Abstract
Background
The global incidence of breast cancer is increasing, mainly due to the sharp rise in breast cancer incidence in Asia. The aim of this study was to evaluate the association of CYP2D6*10 (c.100C>T and c.1039C>T), OATP1B1 A388G, and OATP1B1 T521C polymorphisms with overall survival (OS) for hormone receptor (estrogen receptor or progesterone receptor)-positive tumors (ER+/PR+) breast cancer patients after adjuvant tamoxifen (TAM) therapy.
Material/Method
We included 296 invasive breast cancer patients with hormone receptor-positive tumors during the period 2002–2009. We collected patient data, including clinical features, TAM therapy, and survival status. Archived paraffin blocks from surgery were the source of tissue for genotyping. CYP2D6*10, OATP1B1 A388G, and T521C polymorphisms were detected by direct sequencing of genomic DNA. OS was assessed with Kaplan-Meier analysis, while the Cox proportional hazards model was used to implement multivariate tests for the prognostic significance.
Results
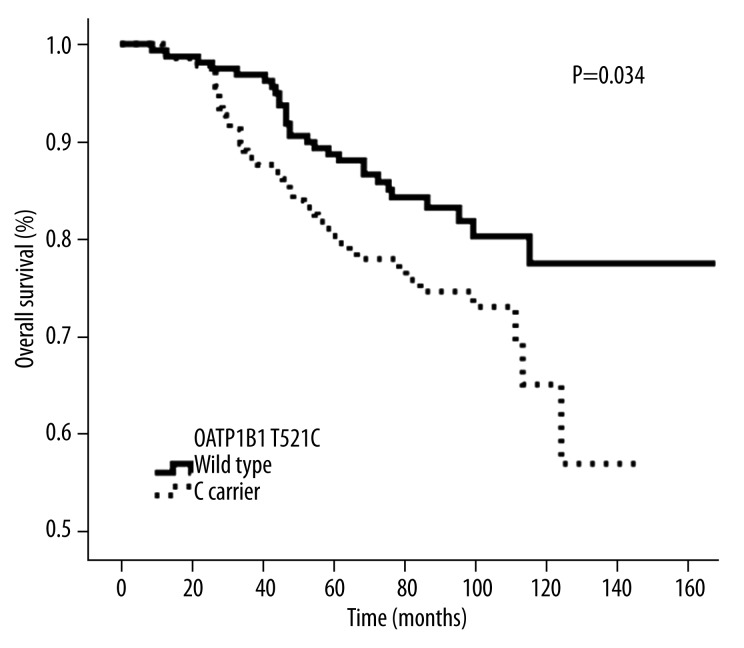
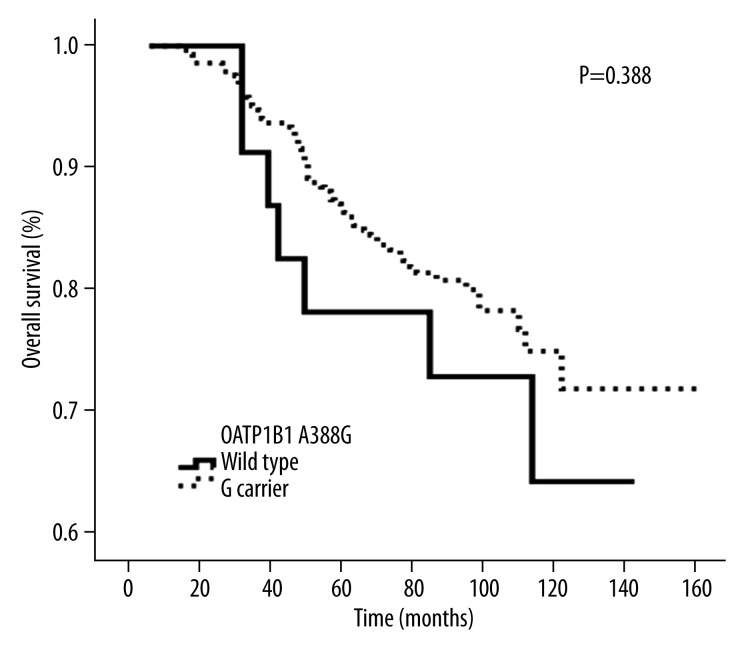
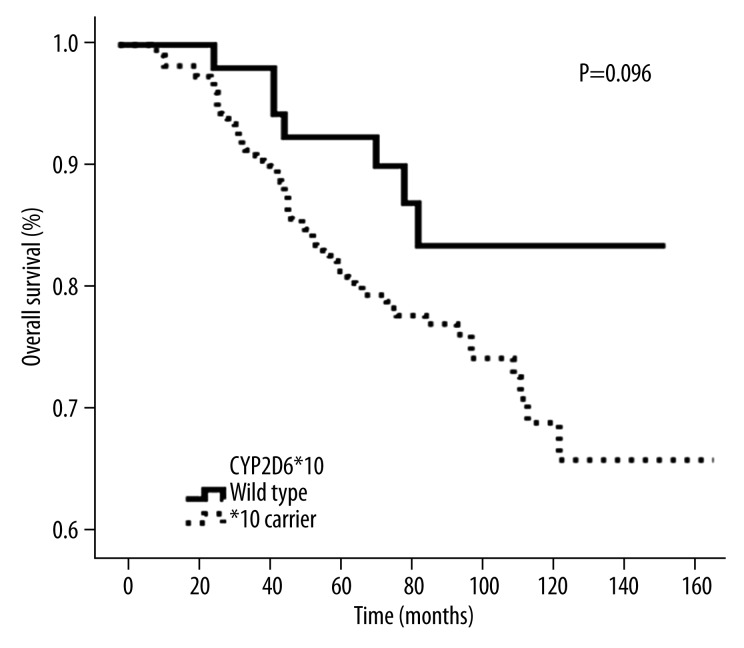
There was a significant difference in OS between OATP1B1 T521C wild-type and the mutant genotype C carrier (P=0.034). However, there was no difference in overall survival between wild-type and carrier groups for CYP2D6*10 (P=0.096) and OATP1B1 A388G (P=0.388), respectively.
Conclusions
These results suggest that the OATP1B1 T521C mutation may be an independent prognostic marker for breast cancer patients using TAM therapy.
