab groups (with a median of 4.0 and 2.0 injections, respectively), compared with 9.3 letters for patients receiving vPDT who could be switched to ranibizumab from month 3 onwards (with a median of 2.0 injections between months 3 and 12). This indicates that patients who previously received vPDT could still gain vision when switched to ranibizumab.52 The results also suggested that either early treatment of myopic CNV with ranibizumab is important in preventing irreversible retinal damage, or that initial treatment with vPDT may have induced retinal damage, since patients in the vPDT arm switched to ranibizumab did not achieve the same visual gains as those treated initially with ranibizumab. Anatomical outcome improvements were also observed with ranibizumab and vPDT; the proportion of patients with CNV leakage and intraretinal oedema decreased substantially in all groups during the study.52
RADIANCE also revealed significant improvements in several quality of life parameters (ie, Visual Functioning Questionnaire 25 composite, general vision, mental health and dependency subscale scores) for patients treated with ranibizumab compared with vPDT, which were maintained through to 12 months (K Ohno-Matsui et al, ARVO Annual Meeting, Seattle, USA, 2013).
RADIANCE confirmed the results of the REPAIR trial (N=65), in which patients received one injection of ranibizumab followed by monthly monitoring and a PRN treatment regimen based on disease activity.53 In REPAIR, after 12 months of treatment, there was a mean BCVA change from baseline of 13.8 letters after receiving a median of 3.0 injections.53 Data from RADIANCE and REPAIR indicate that the safety profile of ranibizumab for treatment of myopic CNV is similar to that for AMD-CNV, retinal vein occlusion and diabetic macular oedema,52 53 with no new safety signals identified. Importantly, there were no retinal detachments, which is a concern in eyes with high myopia.52 53
Bevacizumab
Bevacizumab (Avastin) is not approved for intraocular use, and evidence on its safety and efficacy profile is limited.75 76 Increasing concerns regarding its safety have been raised, particularly with regards to the increased risk of cardiovascular events (eg, stroke) compared with ranibizumab,77 78 and the potential risk of infection after repackaging the drug for intravitreal use.78 79 Despite this, bevacizumab is used by many ophthalmologists, and several retrospective and prospective studies have shown increases in visual acuity of between 4 and 18 letters after 12 months (table 2).64–74 80–82 However, these studies are small and provide lower levels of evidence. Additionally, comparisons between trials should be made with caution, due to differences in study designs, patient populations etc.
Recent data have shown that the initial gains in visual acuity may not be maintained up to 5 years after treatment, and that this is associated with retinal thinning (V Sarao et al, ARVO Annual Meeting, Seattle, USA, 2013). This could indicate the development of chorioretinal atrophy, and like vPDT, it is not yet known whether this is related to treatment. However, a 4-year follow-up of 92 patients treated with bevacizumab (n=68) or ranibizumab (n=24) has shown good long-term outcomes, with changes in visual acuity of 9.4 letters at 12 months and 7.0 letters at 48 months (mean of 4.9 injections).83 Variability in treatment responses with anti-VEGF therapies in eyes with myopic CNV has been attributed to the size of the CNV lesion at baseline and the presence of single nucleotide polymorphisms in the VEGF gene.84 85 Further studies with larger numbers of patients are required to determine long-term outcomes with anti-VEGF therapies and prognostic factors for treatment responses.
As there have been no large prospective, randomised clinical trials with bevacizumab in myopic CNV, the optimal dosing frequency has not been established. In two studies directly comparing bevacizumab with ranibizumab in patients with myopic CNV, there were similar improvements in BCVA,86 87 but the number of bevacizumab injections required was significantly higher in one study (4.7 vs 2.6, p=0.0004).87 This may indicate an increased treatment burden with bevacizumab, but further studies are required.
Aflibercept
The efficacy and safety of aflibercept (Eylea) for myopic CNV was evaluated in the ongoing phase III, multicentre, randomised, sham-controlled, 12-month MYRROR study in Asian patients (N=121; NCT01249664).88 Patients received aflibercept according to a PRN schedule based on visual and anatomical criteria.89 Interim 6-month results reported a 12.1-letter improvement in BCVA compared with a 2-letter loss in those receiving sham injection,89 and recent reports indicate sustained BCVA gains up to 12 months (K Ohno-Matsui et al, AAO Annual Meeting, New Orleans, USA, 2013).
