Abstract
Background
We hypothesized that the American Heart Association's metric of ideal cardiovascular health (CVH) predicts improved long‐term functional status after adjusting for incident stroke and myocardial infarction.
Methods and Results
In the prospective, multiethnic Northern Manhattan Study, stroke‐free individuals in northern Manhattan aged ≥40 years had annual assessments of the primary outcome of functional status with the Barthel index (BI), for a median of 13 years. Ideal CVH was calculated as a composite of 7 measures, each scored on a scale of 0 to 2. Primary predictors were (1) number of ideal CVH metrics, and (2) total score of all CVH metrics. Of 3219 participants, mean age was 69 years (SD 10), 63% were female, 21% were white, 25% were non‐Hispanic black, and 54% were Hispanic. Twenty percent had 0 to 1 ideal CVH metrics, 32% had 2, 30% had 3, 14% had 4, and 4% had 5 to 7. Both number of ideal CVH categories and higher CVH metric scores were associated with higher mean BI scores at 5 and 10 years. 0047 Gradients persisted when results were adjusted for incident stroke and myocardial infarction, when mobility and nonmobility domains of the BI were analyzed separately, and when BI was analyzed dichotomously. At 10 years, in a fully adjusted model, differences in mean BI score were lower for poor versus ideal physical activity (3.48 points, P<0.0001) and fasting glucose (4.58 points, P<0.0001).
Conclusions
Ideal CVH predicts functional status, even after accounting for incident vascular events. Vascular functional impairment is an important outcome that can be reduced by optimizing vascular health.
Keywords: cerebrovascular disorders, epidemiology, stroke
Introduction
Ideal cardiovascular health (CVH) was proposed by the American Heart Association/American Stroke Association in 2010 to identify factors that, considered together, represent a healthy profile associated with longevity without cardiovascular disease.1 Ideal CVH has 7 components: 4 favorable behaviors (nonsmoking, ideal body mass index, physical activity at goal, and dietary patterns that promote cardiovascular health) and 3 favorable factors (ideal levels of total cholesterol, blood pressure, and fasting glucose [FG]). In prior studies, ideal CVH has been associated with reduced vascular and nonvascular mortality2 and nonfatal vascular events,3–4 as well as subclinical disease markers such as arterial stiffness,5 carotid intima media thickness,6 retinal microvascular changes,7 coronary artery calcification,8 and intracranial stenosis.9 Ideal CVH has also been associated with reduced incidence of depression10 and cancer,11 reflecting its potential use to monitor and predict improved health in noncardiovascular diseases that may share common causes with vascular disease.
However, no prior study has examined associations between ideal CVH and disability, which is tightly linked to vascular risk factors and events such as stroke and myocardial infarction (MI). Clinically evident stroke is the leading cause of disability,12 but subclinical infarcts have also been associated with disability and may be as much as 5 times more prevalent as clinically evident strokes.13 Furthermore, white matter disease appears to be caused by vascular risk factors and is associated with functional decline, cognitive impairment, and reduced quality of life.13 Disability is a patient‐centered outcome that may more comprehensively reflect population health than measures of events such as mortality, stroke, or MI.14
In a prior analysis in the stroke‐free cohort of the Northern Manhattan Study,15 there was a mean annual decline of 1.02 points in the Barthel index (BI), and predictors of decline in BI included age, female sex, diabetes, depression, and cholesterol level. In the present study, we modeled the effect of ideal CVH on disability in the Northern Manhattan Study. Although effects of single risk factors on disability have been previously examined, the aggregation of these in the construct of ideal CVH has not yet been studied. This analysis would provide essential population‐based data that is aligned with national goals for health promotion. We hypothesized that progressively improved CVH, measured by the American Heart Association/American Stroke Association's ideal CVH metric, is associated with improved long‐term functional status, even when adjusting for the effect of incident stroke and MI.
Methods
The Northern Manhattan Study, a prospective cohort study of 3298 subjects in a community‐based sample of a racially and ethnically diverse population, was approved by the institutional review boards of Columbia University and the University of Miami, and all participants provided informed consent.
Cohort Selection
Subjects were recruited between 1993 and 200116–17 and were enrolled if they were ≥40 years of age, lived in northern Manhattan for ≥3 months in a household with a telephone, and were stroke‐free. Subjects were contacted by random digit dialing of published and unpublished telephone numbers. The telephone response rate was 91%, 87% of eligible subjects indicated willingness to participate, and enrollment response rate was 75%. Seventy‐nine subjects who were not classified as Hispanic, white, or black were excluded from the present analysis, for a final cohort of 3219 participants.