MeSH Keywords: Breast Diseases; Cytochrome P-450 CYP2D6; Organic Anion Transporters; Polymorphism, Genetic; Tamoxifen
Background
According to the International Agency for Research on Cancer (IARC), the most common cancers worldwide are lung cancer, breast cancer, and colorectal cancer. The current global incidence of breast cancer is increasing, mainly due to the sharp rise in breast cancer incidence in Asia [1]. Compared with Western women, Asian women are more likely to be diagnosed with advanced breast cancer – about 10–25% of patients have metastasis, while the value for European and Americans is only 3–5% [2]. Breast cancer is not just a local tumor, but is also a systemic disease with occurrence and development closely related to abnormalities of estrogen hormones [3,4]. Clinical and experimental studies have shown that estrogen plays a role in the development and progression of breast cancer, mainly through ER signaling pathway [5]. Approximately 75% of breast cancer patients show ER+/PR+ [6]. TAM is a non-steroidal, anti-estrogen drug regulating estrogen receptor selectively, with structure similar to that of estrogen. TAM has been used in more than 120 countries for the treatment and prevention of recurrence of ER+/PR+ breast cancer patients [7]. TAM therapy has become one of the most important means for breast cancer adjuvant endocrine treatment. Due to the use of TAM endocrine adjuvant therapy in the past 30 years, the OS rate of breast cancer patients has been extended [8].