Treatment recommendations for myopic CNV
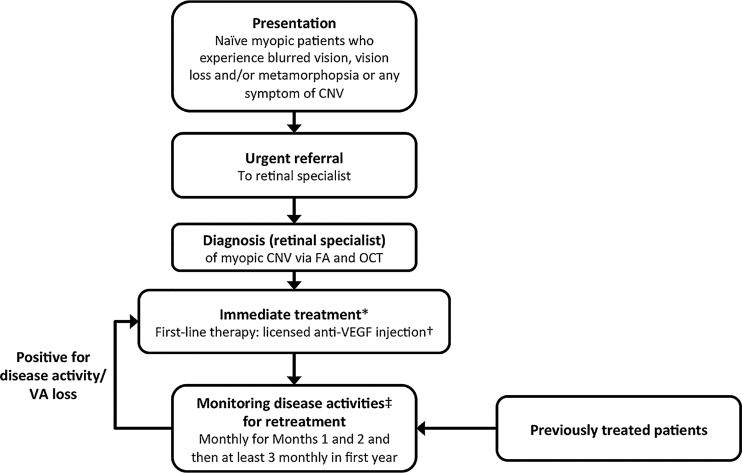
Based on the evidence above, the following clinical management algorithm is proposed for diagnosis and treatment of myopic CNV (figure 3).
Figure 3.
Treatment algorithm for myopic CNV. *Ranibizumab is the only licensed anti-VEGF therapy for myopic CNV. Other anti-VEGFs (eg, bevacizumab and aflibercept) are not currently approved for myopic CNV. †Initiated with a single injection. ‡Monitoring for disease activity may include clinical examination, OCT or FA. If monitoring reveals signs of disease activity (reduced VA, blurred vision, metamorphopsia and/or lesion activity), further treatment is recommended. CNV, choroidal neovascularisation; OCT, optical coherence tomography; FA, fluorescein angiography; VA, visual acuity; VEGF, vascular endothelial growth factor.
Assessment and diagnosis
Patients with myopia and reduced vision, central scotoma and/or metamorphopsia should be referred to a retinal specialist. An early or urgent referral is recommended due to the potential for severe visual loss associated with the active stage of myopic CNV.3 26 31 Myopic CNV may also regress spontaneously resulting in chorioretinal atrophy (figure 3), and the retinal specialist could lose the opportunity to treat the active CNV. Slit lamp biomicroscopy examination and imaging (FA and OCT) should be used to diagnose myopic CNV and differentiate it from other causes of CNV and other causes of visual loss associated with PM. ICGA may be required in selected cases.
Initial treatment
Once diagnosed, prompt treatment with a single intravitreal injection of anti-VEGF therapy is recommended due to the superior efficacy of anti-VEGFs over other treatment modalities.52 Currently, ranibizumab is the only anti-VEGF therapy licensed for myopic CNV, although other agents are being evaluated. While some small studies in myopic CNV involved three initial injections followed by PRN dosing,56 58 the RADIANCE and REPAIR trials support the use of a single ranibizumab injection followed by PRN dosing.52 54 Patients previously treated with vPDT with recurrence of myopic CNV may also be switched to anti-VEGF therapy, as increases in BCVA were observed following a switch in treatment in the RADIANCE trial.52 The results from a recent meta-analysis demonstrated that anti-VEGF therapy was more effective than vPDT in improving BCVA.90
Patients with extrafoveal CNV should also receive immediate treatment, as CNV-related chorioretinal atrophy could develop and affect central vision. In this instance, patients can also receive first-line treatment with anti-VEGF therapy, as the RADIANCE trial included patients with extrafoveal CNV.52 However, vPDT could also be used when anti-VEGF therapy is not available or contraindicated.