Baseline Assessment
Baseline examination included comprehensive medical history, physical examination, medical record review, and fasting blood samples. Standardized questions were adapted from the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System. Race‐ethnicity was self‐identified and modeled after the U.S. Census. Smoking was based on self‐reported age of starting and quitting smoking. Education was dichotomized at high school education. Marital status was classified as married versus other. Insurance status was characterized as Medicare/private insurance versus Medicaid/no insurance.18 Diet was assessed through a structured in‐person interview using questions adapted from the National Cancer Institute food frequency questionnaire.19 Alcohol use was defined as low/no (<1 drink/month), moderate (1 drink/month to 2 drinks/day), and heavy (>2 drinks/day). Blood pressure, height, weight, and FG were measured with standard methods as described previously.20–21 Fasting total cholesterol was measured with a Hitachi 705 automated spectrophotometer (Boehringer Mannheim, Mannheim, Germany). Leisure‐time physical activity was measured with a questionnaire based on the National Health Interview Survey.22 The Hamilton Depression Rating Scale measured symptoms of depression; a score of ≥8 signified depression.
Classification of CVH
Following American Heart Association/American Stroke Association definitions,1 7 CVH factors were classified into ideal, intermediate, or poor categories as previously described3 and as outlined in Table 1. CVH was analyzed according to 2 definitions. For “number of ideal CVH metrics,” we classified participants into 5 groups, defined by number of ideal CVH metrics present at baseline (0 to 1, 2, 3, 4, and 5 to 7). We collapsed 0 with 1 and 5 with 6 and 7 ideal metrics because of relatively few subjects who had 0 (2.3% of total cohort) or 6 (0.5% of total cohort; none had 7). For “score of CVH metrics,” 0 was assigned for a category of “poor,” 1 was assigned for “intermediate,” and 2 for “ideal.” The score was calculated by summing values for each of the 7 CVH metrics (possible range 0 to 14).3
Table 1.
Variable Definitions for the 7 Cardiovascular Health Indicators
| Cardiovascular Health Indicator | Poor | Intermediate | Ideal |
|---|---|---|---|
| Smoking | Current | Quit ≤1 year | Never or quit >1 year |
| Body mass index | ≥30 kg/m2 | 25 to <30 kg/m2 | <25 kg/m2 |
| Physical activity | No moderate or vigorous activity | 1 to 149 min/wk moderate intensity, 1 to 74 min/wk vigorous intensity, or equivalent combination | ≥150 min/wk moderate intensity, ≥75 min/wk vigorous intensity, or equivalent combination |
| Diet* | 0 to 1 healthy components | 2 to 3 healthy components | 4 to 5 healthy components |
| Total cholesterol | ≥240 mg/dL | Treated to <200 or 200 to 239 mg/dL | Untreated and <200 mg/dL |
| Blood pressure | ≥140/90 mm Hg | Treated to <120/<80 mm Hg or 120 to 139/80 to 89 mm Hg | Untreated and <120/<80 mm Hg |
| Fasting plasma glucose | ≥126 mg/dL | Treated to <100 or 100 to 125 mg/dL | Untreated and <100 mg/dL |
Based on 5 health dietary metrics: (1) ≥4.5 cups of fruits and vegetables/d, (2) >2 3.5‐oz servings of fish/wk, (3) >3 1‐oz‐equivalent servings of fiber‐rich whole grains/d, (4) <1500 mg sodium/d, and (5) ≤450 kcal sugar‐sweetened beverages/wk).
Prospective Follow‐Up
Subjects were followed annually by telephone, with average annual contact rate of 99%. The telephone interview assessed change in vital status, neurological symptoms and events, hospitalizations, and functional status via the BI. The BI23–24 measures 10 core activities of daily living and the scale ranges from 0 to 100 in 5‐point increments; 100 indicates normal. The BI has several strengths: it has been extensively used in geriatric populations,25–26 stroke observational studies, and clinical trials as a disability measure.27 Previous research has examined the psychometric properties of the scale and has demonstrated the reliability of telephone BI assessments.28 A limitation of the BI is the ceiling effect due to its lack of sensitivity to small deficits in functioning.29 However, it is a robust and well‐accepted measurement of disability.30
Positive screens for potential neurological or cardiac events were followed by in‐person confirmation. Nearly 70% of vascular events lead to hospitalizations at Columbia University Medical Center. We prospectively screened all admissions and discharges. Hospital records were reviewed to classify all outcomes as previously reported.16 Stroke included ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage, but not transient ischemic attack or venous sinus thrombosis. A consensus of stroke neurologists assessed stroke subtype using modified Stroke Data Bank criteria and all available information, as previously described.31 MI was defined by criteria adapted from the Cardiac Arrhythmia Suppression trial32 and the Lipid Research Clinics Coronary Primary Prevention trial33 as previously described,34 and cardiologists adjudicated all MI cases independently.