Estrogen plays a key role in the pathogenesis of breast cancer, and TAM has been used to treat hormone-dependent breast cancer patients. However, nearly half of patients receiving adjuvant TAM therapy experience relapse and poor responsiveness, indicating large individual differences in response to TAM therapy [7]. The organic anion-transporting polypeptide 1B1 (OATP1B1, encoded by SLCO1B1) is a trans-membrane transporter protein, which is highly expressed in the sinusoidal membrane of human hepatocytes and is one of the main hepatic uptake transporters. It mediates the uptake not only of diverse endogenous substrates, but also adjusts the uptake of cellular substrate to promote the absorption of the liver-derived compounds and some drugs with important pharmacokinetic and pharmacodynamic significance [9,10]. OATP1B1 is highly polymorphic. Its main mutation points are T521C (rs4149056) and A388G (rs2306283) [11–14], in which OATP1B1 T521C carrying C gene is associated with decreased OATP1B1 transport activities [15]. The expression and regulation of organic anion transporting polypeptides (OATPs) affects the development of cancer and is a useful target for anticancer therapy [16]. OATPs is usually expressed in gastric cancer [17], pancreatic cancer [18], and the surface of colon cancer cells [19]. In vitro experiments have demonstrated that OATP1B1 expression can be detected in breast cancer cell lines and normal breast tissue samples [20]. OATPs is a new target for the treatment of hormone-dependent breast cancer, as confirmed by a report of 7 OATPs gene and protein expressions in immortalized breast epithelial cells (MCF10A), hormone-dependent MCF7 and hormone-independent breast cancer cells (MDA/LCC6-435, MDA-MB-231 and MDA-MB-468) by using quantitative polymerase chain reaction and immunoblotting methods [21]. However, few studies are available on the association of OATP1B1 A388G and T521C polymorphisms with treatment response and/or patient OS for breast cancer treated with TAM. Human cytochrome P450 2D6(CYP2D6) is a key enzyme, playing an important role in the biotransformation of TAM, which converts TAM to the 4-hydroxy-N-desmethyl tamoxifen (endoxifen) [22]. CYP2D6 is highly polymorphic, and more than 100 different genotypes have been found (http://www.cypalleles.ki.se/cyp2d6.htm). The most common allelic variation in Caucasians is non-functional allele *4, while Asians it is allele *10 (rs1065852) [23]. Experiments have confirmed that CYP2D6*10/*10 genotype can reduce enzyme activity, thereby reducing the metabolism of TAM [24]. In 2006, the Food and Drug Administration (FDA) of the United States recommended that CYP2D6 genotyping is necessary prior to the determination of TAM anti-cancer treatment to improve clinical efficacy and the safety [25]; therefore, CYP2D6*10 polymorphism was also included in the present study.
We hypothesized that breast cancer patients with CYP2D6*10, OATP1B1 A388G, and T521C variant genotype may respond differently to TAM treatment. Our study aimed to evaluate the correlation between genetic polymorphism and overall survival of ER+/PR+ breast cancer patients and to provide a reference value for the treatment and prognosis for breast cancer patients.
Material and Methods
Study population
We retrieved data on 1236 breast cancer patients who were diagnosed by biopsy and received surgical treatment from The First Affiliated Hospital (Yijishan Hospital) of Wannan Medical College, with surgeries performed between January 2002 and December 2009. The inclusion criteria included: (1) Chinese females of Han Chinese ethnicity; (2) histological diagnosis of ER+/PR+; (3) diagnosed with invasive breast cancer; and (4) received TAM as monotherapy after surgery with CMF (cyclophosphamide, methotrexate, and fluorouracil) chemotherapy, with no co-administered drugs that might influence the efficacy TAM, such as antipsychotics, antihypertensives, and antidiabetic drugs. We enrolled 296 patients who met our inclusion criteria: 176 cases of modified radical mastectomy, 38 cases of radical resection, 40 cases of breast cancer palliative resection, 22 cases of breast-conserving surgery for breast cancer, 13 cases of breast lesions localized resection, 1 case of breast needle aspiration, and 6 cases of radical mastectomy. The patients took TAM 20 mg/d after surgery for 2–5 years (3.64 years on average), and the median follow-up time was 83 months (9–155 months). All subjects signed an informed consent before study entry. This study was approved by the Ethics Committee of The First Affiliated Hospital of Wannan Medical College.
Genotyping
Genomic DNA was extracted from archived paraffin blocks from surgery of each patient using the QIAamp DNA FFPE Tissue Kit (QIAGEN, Germany). For each subject, 1–3 pieces (10 μm each) of tissue blocks were cut and placed in sterile tubes for DNA extraction. We kept the DNA at −80°C after extraction. The design and synthesis of primer were by Boshang Biotechnology Corporation (Shanghai, China), nested PCR was used to amplify fragments in this experiment, and mutation analysis was performed by direct sequencing of PCR products (Table 1).
Table 1.