Follow-up
After the initial anti-VEGF injection, patients should be monitored monthly for the first 2 months for disease activity, with clinical evaluation and appropriate imaging (OCT and/or FA).91 To assess the progression of myopic CNV, fundus autofluorescence may also be informative.4 Disease activity is defined as a drop in vision, new or persistent visual symptoms (eg, metamorphopsia) or signs of myopic CNV disease activity on FA/OCT (eg, intraretinal or subretinal fluid or active leakage). If disease activity is present, the patient should receive another anti-VEGF injection. This algorithm is supported by the efficacious outcomes of the REPAIR and RADIANCE studies, where patients were treated based on disease activity, defined as any leak on OCT/FA and/or a drop in BCVA associated with CNV activity.52–54 An alternative retreatment approach of treating based on visual acuity stability (also assessed in the RADIANCE trial) resulted in a similar visual acuity gain benefit relative to treating the morphology but required more treatments.52
If there is no disease activity after the initial injection and the two successive monthly visits, three-monthly visits may be considered for the first year.91 For some patients, quarterly monitoring may result in under treatment, so patients should be educated to re-present to the retinal specialist if they experience any decrease in vision or recurrence of metamorphopsia. More frequent monitoring and treatment could be established by the treating retinal specialist if there is evidence of disease activity. After 1 year, the monitoring frequency should be established by the retinal specialist in consultation with the patient,91 and the patient should be advised to return if they experience any drop in vision.
During monitoring, the treating retinal specialist should also check for additional pathologies, such as myopic traction maculopathy (foveoschisis), macular hole, retinal tears and rhegmatogenous detachments, which can also be causes of visual loss and require different treatments.92–95 This is particularly important for myopic traction maculopathy, as the acute shrinkage of CNV by anti-VEGF therapy can worsen a pre-existing retinoschisis.96 Patients with PM and myopic CNV should also be educated about the symptoms of these other retinal complications.
Of note, the number of anti-VEGF injections needed to treat myopic CNV is substantially lower than for other conditions such as AMD-CNV. The RADIANCE trial suggested patients received a median of 2.0 (mean 3.5) injections in the first 12 months under the PRN disease activity dosing regimen.52 Indeed, during months 6–12 of this trial, >60% of patients receiving ranibizumab did not require any injections.52
Conclusion
Treatment of myopic CNV with anti-VEGF agents allows promise of substantial visual acuity gain and quality of life. In particular, there is now a high level of evidence for the use of ranibizumab, the only anti-VEGF agent currently licensed, for treatment of myopic CNV, although others such as aflibercept are being evaluated in clinical trials. While the proposed treatment algorithm is based on the current knowledge and experience gained to date, further research will establish the best management strategy, dosing frequency and timing of injection and monitoring.
Acknowledgments
The authors thank Elizabeth Hutchinson (Fishawack Communications Ltd, UK) for medical writing and editorial assistance towards development of this manuscript, supported by Novartis Pharma AG.
Footnotes
Contributors: At all stages the authors have had control over the content of this manuscript, for which they have given final approval and take full responsibility. TYW was the lead author and took overall responsibility for the scientific accuracy, flow and content of the manuscript. AT was responsible for the overall integrity of the manuscript and acts as the guarantor in accordance with ICJME requirements.
Funding: Medical writing services and publication costs were supported by Novartis Pharma AG.