Statistical Analysis
Mean BI score was calculated for categories of demographic variables, risk factors, number of ideal CVH metrics, and score of ideal CVH metrics. Age‐adjusted mean differences were estimated using linear regression.
For the association of CVH metrics with functional change, the BI was primarily analyzed as a continuous variable. Linear mixed models were used to assess associations of predictor variables with repeated BI measures over time. All available BI measurements were used, and missing values were not imputed. We reported difference in mean BI score based on 2 categories of CVH: (1) number of ideal CVH metrics (reference 0 to 1) and (2) score of CVH metrics (reference 0 to 5). We selected covariates for adjustment that are known confounders of the relationship between vascular risk factors and disability from previous studies in this cohort.15,18 Model 1 adjusted for age, sex, race‐ethnicity, education, health insurance, marital status, number of friends, moderate alcohol drinking, depression, history of MI, coronary artery disease, and peripheral vascular disease, and Model 2 additionally adjusted for incident stroke and MI occurring during follow‐up. The differences in mean BI score at 5 and 10 years of follow‐up were presented separately.
Although the BI measures a single disability construct, it consists of mobility and nonmobility domains, and we examined whether CVH predictor profiles differed for mobility (transfers, mobility, and stair use) and nonmobility (feeding, bathing, grooming, dressing, bowels, bladder, and toilet use) domains.15 We also tested for interactions among time, CVH scores, and race‐ethnicity, as well as interactions among time, CVH scores, and sex.
In secondary analysis, we modeled time to a dichotomous definition of disability (incident BI score of <95 among those with baseline BI ≥95. We selected this cutoff based upon prior research in our cohort.18,35 For each participant, time at risk for disability was computed from date of enrollment to occurrence of incident disability, or the most recent follow‐up date, whichever came first. Cox proportional hazards models were used to estimate the cumulative hazard function, hazard ratio, and 95% CIs after adjusting for the same covariates in Model 1, and additionally with time censored at incident stroke and MI in Model 2. We calculated hazard ratio and 95% CI for each category of number of ideal CVH metrics and score of CVH metrics, as well as a P‐value to test significance of the trend across categories. All models met the proportional hazards assumption.
In supplementary analyses, we calculated mean BI and age‐adjusted mean difference in BI among categories (poor, intermediate, and ideal) of the 7 CVH metrics. Using Models 1 and 2, we also calculated the difference in mean BI for each category, and calculated hazard ratio for dichotomous definitions of the BI, as above.
Regression analyses were also performed to show the combined effects of the numbers of 4 ideal CVH behaviors (smoking, body mass index, physical activity, and diet) and 3 ideal CVH factors (blood pressure, total cholesterol, and FG) on mean BI score (using mixed‐effects models) and disability (using a Poisson model). All data analyses were performed with SAS version 9.2 (SAS Institute Inc, Cary, NC).
Results
Among 3219 participants, median follow‐up was 13 years (interquartile range, 7 to 15 years); there were a total of 37 081 BI assessments (median 13 per participant, interquartile range, 7 to 15). Mean baseline BI score was 97.1. There were significant age‐adjusted mean differences in BI scores between categories of baseline variables including sex, race‐ethnicity, education, and other risk factors. There was a gradient of progressively higher mean BI scores with higher numbers of ideal CVH and higher scores of CVH metrics (Table 2).
Table 2.