The OATP1B1 A388G, T521C and CYP2D6*10’s primer sequences.
| Genotype | Primer | Primer sequence | Length |
|---|---|---|---|
| OAPT1B1 A388G | F | CTTAAAACACATGCTGGGAAATTG | 224 |
| R | CTGTGTTGTTAATGGGCGAACTG | ||
| FN | TAATGGTGCAAATAAAGGGGAAT | ||
| RN | TCTTACCTTTTCCCACTATCTCAG | ||
| F | GCAGCATAAGAATGGACTAATACAC | ||
| OATP1B1 T521C | R | CAATTTTACTAGATGCCAAGAATGC | 222 |
| FN | TAAAATGAAACACTCTCTTATCTACATAGG | ||
| RN | GACAAAGGGAAAGTGATCATACAAT | ||
| CYP2D6*10 | F | CTGCTTCCCCTTCTCAGCCT | 227 |
| R | CGGTGTGCTGAGAGTGTCCT | ||
| FN | ACCTCCTCCCTCACCTGGTC | ||
| RN | CAGAGGAGCCCATTTGGTAG |
F – forward outer primer; R – reverse outer primer; FN – forward internal primer; RN – reverse internal primer.
PCR was performed in 30-μL reactions containing 1 μL DNA, 1 μL of each primer, 2.5 μL×PCR buffer, 0.5 μL deoxy-ribonucleoside triphosphate (dNTP), and 0.15 μL rTaq DNA polymerase. Reaction mixtures with the outer primer sets were thermally cycled once at 95°C for 5 min; 30 times at 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s; and once at 75°C for 10 min. Then we put 5 μL of the first-reaction product in the next PCR with 1 μL of each primer, 5 μL×PCR buffer, 1 μL dNTP, and 0.3 μL rTaq in a total volume of 50 μL. The reaction with the inner primer set was identical. Then fluorescent tags were placed to track the specific base-pair. The final products were analyzed on an ABI 3730XL (Applied Biosystems) automated sequence analyzer. A gene scan program was used for genotypes in each sample.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software, and chi-square test and Fisher’s exact probability test were used to compare the patients who had different genotypes and clinical characteristics. Kaplan-Meier analysis was used to make the survival curve diagram, and the log-rank test was used for performed. The Cox regression model was used for univariate and multivariate analysis to determine if genetic polymorphism was an independent prognostic influential clinical factor. P<0.05 was considered as statistically significant.
Results
Patient characteristics
We included 296 female invasive breast cancer patients taking TAM after surgery, whose median age was 50 years (range: 25–82 years); 122 patients were menopausal and 174 were premenopausal. Lymph node metastasis occurred in 126 patients, and 170 patients had no metastasis. The relationship between clinical characteristics of patients and each genotype are shown in Table 2. These genotyping and clinicopathological parameters were of no statistical significance. Overall, baseline characteristics of the patients were comparable (P>0.05), including menopausal status, tumor size, and lymph node metastasis.
Table 2.
Associations between the OATP1B1 C521T, A388G, CYP2D6*10 genotype and patient’s characteristics.
| Characteristic | Patients n=296 |
OATP1B1 521 | OATP1B1 388 | CYP2D6*10 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carrier n=137 |
Wt n=159 |
P | Carrier n=274 |
Wt n=22 |
P | Carrier n=241 |
Wt n=55 |
P | ||
| Median age (range), y | 50 (25–82) | 51 (25–82) | 50 (28–77) | 50 (25–82) | 48 (33–69) | 51 (28–82) | 46 (25–78) | |||
| Menopausal status | 0.716** | 0.822* | 0.291* | |||||||
| Premenopausal | 174 | 79 | 95 | 160 | 14 | 138 | 36 | |||
| Postmenopausal | 122 | 58 | 64 | 114 | 8 | 103 | 19 | |||
| Tumor size, cm | 0.387** | 1.000* | 0.445* | |||||||
| ≤2.0 | 136 | 59 | 77 | 126 | 10 | 113 | 23 | |||
| >2.0 | 140 | 68 | 72 | 130 | 10 | 111 | 29 | |||
| Unknown | 20 | |||||||||
| Nuclear grade | 0.828* | 0.362* | 0.711* | |||||||
| 1 | 55 | 26 | 29 | 49 | 6 | 44 | 11 | |||
| 2 | 183 | 79 | 104 | 172 | 11 | 151 | 32 | |||
| 3 | 38 | 18 | 20 | 35 | 3 | 28 | 10 | |||
| Unknown | 20 | |||||||||
| Lymph node status | 0.940** | 1.000* | 0.763* | |||||||
| Positive | 126 | 58 | 68 | 117 | 9 | 104 | 22 | |||
| Negative | 170 | 79 | 91 | 157 | 13 | 137 | 33 | |||
| ER status | 0.423* | 0.759* | 0.214* | |||||||
| Positive | 250 | 113 | 137 | 232 | 18 | 200 | 50 | |||
| Negative | 46 | 24 | 22 | 42 | 4 | 41 | 5 | |||
| PR status | 0.743* | 0.540* | 0.288* | |||||||
| Positive | 253 | 116 | 137 | 235 | 18 | 203 | 50 | |||
| Negative | 43 | 21 | 22 | 39 | 4 | 38 | 5 | |||
| HER-2 status | 0.570** | 0.070* | 0.363* | |||||||
| Positive | 118 | 57 | 61 | 105 | 13 | 93 | 25 | |||
| Negative | 178 | 80 | 98 | 169 | 9 | 148 | 30 | |||
Fisher’s exact test;
Pearson’s Chi-squared test.