Competing interests: TYW reports grants, personal fees, travel support and writing/reviewing fees from Novartis and Bayer, and has served as a consultant for Abbott, Allergan, Bayer, Genentech, Novartis, Roche, and Pfizer; KO-M has nothing to disclose; NL reports personal fees and non-financial support from Allergan, Bayer and Novartis, and grants from Théa; FGH reports advisory board remuneration from Acucela, Alcon, Bayer, Genentech, Heidelberg Engineering, Merz, Novartis and Roche; TYL reports advisory board and consultancy remuneration from Allergan, Bayer, Novartis, and lecture fees from Alcon, Allergan, Bausch & Lomb, Bayer Healthcare, Heidelberg Engineering and Novartis; HGY reports grants from Allergan, Bayer and Novartis; PL is a consultant for Alcon, Allergan, Bausch & Lomb, Bayer, Novartis, Roche and Teva; YC reports advisory board remuneration from Novartis; AT reports advisory board remuneration from Alcon, Allergan, Bayer, Novartis, Roche, Genentech, Heidelberg Engineering, Pfizer and ThromboGenics.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Avila MP, Weiter JJ, Jalkh AE, et al. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology 1984;91:1573–81. [DOI] [PubMed] [Google Scholar]
- 2.Fried M, Siebert A, Meyer-Schwickerath G, et al. Natural history of Fuchs’ spot: a long-term follow-up study. Doc Ophthal Proc Series 1981;28:215–21. [Google Scholar]
- 3.Hampton GR, Kohen D, Bird AC. Visual prognosis of disciform degeneration in myopia. Ophthalmology 1983;90:923–6. [DOI] [PubMed] [Google Scholar]
- 4.Neelam K, Cheung CM, Ohno-Matsui K, et al. Choroidal neovascularization in pathological myopia. Prog Retin Eye Res 2012;31:495–525. [DOI] [PubMed] [Google Scholar]
- 5.Leveziel N, Yu Y, Reynolds R, et al. Genetic factors for choroidal neovascularization associated with high myopia. Invest Ophthalmol Vis Sci 2012;53:5004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikuno Y, Jo Y, Hamasaki T, et al. Ocular risk factors for choroidal neovascularization in pathologic myopia. Invest Ophthalmol Vis Sci 2010;51:3721–5. [DOI] [PubMed] [Google Scholar]
- 7.Seko Y, Fujikura H, Pang J, et al. Induction of vascular endothelial growth factor after application of mechanical stress to retinal pigment epithelium of the rat in vitro. Invest Ophthalmol Vis Sci 1999;40:3287–91. [PubMed] [Google Scholar]
- 8.Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol 2003;87:570–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology 2010;117:1595–611. [DOI] [PubMed] [Google Scholar]
- 10.Fredrick DR. Myopia. BMJ 2002;324:1195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akagi-Kurashige Y, Kumagai K, Yamashiro K, et al. Vascular endothelial growth factor gene polymorphisms and choroidal neovascularization in highly myopic eyes. Invest Ophthalmol Vis Sci 2012;53:2349–53. [DOI] [PubMed] [Google Scholar]
- 12.Miyake M, Yamashiro K, Nakanishi H, et al. Evaluation of pigment epithelium-derived factor and complement factor I polymorphisms as a cause of choroidal neovascularization in highly myopic eyes. Invest Ophthalmol Vis Sci 2013;54:4208–12. [DOI] [PubMed] [Google Scholar]
- 13.Wakabayashi T, Ikuno Y. Choroidal filling delay in choroidal neovascularisation due to pathological myopia. Br J Ophthalmol 2010;94:611–15. [DOI] [PubMed] [Google Scholar]
- 14.Steidl SM, Pruett RC. Macular complications associated with posterior staphyloma. Am J Ophthalmol 1997;123:181–7. [DOI] [PubMed] [Google Scholar]
- 15.Chan WM, Ohji M, Lai TY, et al. Choroidal neovascularisation in pathological myopia: an update in management. Br J Ophthalmol 2005;89:1522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller DG, Singerman LJ. Natural history of choroidal neovascularization in high myopia. Curr Opin Ophthalmol 2001;12:222–4. [DOI] [PubMed] [Google Scholar]
- 17.Soubrane G. Choroidal neovascularization in pathologic myopia: recent developments in diagnosis and treatment. Surv Ophthalmol 2008;53:121–38. [DOI] [PubMed] [Google Scholar]
- 18.Verteporfin in Photodynamic Therapy Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 1-year results of a randomized clinical trial—VIP report no. 1. Ophthalmology 2001;108:841–52. [DOI] [PubMed] [Google Scholar]