BI Score and Sample Characteristics at Baseline
| Characteristics | N (%) | BI Score | Age‐Adjusted Mean Difference (SE) | P Value |
|---|---|---|---|---|
| Mean±SD | ||||
| All | 3219 (100) | 97.1±8.1 | — | — |
| Age at baseline (mean±SD: 69±10), y | ||||
| <70 | 1686 (52) | 98.5±5.1 | Reference | |
| ≥70 | 1533 (48) | 95.6±10.2 | −2.93 (0.28) | <0.0001 |
| Sex | ||||
| Female | 2027 (63) | 96.4±9.1 | Reference | |
| Male | 1192 (37) | 98.4±5.8 | 1.50 (0.29) | <0.0001 |
| Ethnicity | ||||
| Non‐Hispanic white | 690 (21) | 97.1±8.8 | Reference | |
| Non‐Hispanic black | 803 (25) | 96.7±8.4 | −0.85 (0.41) | 0.04 |
| Hispanic | 1726 (54) | 97.4±7.6 | −1.29 (0.37) | 0.0004 |
| High school education or higher | ||||
| No | 1763 (55) | 96.7±8.8 | Reference | |
| Yes | 1456 (45) | 97.7±7.0 | 1.15 (0.28) | <0.0001 |
| Medicaid or uninsured | ||||
| No | 1783 (56) | 98.0±6.3 | Reference | |
| Yes | 1436 (44) | 96.1±9.8 | −2.71 (0.28) | <0.0001 |
| Marital status | ||||
| Other | 2200 (68) | 96.6±9.0 | Reference | |
| Married | 1018 (32) | 98.3±5.5 | 0.99 (0.30) | 0.001 |
| Number of friends | ||||
| <3 | 481 (15) | 94.8±12.6 | Reference | |
| 3+ | 2737 (85) | 97.6±6.9 | 2.37 (0.39) | <0.0001 |
| Moderate alcohol use | ||||
| No | 2160 (67) | 96.5±9.1 | Reference | |
| Yes | 1059 (33) | 98.5±5.0 | 1.49 (0.29) | <0.0001 |
| Depression | ||||
| No | 2890 (90) | 97.6±7.1 | Reference | |
| Yes | 329 (10) | 93.4±13.5 | −4.35 (0.45) | <0.0001 |
| History of coronary artery disease | ||||
| No | 2530 (79) | 97.5±7.7 | Reference | |
| Yes | 689 (21) | 95.7±9.2 | −1.16 (0.34) | 0.0006 |
| History of peripheral vascular disease | ||||
| No | 2711 (84) | 97.5±7.6 | Reference | |
| Yes | 508 (16) | 95.0±9.8 | −2.55 (0.37) | <0.0001 |
| No. of ideal CVH metrics | ||||
| 0 to 1 | 633 (20) | 96.5±8.9 | Reference | |
| 2 | 1037 (32) | 97.0±8.2 | 0.61 (0.39) | 0.12 |
| 3 | 960 (30) | 97.1±8.5 | 0.92 (0.40) | 0.02 |
| 4 | 447 (14) | 98.3±6.1 | 2.26 (0.48) | <0.0001 |
| 5 to 7 | 142 (4) | 98.1±5.1 | 1.56 (0.72) | 0.03 |
| Score of CVH metrics | ||||
| 0 to 5 | 718 (22) | 96.1±9.4 | Reference | |
| 6 | 528 (16) | 96.8±8.3 | 0.91 (0.45) | 0.04 |
| 7 | 623 (19) | 96.8±9.2 | 0.90 (0.43) | 0.03 |
| 8 | 550 (17) | 97.5±7.3 | 1.64 (0.44) | 0.0002 |
| 9 to 13 | 800 (25) | 98.2±5.7 | 2.56 (0.40) | <0.0001 |
BI indicates Barthel index; CVH, cardiovascular health.
Table 3 and Figure 1 show adjusted relationships between number of ideal CVH metrics, scores of CVH metrics, and follow‐up BI scores. There was a gradient of progressively higher total BI scores with progressively higher numbers of ideal CVH metrics, in both Model 1 and Model 2, and the magnitude of difference was higher with 10 years of follow‐up compared to 5 years. For example, in Model 1, adjusted mean BI score at 5 years was 2.26 points higher among individuals with 5 to 7 ideal CVH metrics compared to those with 0 to 1. This difference was 4.21 points at 10 years. There was a similar gradient when the score of CVH metrics was examined; in Model 1, mean BI score at 10 years for a score of 9 to 13 was 3.46 points higher compared to a score of 0 to 5. The gradients persisted when the mobility and nonmobility domains of the BI were analyzed separately.
Table 3.