Wt – wild type; HER-2 – human epidermal growth factor receptor 2.
Frequencies of genotypes
OATP1B1 A388G genotype was assessed for all 296 patients, of which 22 (7.4%) were homozygous (A/A) for the genotype and 274 (92.6%) were mutational (G carrier) for the genotype. In terms of polymorphism of OATP1B1 T521C, wild genotype (T/T) was present in 159 of 296 patients (53.7%) and variant genotypes (C carrier) were present in 137 (46.3%). The polymorphisms of OATP1B1 A388G and OATP1B1 T521C can be indicated according to their chain models SLCO1B1*1a (c.388A-c.521T), SLCO1B1*1b (c.388G-c.521T), SLCO1B1*5 (c.388A-c.521C), and SLCO1B1*15 (c.388G-c.521C), constituting 4 haplotypes with different functions. In this experiment, the gene frequencies were 25.5%, 48.7%, 8.8%, and 17.0% respectively.
The relationship between each genotype and survival rate of patients receiving therapy of TAM
When the follow-up was terminated, there were 65 deaths among 296 breast cancer patients taking TAM after surgery, with a 5-year OS of 84.5%, and 10-year OS of 72.5%. The OATP1B1 T521C genotype was significantly associated with OS in this subgroup, and patients with the homozygous wild-type T/T genotype had a higher OS rate than that of the homozygous variant C/C and heterozygous C/T genotype (C carrier) (P=0.034, Figure 1). The intermediate survival times were 87 months (range: 9–155 months, n=159) and 81 months (range: 12–148 months, n=137) for T521C wild-type and C carriers, respectively. In multivariate analysis, as compared with the wild-type genotype, the C carrier remained an independent prognostic marker of OS (hazard ratio=0.593; 95% confidence interval=0.363–0.971; P=0.038) (Table 3) after adjusting for age, lymph node involvement, tumor size, and ER or PR status in this subgroup of 296 patients. The results show that there was no statistical significance between the OATP1B1 A388G genotype and OS in these 296 patients (P=0.388, Figure 2). The intermediate survival time of OATP1B1 A388G wild-type was 110 months (range: 28–147 months, n=22). The intermediate mutant survival time of OATP1B1 A388G was 82 months (range: 9–155 months, n=174). Likewise, the CYP2D6 *10 genotype was not significantly associated with OS in this subgroup (P=0.096, Figure 3). The intermediate survival time of *10 carriers was 84 months (range: 9–150 months, n=241). The intermediate survival time of wild-type was 81 months (range: 27–155 months, n=55).
Figure 1.
Kaplan-Meier probabilities of overall survival in patients treated with adjuvant TAM in relation with OATP1B1 521 genotype.
Table 3.
Cox proportional hazards regression(Adjusted Cox Proportional HR of CYP2D6*10, OATP1B1 A388G and T521C polymorphisms).
| Hazard ratio (95% CI) 3 | P-value | |
|---|---|---|
| CYP2D6*10 | 0.102 | |
| Wt | 0.520 (0.237–1.139) | |
| T carrier | 1 | |
| OATP1B1 A388G | 0.396 | |
| Wt | 1.408 (0.640–3.098) | |
| G carrier | 1 | |
| OATP1B1 T521C | 0.038 | |
| Wt | 0.593 (0.363–0.971) | |
| C carrier | 1 |
1) If the relative risk is less than 1, the relative risk can be thought of as the average decreased risk of dying at any point in time compared with the reference group. If the relative risk is greater than 1, the relative risk can be thought of as the average increased risk of dying at any point in time compared with the reference group. [The group with the ratio equal to 1.00 is the reference group]. 2) P value based on log-rank test. 3) Hazard ratios from Cox proportional hazards model, adjusted for age, menopausal status, Tumor size, lymph node status, ER status, PR status, HER-2 status. CI – confidence interval; Wt – wild type.