- 19.Channa R, Ibrahim M, Sepah Y, et al. Characterization of macular lesions in punctate inner choroidopathy with spectral domain optical coherence tomography. J Ophthalmic Inflamm Infect 2012;2:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vance SK, Khan S, Klancnik JM, et al. Characteristic spectral-domain optical coherence tomography findings of multifocal choroiditis. Retina 2011;31:717–23. [DOI] [PubMed] [Google Scholar]
- 21.Watzke RC, Packer AJ, Folk JC, et al. Punctate inner choroidopathy. Am J Ophthalmol 1984;98:572–84. [DOI] [PubMed] [Google Scholar]
- 22.Silva R. Myopic maculopathy: a review. Ophthalmologica 2012;228:197–213. [DOI] [PubMed] [Google Scholar]
- 23.Wong TY, Ferreira A, Hughes R, et al. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol 2014;157:9–25 e12. [DOI] [PubMed] [Google Scholar]
- 24.Leveziel N, Caillaux V, Bastuji-Garin S, et al. Angiographic and optical coherence tomography characteristics of recent myopic choroidal neovascularization. Am J Ophthalmol 2013;155:913–19. [DOI] [PubMed] [Google Scholar]
- 25.Jonas JB, Jonas SB, Jonas RA, et al. Parapapillary atrophy: histological gamma zone and delta zone. PLoS One 2012;7:e47237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida T, Ohno-Matsui K, Yasuzumi K, et al. Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology 2003;110:1297–305. [DOI] [PubMed] [Google Scholar]
- 27.Kojima A, Ohno-Matsui K, Teramukai S, et al. Estimation of visual outcome without treatment in patients with subfoveal choroidal neovascularization in pathologic myopia. Graefes Arch Clin Exp Ophthalmol 2006;244:1474–9. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida T, Ohno-Matsui K, Ohtake Y, et al. Long-term visual prognosis of choroidal neovascularization in high myopia: a comparison between age groups. Ophthalmology 2002;109:712–19. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi K, Ohno-Matsui K, Yoshida T, et al. Characteristics of patients with a favorable natural course of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 2005;243:13–19. [DOI] [PubMed] [Google Scholar]
- 30.Hotchkiss ML, Fine SL. Pathologic myopia and choroidal neovascularization. Am J Ophthalmol 1981;91:177–83. [DOI] [PubMed] [Google Scholar]
- 31.Tabandeh H, Flynn HW, Jr, Scott IU, et al. Visual acuity outcomes of patients 50 years of age and older with high myopia and untreated choroidal neovascularization. Ophthalmology 1999;106:2063–7. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Moreno JM, Montero JA. Long-term visual acuity after argon green laser photocoagulation of juxtafoveal choroidal neovascularization in highly myopic eyes. Eur J Ophthalmol 2002;12:117–22. [DOI] [PubMed] [Google Scholar]
- 33.Tano Y. Pathologic myopia: where are we now? Am J Ophthalmol 2002;134:645–60. [DOI] [PubMed] [Google Scholar]
- 34.Virgili G, Menchini F. Laser photocoagulation for choroidal neovascularisation in pathologic myopia. Cochrane Database Syst Rev 2005:CD004765. [DOI] [PubMed] [Google Scholar]
- 35.Secretan M, Kuhn D, Soubrane G, et al. Long-term visual outcome of choroidal neovascularization in pathologic myopia: natural history and laser treatment. Eur J Ophthalmol 1997;7:307–16. [DOI] [PubMed] [Google Scholar]
- 36.Altan T, Acar N, Kapran Z, et al. Outcome of photodynamic therapy in choroidal neovascularization due to pathologic myopia and related factors. Int Ophthalmol 2012;32:119–25. [DOI] [PubMed] [Google Scholar]
- 37.Chan WM, Lai TY, Wong AL, et al. Combined photodynamic therapy and intravitreal triamcinolone injection for the treatment of choroidal neovascularisation secondary to pathological myopia: a pilot study. Br J Ophthalmol 2007;91:174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen YS, Lin JY, Tseng SY, et al. Photodynamic therapy for Taiwanese patients with pathologic myopia: a 2-year follow-up. Retina 2007;27:839–45. [DOI] [PubMed] [Google Scholar]