CVH Status and BI Score at Follow‐Up
| CVH Metrics | Year 5 of Follow‐Up | Year 10 of Follow‐Up | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| Estimate* (SE) | P Value | Estimate* (SE) | P Value | Estimate* (SE) | P Value | Estimate* (SE) | P Value | |
| BI score, total | ||||||||
| No. of ideal CVH metrics | ||||||||
| 0 to 1 vs 2 | −1.26 (0.57) | 0.027 | −0.89 (0.56) | 0.108 | −2.26 (0.59) | 0.0001 | −1.56 (0.58) | 0.007 |
| 0 to 1 vs 3 | −1.60 (0.58) | 0.006 | −0.91 (0.57) | 0.108 | −2.46 (0.60) | <0.0001 | −1.28 (0.59) | 0.030 |
| 0 to 1 vs 4 | −2.24 (0.71) | 0.002 | −1.34 (0.69) | 0.054 | −2.78 (0.73) | 0.0002 | −1.28 (0.72) | 0.074 |
| 0 to 1 vs 5 to 7 | −2.26 (1.06) | 0.033 | −1.24 (1.03) | 0.231 | −4.21 (1.08) | 0.0001 | −2.32 (1.05) | 0.028 |
| Score of CVH metrics | ||||||||
| 0 to 5 vs 6 | −1.10 (0.65) | 0.090 | −0.65 (0.63) | 0.299 | −1.13 (0.67) | 0.092 | −0.45 (0.65) | 0.493 |
| 0 to 5 vs 7 | −1.38 (0.62) | 0.027 | −0.77 (0.61) | 0.205 | −1.98 (0.64) | 0.002 | −0.97 (0.63) | 0.123 |
| 0 to 5 vs 8 | −2.26 (0.64) | 0.0005 | −1.39 (0.63) | 0.027 | −3.18 (0.67) | <0.0001 | −1.66 (0.65) | 0.011 |
| 0 to 5 vs 9 to 13 | −2.86 (0.60) | <0.0001 | −1.80 (0.58) | 0.002 | −3.46 (0.62) | <0.0001 | −1.71 (0.60) | 0.005 |
| BI score, mobility domain | ||||||||
| No. of ideal CVH metrics | ||||||||
| 0 to 1 vs 2 | −0.58 (0.25) | 0.023 | −0.43 (0.25) | 0.086 | −1.11 (0.26) | <0.0001 | −0.82 (0.26) | 0.001 |
| 0 to 1 vs 3 | −0.80 (0.26) | 0.002 | −0.52 (0.25) | 0.040 | −1.31 (0.27) | <0.0001 | −0.83 (0.26) | 0.002 |
| 0 to 1 vs 4 | −1.27 (0.32) | <0.0001 | −0.89 (0.31) | 0.004 | −1.62 (0.33) | <0.0001 | −1.00 (0.32) | 0.002 |
| 0 to 1 vs 5 to 7 | −1.03 (0.47) | 0.028 | −0.61 (0.46) | 0.183 | −2.06 (0.48) | <0.0001 | −1.28 (0.47) | 0.007 |
| Score of CVH metrics | ||||||||
| 0 to 5 vs 6 | −0.40 (0.29) | 0.165 | −0.22 (0.28) | 0.440 | −0.49 (0.30) | 0.104 | −0.20 (0.29) | 0.486 |
| 0 to 5 vs 7 | −0.64 (0.28) | 0.020 | −0.39 (0.27) | 0.147 | −0.84 (0.29) | 0.003 | −0.42 (0.28) | 0.130 |
| 0 to 5 vs 8 | −1.19 (0.29) | <0.0001 | −0.83 (0.28) | 0.003 | −1.60 (0.30) | <0.0001 | −0.97 (0.29) | 0.0008 |
| 0 to 5 vs 9 to 13 | −1.44 (0.27) | <0.0001 | −1.00 (0.26) | 0.0001 | −1.80 (0.28) | <0.0001 | −1.08 (0.27) | <0.0001 |
| BI score, nonmobility domain | ||||||||
| No. of ideal CVH metrics | ||||||||
| 0 to 1 vs 2 | −0.68 (0.34) | 0.044 | −0.46 (0.33) | 0.159 | −1.14 (0.35) | 0.001 | −0.73 (0.34) | 0.034 |
| 0 to 1 vs 3 | −0.79 (0.34) | 0.023 | −0.39 (0.34) | 0.249 | −1.13 (0.36) | 0.002 | −0.45 (0.35) | 0.202 |
| 0 to 1 vs 4 | −0.97 (0.42) | 0.021 | −0.44 (0.41) | 0.281 | −1.15 (0.44) | 0.008 | −0.28 (0.43) | 0.511 |
| 0 to 1 vs 5 to 7 | −1.21 (0.62) | 0.053 | −0.61 (0.61) | 0.315 | −2.12 (0.64) | 0.001 | −1.02 (0.63) | 0.104 |
| Score of CVH metrics | ||||||||
| 0 to 5 vs 6 | −0.69 (0.38) | 0.072 | −0.43 (0.37) | 0.251 | −0.63 (0.40) | 0.113 | −0.23 (0.39) | 0.549 |
| 0 to 5 vs 7 | −0.73 (0.37) | 0.047 | −0.37 (0.36) | 0.297 | −1.13 (0.38) | 0.003 | −0.54 (0.37) | 0.148 |
| 0 to 5 vs 8 | −1.06 (0.38) | 0.005 | −0.55 (0.37) | 0.138 | −1.56 (0.40) | <0.0001 | −0.68 (0.39) | 0.081 |
| 0 to 5 vs 9 to 13 | −1.41 (0.35) | <0.0001 | −0.79 (0.35) | 0.022 | −1.64 (0.37) | <0.0001 | −0.62 (0.36) | 0.084 |
Model 1: adjusted for age, sex, race‐ethnicity, education, health insurance, marital status, number of friends, moderate alcohol drinking, depression, history of coronary artery disease, and history of peripheral vascular disease. Model 2: model 1 additionally adjusted for incident stroke and myocardial infarction at follow‐up. BI indicates Barthel index; CVH, cardiovascular health.