Figure 2.
Kaplan-Meier probabilities of overall survival in patients treated with adjuvant TAM in relation with OATP1B1 388 genotype.
Figure 3.
Kaplan-Meier probabilities of overall survival in patients treated with adjuvant TAM in relation with CYP2D6 genotype.
Discussion
Muto et al. [26] collected 102 cases of parameters and survival conditions of breast cancer patients, and tested the expression of organic anion transporter-2 (OATP1B3, LST-2) by immunohistochemical methods, and also analyzed the disease-free survival and OS of the patients with OATP1B3 immune negative and positive response. They found that LST-2 immunoreactivity significantly reduced breast cancer recurrence and improved the prognosis. In 2011, Justenhoven et al. [27] studied the potential functions of 31 polymorphisms of OATPs and PXR in breast cancer risk. To our knowledge, there has been no information reported about the efficacy of A388G and T521C polymorphism on postoperative prognosis of breast cancer patients taking TAM. In the present study we found there was a tendency that OATP1B1 A388G mutations lead to increased efficacy of TAM treatment for ER+/PR+ breast cancer patients, but with no statistical significance. However, T521C polymorphism has a significant impact on TAM efficacy in ER+/PR+ breast cancer patients.
The frequencies of the OATP1B1 A388G and T521C variant alleles in Chinese were 73.4% and 14.0%, respectively [28]. There are growing concerns about the influence of OATP1B1 polymorphisms on pharmacokinetic and pharmacodynamic profiles of certain drugs. At present, the FDA recommends against 80 mg daily simvastatin dosage. In patients with C allele at OATP1B1 T521C, there are modest increases in myopathy risk even at lower simvastatin doses (40 mg daily). If optimal efficacy is not achieved with a lower dose, alternative agents should be considered to control drug adverse reaction caused by OATP1B1 T521C polymorphism, and to improve the safety of medication [29,30]. The OATP1B1 T521C polymorphism plays an important role in the interindividual variability of plasma concentrations of nateglinide and repaglinide, and OATP1B1 A388G genotype is associated with reduced pharmacokinetic exposure after single-dose oral administration of 2 mg repaglinide, including decreased AUC and increased clearance of repaglinide [31,32]. Extensive data show OATP1B1 genotypes were associated with drug adverse reactions and drug-drug interactions, and polymorphisms can increase risk. Recent studies have found that the OATP1B1 genotypes were associated with methotrexate clearance [33]. OATP1B1 polymorphisms play more and more important roles in clinical drug use.
CYP2D6 gene mutations can cause changes in the number and activity of the enzymes, resulting in differences in specific drug metabolism and efficacy, and significant differences can be seen between individuals and races. CYP2D6*10 is a key enzyme, playing an important role in the biotransformation of TAM [34]. In vitro experiments show that an unstable enzyme is synthesized in the carrier of CYP2D6*10/*10 gene, of which the half-life is relatively short and the activity is relatively weak – about 1/40 of the wild-type CYP2D6*10 [34]. Our study shows that the OS of mutant-type CYP2D6 is lower than that of wild-type CYP2D6; the survival rate of wild-type is the highest. We found that the influence of the wild-type CYP2D6 on the OS of breast cancer patients who received TAM treatment is similar to that of mutant CYP2D6. There is a trend of CYP2D6*10 mutation leading to decreased efficacy of TAM treatment in ER+/PR+ patients, but with no statistical significance, which is consistent with the relevant literature [35]. We expect to conduct multi -center or larger-sample studies in the future.
Conclusions
Our study suggests that OATP1B1 T521C polymorphism might be useful in predicting TAM efficacy and clinical outcomes in breast cancer patients receiving adjuvant TAM therapy. In this field, further research could contribute to more individualized treatment strategies.
Footnotes
Statement
None of the authors has any proprietary interest.