- 39.Costa RA, Williams GA. Twofold illumination photodynamic therapy scheme for subfoveal choroidal neovascularization in pathologic myopia: results from a randomized pilot study. Retina 2006;26:757–64. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term results of photodynamic therapy for choroidal neovascularization in Japanese patients with pathologic myopia. Am J Ophthalmol 2011;151:137–47 e1. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi K, Ohno-Matsui K, Teramukai S, et al. Photodynamic therapy with verteporfin for choroidal neovascularization of pathologic myopia in Japanese patients: comparison with nontreated controls. Am J Ophthalmol 2008;145:518–26. [DOI] [PubMed] [Google Scholar]
- 42.Krebs I, Binder S, Stolba U, et al. Choroidal neovascularization in pathologic myopia: three-year results after photodynamic therapy. Am J Ophthalmol 2005;140:416–25. [DOI] [PubMed] [Google Scholar]
- 43.Lam DS, Chan WM, Liu DT, et al. Photodynamic therapy with verteporfin for subfoveal choroidal neovascularisation of pathologic myopia in Chinese eyes: a prospective series of 1 and 2 year follow up. Br J Ophthalmol 2004;88:1315–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montero JA, Ruiz-Moreno JM. Verteporfin photodynamic therapy in highly myopic subfoveal choroidal neovascularisation. Br J Ophthalmol 2003;87:173–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pece A, Isola V, Vadala M, et al. Photodynamic therapy with verteporfin for subfoveal choroidal neovascularization secondary to pathologic myopia: long-term study. Retina 2006;26:746–51. [DOI] [PubMed] [Google Scholar]
- 46.Pece A, Milani P, Isola V, et al. A long-term study of photodynamic therapy with verteporfin for choroidal neovascularization at the edge of chorioretinal atrophy in pathologic myopia. Ophthalmologica 2011;225:161–8. [DOI] [PubMed] [Google Scholar]
- 47.Pece A, Vadala M, Isola V, et al. Photodynamic therapy with verteporfin for juxtafoveal choroidal neovascularization in pathologic myopia: a long-term follow-up study. Am J Ophthalmol 2007;143:449–54. [DOI] [PubMed] [Google Scholar]
- 48.Coutinho AM, Silva RM, Nunes SG, et al. Photodynamic therapy in highly myopic eyes with choroidal neovascularization: 5 years of follow-up. Retina 2011;31:1089–94. [DOI] [PubMed] [Google Scholar]
- 49.Giansanti F, Virgili G, Donati MC, et al. Long-term results of photodynamic therapy for subfoveal choroidal neovascularization with pathologic myopia. Retina 2012;32:1547–52. [DOI] [PubMed] [Google Scholar]
- 50.Schnurrbusch UE, Jochmann C, Wiedemann P, et al. Quantitative assessment of the long-term effect of photodynamic therapy in patients with pathologic myopia. Graefes Arch Clin Exp Ophthalmol 2005;243:829–33. [DOI] [PubMed] [Google Scholar]
- 51.Blinder KJ, Blumenkranz MS, Bressler NM, et al. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial—VIP report no. 3. Ophthalmology 2003;110:667–73. [DOI] [PubMed] [Google Scholar]
- 52.Wolf S, Balciuniene VJ, Laganovska G, et al. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology 2014;121:682–92. [DOI] [PubMed] [Google Scholar]
- 53.Tufail A, Narendran N, Patel PJ, et al. Ranibizumab in myopic choroidal neovascularization: the 12-month results from the REPAIR study. Ophthalmology 2013;120:1944–5. [DOI] [PubMed] [Google Scholar]
- 54.Tufail A, Patel PJ, Sivaprasad S, et al. Ranibizumab for the treatment of choroidal neovascularisation secondary to pathological myopia: interim analysis of the REPAIR study. Eye (Lond) 2013;27:709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2. Oxford Centre for Evidence-Based Medicine. 2011. http://www.cebm.net/index.aspx?o=5653 (accessed 13 Nov 2013).
- 56.Calvo-Gonzalez C, Reche-Frutos J, Donate J, et al. Intravitreal ranibizumab for myopic choroidal neovascularization: factors predictive of visual outcome and need for retreatment. Am J Ophthalmol 2011;151:529–34. [DOI] [PubMed] [Google Scholar]
- 57.Franqueira N, Cachulo ML, Pires I, et al. Long-term follow-up of myopic choroidal neovascularization treated with ranibizumab. Ophthalmologica 2012;227:39–44. [DOI] [PubMed] [Google Scholar]
- 58.Lai TY, Luk FO, Lee GK, et al. Long-term outcome of intravitreal anti-vascular endothelial growth factor therapy with bevacizumab or ranibizumab as primary treatment for subfoveal myopic choroidal neovascularization. Eye (Lond) 2012;26:1004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monés JM, Amselem L, Serrano A, et al. Intravitreal ranibizumab for choroidal neovascularization secondary to pathologic myopia: 12-month results. Eye (Lond) 2009;23:1275–80, quiz 81. [DOI] [PubMed] [Google Scholar]