Mean difference.
Figure 1.
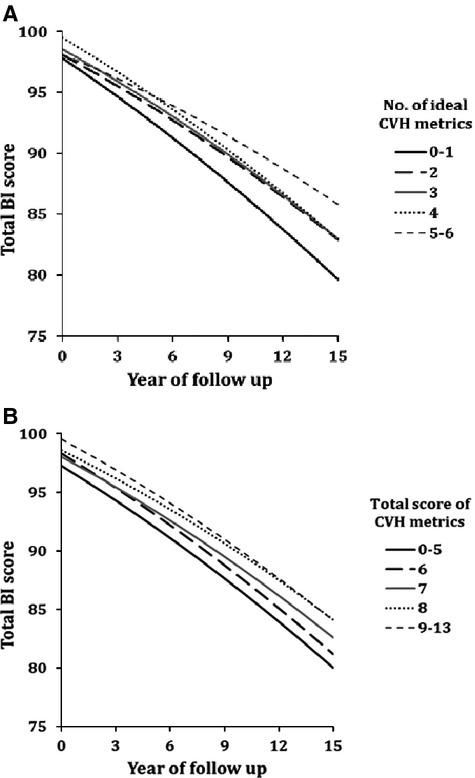
Adjusted mean Barthel index (BI) scores over time by the number of ideal cardiovascular health (CVH) metrics (A) or by total score of CVH metrics (B). Mean BI scores were adjusted for age, sex, race‐ethnicity, education, health insurance, marital status, number of friends, moderate alcohol drinking, depression, history of coronary artery disease, and history of peripheral vascular disease.
These gradients also persisted after adjusting for incident stroke and MI as a time‐varying covariate, although the absolute mean differences were reduced. The effect of incident stroke or MI occurring during follow‐up was to reduce mean BI score by 17.0 points (95% CI −17.7 to −16.3, P<0.0001), for both definitions of CVH. As a comparison, in Model 2, the effect of age on total BI score was −0.55 points per year (95% CI −0.60 to −0.51, P<0.0001) for number of ideal CVH metrics and −0.60 points per year (95% CI −0.65 to −0.56, P<0.0001) for score of CVH metrics.
Table 4 and Figure 2 show associations between CVH metrics and the BI dichotomized at a score of 95. Even with a dichotomous BI definition, a gradient remained; increasing numbers of ideal CVH metrics, and higher scores of CVH metrics, were associated with reduced incidence of disability.
Table 4.
CVH Status at Baseline and Disability at Follow‐Up
| CVH Metrics | BI Score <95 at Follow‐Up | |||
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| No. of ideal CVH metrics | ||||
| 0 to 1 | Reference | Reference | ||
| 2 | 0.83 (0.72 to 0.94) | 0.005 | 0.86 (0.75 to 0.99) | 0.047 |
| 3 | 0.77 (0.67 to 0.89) | 0.0002 | 0.82 (0.71 to 0.95) | 0.010 |
| 4 | 0.67 (0.57 to 0.80) | <0.0001 | 0.69 (0.58 to 0.83) | <0.0001 |
| 5 to 7 | 0.55 (0.42 to 0.73) | <0.0001 | 0.59 (0.44 to 0.80) | 0.0005 |
| Trend | <0.0001 | <0.0001 | ||
| Score of CVH metrics | ||||
| 0 to 5 | Reference | Reference | ||
| 6 | 0.96 (0.82 to 1.12) | 0.578 | 1.01 (0.86 to 1.19) | 0.871 |
| 7 | 0.79 (0.68 to 0.92) | 0.002 | 0.83 (0.71 to 0.97) | 0.022 |
| 8 | 0.71 (0.61 to 0.83) | <0.0001 | 0.72 (0.63 to 0.88) | 0.0006 |
| 9 to 13 | 0.65 (0.57 to 0.76) | <0.0001 | 0.69 (0.59 to 0.81) | <0.0001 |
| Trend | <0.0001 | <0.0001 | ||
Model 1: adjusted for age, sex, race‐ethnicity, education, health insurance, marital status, number of friends, moderate alcohol drinking, depression, history of coronary artery disease, and history of peripheral vascular disease. Model 2: model 1 but censored at incident stroke and myocardial infarction at follow‐up. BI indicates Barthel index; CVH, cardiovascular health; HR, hazard ratio.