Source of support: This work was supported by the National Natural Science Foundation of China (Grant No: 81173134)
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Aziz SA, Miao H, Hartman M, et al. Predicting survival of de novo metastatic breast cancer in Asian women: systematic review and validation study. PLoS ONE. 2014;9(4):1–12. doi: 10.1371/journal.pone.0093755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen WY, Hankinson SE, Schnitt SJ, et al. Association of hormone replacement therapy to estrogen and progesterone receptor status in invasive breast carcinoma. Cancer. 2004;101(7):1490–500. doi: 10.1002/cncr.20499. [DOI] [PubMed] [Google Scholar]
- 4.Manavathi B, Dey O, Gajulapalli VN, et al. Derailed estrogen signaling and breast cancer: an authentic couple. Endocr Rev. 2013;34(1):1–32. doi: 10.1210/er.2011-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali S, Coombes RC. Estrogen receptor alpha in human breast cancer: occurrence and significance. J Mammary Gland Biol Neoplasia. 2000;5(3):271–81. doi: 10.1023/a:1009594727358. [DOI] [PubMed] [Google Scholar]
- 6.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;5(17):1474–81. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 7.Kiyotani K, Mushiroda T, Nakamura Y, Zembutsu H. Pharmacogenomics of tamoxifen: roles of drug metabolizing enzymes and transporters. Drug Metab Pharmacokinet. 2012;27(1):122–31. doi: 10.2133/dmpk.dmpk-11-rv-084. [DOI] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) PR, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–44. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues AC. Efflux and uptake transporters as determinants of statin response. Expert Opin Drug Metab Toxicol. 2010;6(5):621–32. doi: 10.1517/17425251003713519. [DOI] [PubMed] [Google Scholar]
- 10.van de Steeg E, van Esch A, Wagenaar E, et al. Influence of human OATP1B1, OATP1B3, and OATP1A2 on the pharmacokinetics of methotrexate and paclitaxel in humanized transgenic mice. Clin Cancer Res. 2013;19(4):821–32. doi: 10.1158/1078-0432.CCR-12-2080. [DOI] [PubMed] [Google Scholar]
- 11.Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European-and African-Americans. J Biol Chem. 2001;276(38):35669–75. doi: 10.1074/jbc.M103792200. [DOI] [PubMed] [Google Scholar]
- 12.Nozawa T, Nakajima M, Tamai I, et al. Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J Pharmacol Exp Ther. 2002;302(2):804–13. doi: 10.1124/jpet.302.2.804. [DOI] [PubMed] [Google Scholar]
- 13.Nishizato Y, Ieiri I, Suzuki H, et al. Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics. Clin Pharmacol Ther. 2003;73(6):554–65. doi: 10.1016/S0009-9236(03)00060-2. [DOI] [PubMed] [Google Scholar]
- 14.Niemi M, Schaeffeler E, Lang T, et al. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1) Pharmacogenetics. 2004;14(7):429–40. doi: 10.1097/01.fpc.0000114750.08559.32. [DOI] [PubMed] [Google Scholar]
- 15.Giannakopoulou E, Ragia G, Kolovou V, et al. No impact of SLCO1B1 521T>C, 388A>G and 411G>A polymorphisms on response to statin therapy in the Greek population. Mol Biol Rep. 2014;41(7):4631–38. doi: 10.1007/s11033-014-3334-z. [DOI] [PubMed] [Google Scholar]
- 16.Obaidat A, Roth M, Hagenbuch B. The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annu Rev Pharmacol Toxicol. 2012;52:135–51. doi: 10.1146/annurev-pharmtox-010510-100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abe T, Unno M, Onogawa T, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology. 2001;120(7):1689–99. doi: 10.1053/gast.2001.24804. [DOI] [PubMed] [Google Scholar]