- 60.Silva RM, Ruiz-Moreno JM, Rosa P, et al. Intravitreal ranibizumab for myopic choroidal neovascularization: 12-month results. Retina 2010;30:407–12. [DOI] [PubMed] [Google Scholar]
- 61.Vadala M, Pece A, Cipolla S, et al. Is ranibizumab effective in stopping the loss of vision for choroidal neovascularisation in pathologic myopia? A long-term follow-up study. Br J Ophthalmol 2011;95:657–61. [DOI] [PubMed] [Google Scholar]
- 62.Lai TY, Chan WM, Liu DT, et al. Intravitreal ranibizumab for the primary treatment of choroidal neovascularization secondary to pathologic myopia. Retina 2009;29:750–6. [DOI] [PubMed] [Google Scholar]
- 63.Yoon JU, Byun YJ, Koh HJ. Intravitreal anti-VEGF versus photodynamic therapy with verteporfin for treatment of myopic choroidal neovascularization. Retina 2010;30:418–24. [DOI] [PubMed] [Google Scholar]
- 64.Ikuno Y, Sayanagi K, Soga K, et al. Intravitreal bevacizumab for choroidal neovascularization attributable to pathological myopia: one-year results. Am J Ophthalmol 2009;147:94–100 e1. [DOI] [PubMed] [Google Scholar]
- 65.Chan WM, Lai TY, Liu DT, et al. Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularisation: 1-year results of a prospective pilot study. Br J Ophthalmol 2009;93:150–4. [DOI] [PubMed] [Google Scholar]
- 66.Gharbiya M, Allievi F, Mazzeo L, et al. Intravitreal bevacizumab treatment for choroidal neovascularization in pathologic myopia: 12-month results. Am J Ophthalmol 2009;147:84–93 e1. [DOI] [PubMed] [Google Scholar]
- 67.Ruiz-Moreno JM, Montero JA, Amat-Peral P. Myopic choroidal neovascularization treated by intravitreal bevacizumab: comparison of two different initial doses. Graefes Arch Clin Exp Ophthalmol 2011;249:595–9. [DOI] [PubMed] [Google Scholar]
- 68.Ruiz-Moreno JM, Montero JA, Arias L, et al. Twelve-month outcome after one intravitreal injection of bevacizumab to treat myopic choroidal neovascularization. Retina 2010;30:1609–15. [DOI] [PubMed] [Google Scholar]
- 69.Ruiz-Moreno JM, Lopez-Galvez MI, Donate J, et al. Myopic choroidal neovascularization. Ophthalmology 2011;118:2521–3. [DOI] [PubMed] [Google Scholar]
- 70.Ruiz-Moreno JM, Lopez-Galvez MI, Montero Moreno JA, et al. Intravitreal bevacizumab in myopic neovascular membranes: 24-month results. Ophthalmology 2013;120:1510–11 e1. [DOI] [PubMed] [Google Scholar]
- 71.Iacono P, Parodi MB, Papayannis A, et al. Intravitreal bevacizumab therapy on an as-per-needed basis in subfoveal choroidal neovascularization secondary to pathological myopia: 2-year outcomes of a prospective case series. Retina 2011;31:1841–7. [DOI] [PubMed] [Google Scholar]
- 72.Gharbiya M, Cruciani F, Parisi F, et al. Long-term results of intravitreal bevacizumab for choroidal neovascularisation in pathological myopia. Br J Ophthalmol 2012;96:1068–72. [DOI] [PubMed] [Google Scholar]
- 73.Hayashi K, Shimada N, Moriyama M, et al. Two-year outcomes of intravitreal bevacizumab for choroidal neovascularization in Japanese patients with pathologic myopia. Retina 2012;32:687–95. [DOI] [PubMed] [Google Scholar]
- 74.Hayashi K, Ohno-Matsui K, Teramukai S, et al. Comparison of visual outcome and regression pattern of myopic choroidal neovascularization after intravitreal bevacizumab or after photodynamic therapy. Am J Ophthalmol 2009;148:396–408. [DOI] [PubMed] [Google Scholar]
- 75.Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013;382:1258–67. [DOI] [PubMed] [Google Scholar]
- 76.Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Curtis LH, Hammill BG, Schulman KA, et al. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol 2010;128:1273–9. [DOI] [PubMed] [Google Scholar]
- 78.Cruess AF, Giacomantonio N. Cardiac issues of noncardiac drugs: the rising story of Avastin in age-related macular degeneration. Ophthalmologica 2014;231:75–9. [DOI] [PubMed] [Google Scholar]
- 79.US Food and Drug Administration. Potential use of Avastin in the treatment of wet age-related macular degeneration. 2011. http://www.fda.gov/Drugs/DrugSafety/ucm270296.htm (accessed 11 Jul 2013).