Figure 2.
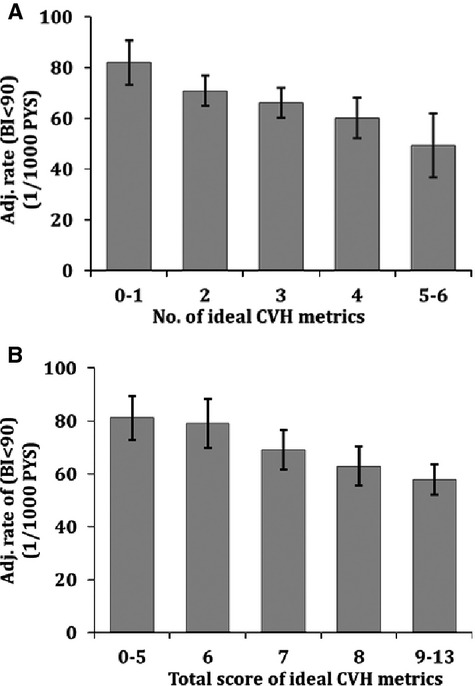
Adjusted incidence rates of BI<95 by the number of ideal health metrics (A) and by total score of CVH metrics for BI<95 (B). Incidence rates were adjusted for age, sex, race‐ethnicity, education, health insurance, marital status, number of friends, moderate alcohol drinking, depression, history of coronary artery disease, and history of peripheral vascular disease. BI indicates Barthel index; CVH, cardiovascular health; PYS, person years. Errors bars are significant at P < 0.05.
Table 5 shows mean BI scores by categories of the 7 CVH metrics. There were significant mean differences among categories of body mass index (1.62 points higher score for ideal versus poor body mass index), physical activity (2.80 points higher score for ideal versus poor activity), FG (1.01 points higher score for ideal versus poor glucose), and total cholesterol (0.81 points lower score for ideal versus poor cholesterol). Table 6 shows adjusted mean BI scores by categories of the 7 CVH metrics, at 5 and 10 years. At 10 years, in a fully adjusted model, there were significant and large differences in mean BI score for poor versus ideal physical activity (3.37 points lower, P<0.0001) and FG (4.57 points lower, P<0.0001). Figure 3 demonstrates a gradient in incidence rates of disability stratified by number of ideal health factors and behaviors. There were significant interactions among CVH scores, time, and race‐ethnicity (all interaction P‐values <0.0015). Overall, there were significant, increasing magnitudes of effect with higher numbers of ideal CVH and higher scores of CVH metrics among non‐Hispanic whites, Hispanics, and women, but less definite gradients among non‐Hispanic blacks and men (Table 7).
Table 5.
BI Score by CVH Metrics at Baseline
| Characteristics | N (%) | BI Score | Age‐Adjusted Mean Difference (SE) | P Value |
|---|---|---|---|---|
| Mean±SD | ||||
| Smoking | ||||
| Poor | 556 (17) | 98.3±4.8 | Reference | |
| Intermediate | 67 (2) | 97.7±7.5 | −0.51 (1.01) | 0.61 |
| Ideal | 2587 (81) | 96.9±8.6 | −0.58 (0.37) | 0.11 |
| Body mass index | ||||
| Poor | 882 (28) | 96.6±8.5 | Reference | |
| Intermediate | 1346 (42) | 97.6±7.4 | 1.48 (0.33) | <0.0001 |
| Ideal | 974 (30) | 97.2±8.1 | 1.62 (0.36) | <0.0001 |
| Physical activity | ||||
| Poor | 1358 (42) | 95.7±10.5 | Reference | |
| Intermediate | 794 (25) | 97.7±6.2 | 2.03 (0.34) | <0.0001 |
| Ideal | 1067 (33) | 98.5±4.8 | 2.80 (0.32) | <0.0001 |
| Diet | ||||
| Poor | 2151 (75) | 97.3±7.7 | Reference | |
| Intermediate | 718 (25) | 97.2±7.6 | 0.07 (0.32) | 0.82 |
| Ideal | 11 (0) | 97.3±5.2 | 0.12 (2.24) | 0.96 |
| Blood pressure | ||||
| Poor | 1211 (38) | 97.0±7.9 | Reference | |
| Intermediate | 1804 (56) | 97.2±8.2 | −0.07 (0.29) | 0.82 |
| Ideal | 189 (6) | 97.3±7.9 | −0.48 (0.61) | 0.43 |
| Fasting glucose | ||||
| Poor | 505 (16) | 96.6±8.2 | Reference | |
| Intermediate | 653 (21) | 97.0±8.6 | 0.64 (0.44) | 0.15 |
| Ideal | 1912 (62) | 97.6±7.3 | 1.01 (0.37) | 0.007 |
| Total cholesterol | ||||
| Poor | 525 (17) | 97.9±6.7 | Reference | |
| Intermediate | 1289 (42) | 97.3±7.1 | −0.50 (0.38) | 0.19 |
| Ideal | 1284 (41) | 97.2±8.1 | −0.81 (0.38) | 0.03 |
BI indicates Barthel index; CVH, cardiovascular health.