- 18.Lee W, Belkhiri A, Lockhart AC, et al. Overexpression of OATP1B3 confers apoptotic resistance in colon cancer. Cancer Res. 2008;68(24):10315–23. doi: 10.1158/0008-5472.CAN-08-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hays A, Apte U, Hagenbuch B. Organic anion transporting polypeptides expressed in pancreatic cancer may serve as potential diagnostic markers and therapeutic targets for early stage adenocarcinomas. Pharm Res. 2013;30(9):2260–69. doi: 10.1007/s11095-012-0962-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wlcek K, Svoboda M, Thalhammer T, et al. Altered expression of organic anion transporter polypeptide (OATP) genes in human breast carcinoma. Cancer Biol Ther. 2008;7(8):1450–55. doi: 10.4161/cbt.7.9.6282. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee N, Allen C, Bendayan R. Differential role of organic anion-transporting polypeptides in estrone-3-sulphate uptake by breast epithelial cells and breast cancer cells. J Pharmacol Exp Ther. 2012;342(2):510–19. doi: 10.1124/jpet.112.192344. [DOI] [PubMed] [Google Scholar]
- 22.Hertz DL, McLeod HL, Irvin WJ. Tamoxifen and CYP2D6: a contradiction of data. Oncologist. 2012;17(5):620–30. doi: 10.1634/theoncologist.2011-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005;5(1):6–13. doi: 10.1038/sj.tpj.6500285. [DOI] [PubMed] [Google Scholar]
- 24.Okishiro M, Taguchi T, Jin Kim S, et al. Genetic polymorphisms of CYP2D6*10 and CYP2C19*2,*3 are not associated with prognosis, endometrial thickness, or bone mineral density in Japanese breast cancer patients treated with adjuvant tamoxifen. Cancer. 2009;115(5):952–61. doi: 10.1002/cncr.24111. [DOI] [PubMed] [Google Scholar]
- 25.Dezentje VO, Guchelaar HJ, Nortier JW, et al. Clinical implications of CYP2D6 genotyping in tamoxifen treatment for breast cancer. Clin Cancer Res. 2009;15(1):15–21. doi: 10.1158/1078-0432.CCR-08-2006. [DOI] [PubMed] [Google Scholar]
- 26.Muto M, Onogawa T, Suzuki T, et al. Human liver-specific organic anion transporter-2 is a potent prognostic factor for human breast carcinoma. Cancer Sci. 2007;98(10):1570–76. doi: 10.1111/j.1349-7006.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Justenhoven C, Schaeffeler E, Winter S, et al. Polymorphisms of the nuclear receptor pregnane X receptor and organic anion transporter polypeptides 1A2, 1B1, 1B3, and 2B1 are not associated with breast cancer risk. Breast Cancer Res Treat. 2011;125(2):563–69. doi: 10.1007/s10549-010-1046-1. [DOI] [PubMed] [Google Scholar]
- 28.Xu LY, He YJ, Zhang W, et al. Organic anion transporting polypeptide-1B1 haplotypes in Chinese patients. Acta Pharmacol Sin. 2007;28(10):1693–97. doi: 10.1111/j.1745-7254.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- 29.Pasanen MK, Neuvonen M, Neuvonen PJ, Niemi M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet Genomics. 2006;16(12):873–79. doi: 10.1097/01.fpc.0000230416.82349.90. [DOI] [PubMed] [Google Scholar]
- 30.Search Collaborative Group. Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy-a genomewide study. N Engl J Med. 2008;359(8):789–99. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 31.Kalliokoski A, Neuvonen M, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics and pharmacodynamics of repaglinide and nateglinide. J Clin Pharmacol. 2008;48(3):311–21. doi: 10.1177/0091270007311569. [DOI] [PubMed] [Google Scholar]
- 32.Kalliokoski A, Neuvonen P, Niemi M. SLCO1B1 polymorphism and oral antidiabetic drugs. Basic Clin Pharmacol Toxicol. 2010;107(4):775–81. doi: 10.1111/j.1742-7843.2010.00581.x. [DOI] [PubMed] [Google Scholar]
- 33.Trevino LR, Shimasaki N, Yang W, et al. Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J Clin Oncol. 2009;27(35):5972–78. doi: 10.1200/JCO.2008.20.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Sun Y, Yao L, et al. Association between CYP2D6 *10 genotype and survival of breast cancer patients receiving tamoxifen treatment. Ann Oncol. 2008;19(18):1423–29. doi: 10.1093/annonc/mdn155. [DOI] [PubMed] [Google Scholar]
- 35.Chamnanphon M, Pechatanan K, Sirachainan E, et al. Association of CYP2D6 and CYP2C19 polymorphisms and disease-free survival of Thai post-menopausal breast cancer patients who received adjuvant tamoxifen. Pharmacogenomics Pers Med. 2013;6:37–48. doi: 10.2147/PGPM.S42330. [DOI] [PMC free article] [PubMed] [Google Scholar]