- 80.Nakanishi H, Tsujikawa A, Yodoi Y, et al. Prognostic factors for visual outcomes 2-years after intravitreal bevacizumab for myopic choroidal neovascularization. Eye (Lond) 2011;25:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parodi MB, Iacono P, Papayannis A, et al. Laser photocoagulation, photodynamic therapy, and intravitreal bevacizumab for the treatment of juxtafoveal choroidal neovascularization secondary to pathologic myopia. Arch Ophthalmol 2010;128:437–42. [DOI] [PubMed] [Google Scholar]
- 82.Yodoi Y, Tsujikawa A, Nakanishi H, et al. Central retinal sensitivity after intravitreal injection of bevacizumab for myopic choroidal neovascularization. Am J Ophthalmol 2009;147:816–24, 24 e1. [DOI] [PubMed] [Google Scholar]
- 83.Ruiz-Moreno JM, Arias L, Montero JA, et al. Intravitreal anti-VEGF therapy for choroidal neovascularisation secondary to pathological myopia: 4-year outcome. Br J Ophthalmol 2013;97:1447–50. [DOI] [PubMed] [Google Scholar]
- 84.Yang HS, Kim JG, Kim JT, et al. Prognostic factors of eyes with naïve subfoveal myopic choroidal neovascularization after intravitreal bevacizumab. Am J Ophthalmol 2013;156:1201–10 e2. [DOI] [PubMed] [Google Scholar]
- 85.Miyake M, Yamashiro K, Akagi-Kurashige Y, et al. Vascular endothelial growth factor gene and the response to anti-vascular endothelial growth factor treatment for choroidal neovascularization in high myopia. Ophthalmology 2014;121:225–33. [DOI] [PubMed] [Google Scholar]
- 86.Gharbiya M, Giustolisi R, Allievi F, et al. Choroidal neovascularization in pathologic myopia: intravitreal ranibizumab versus bevacizumab—a randomized controlled trial. Am J Ophthalmol 2010;149:458–64 e1. [DOI] [PubMed] [Google Scholar]
- 87.Iacono P, Parodi MB, Papayannis A, et al. Intravitreal ranibizumab versus bevacizumab for treatment of myopic choroidal neovascularization. Retina 2012;32:1539–46. [DOI] [PubMed] [Google Scholar]
- 88.ClinicalTrials.gov. VEGF trap-eye in choroidal neovascularization secondary to pathologic myopia (mCNV) (MYRROR). 2012. http://clinicaltrials.gov/ct2/show/NCT01249664?term=aflibercept+AND+myopia&rank=1 (accessed 1 Jul 2013).
- 89.Bayer HealthCare. Positive phase 3 results for VEGF Trap-Eye (intravitreal aflibercept) in myopic choroidal neovascularization (mCNV). 2013. http://press.healthcare.bayer.com/en/press/auth/news-details-page.php/15070/2013–0317 (accessed 25 Jul 2013).
- 90.Wang E, Chen Y. Intravitreal anti-vascular endothelial growth factor for choroidal neovascularization secondary to pathologic myopia: systematic review and meta-analysis. Retina 2013;33:1375–92. [DOI] [PubMed] [Google Scholar]
- 91.Novartis. Lucentis summary of product characteristics. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000715/WC500043546.pdf (accessed 13 Nov 2013).
- 92.Akiba J. Prevalence of posterior vitreous detachment in high myopia. Ophthalmology 1993;100:1384–8. [DOI] [PubMed] [Google Scholar]
- 93.Kobayashi H, Kobayashi K, Okinami S. Macular hole and myopic refraction. Br J Ophthalmol 2002;86:1269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lewis H. Peripheral retinal degenerations and the risk of retinal detachment. Am J Ophthalmol 2003;136:155–60. [DOI] [PubMed] [Google Scholar]
- 95.Tang J, Rivers MB, Moshfeghi AA, et al. Pathology of macular foveoschisis associated with degenerative myopia. J Ophthalmol 2010;2010:175613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shimada N, Ohno-Matsui K, Hayashi K, et al. Macular detachment after successful intravitreal bevacizumab for myopic choroidal neovascularization. Jpn J Ophthalmol 2011;55:378–82. [DOI] [PubMed] [Google Scholar]