Table 6.
CVH Metrics and Total BI Score at Follow‐Up
| CVH Metrics | Year 5 of Follow‐Up | Year 10 of Follow‐Up | ||
|---|---|---|---|---|
| Estimate* (SE) | P Value | Estimate* (SE) | P Value | |
| Smoking | ||||
| Poor vs intermediate | −0.32 (1.46) | 0.827 | −0.42 (1.52) | 0.784 |
| Poor vs ideal | −0.64 (0.54) | 0.242 | 1.27 (0.56) | 0.025 |
| Body mass index | ||||
| Poor vs intermediate | −1.32 (0.49) | 0.007 | −1.60 (0.50) | 0.002 |
| Poor vs ideal | −1.28 (0.54) | 0.018 | −0.72 (0.56) | 0.197 |
| Physical activity | ||||
| Poor vs intermediate | −1.30 (0.50) | 0.010 | −0.97 (0.52) | 0.064 |
| Poor vs ideal | −2.91 (0.47) | <0.0001 | −3.37 (0.49) | <0.0001 |
| Diet | ||||
| Poor vs intermediate | −0.50 (0.46) | 0.280 | 0.90 (0.48) | 0.060 |
| Poor vs ideal | −2.77 (3.20) | 0.386 | −2.03 (3.33) | 0.542 |
| Blood pressure | ||||
| Poor vs intermediate | −0.15 (0.42) | 0.714 | −1.11 (0.44) | 0.011 |
| Poor vs ideal | 1.44 (0.90) | 0.108 | −0.72 (0.92) | 0.436 |
| Fasting glucose | ||||
| Poor vs intermediate | −1.75 (0.67) | 0.009 | −3.61 (0.70) | <0.0001 |
| Poor vs ideal | −2.35 (0.57) | <0.0001 | −4.57 (0.59) | <0.0001 |
| Total cholesterol | ||||
| Poor vs intermediate | 0.02 (0.57) | 0.973 | −0.61 (0.59) | 0.297 |
| Poor vs ideal | 1.27 (0.59) | 0.030 | 0.31 (0.60) | 0.612 |
BI indicates Barthel index; CVH, cardiovascular health.
Mean difference was adjusted for age, sex, race‐ethnicity, education, health insurance, marital status, number of friends, moderate alcohol drinking, depression, history of coronary artery disease, and history of peripheral vascular disease.
Figure 3.
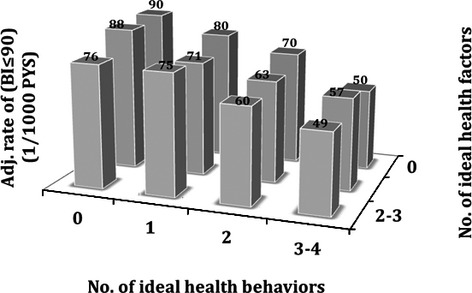
Adjusted incidence rates of Barthel index (BI)<95 by the numbers of ideal health behaviors (smoking, obesity, physical activity, and diet) and health factors (blood pressure, cholesterol, and glucose). Incidence rates were adjusted for age, sex, race‐ethnicity, education, health insurance, marital status, number of friends, moderate alcohol drinking, depression, history of coronary artery disease, and history of peripheral vascular disease.
Table 7.
CVH Status and Total BI Score at Follow‐Up by Race‐Ethnicity and Sex
| CVH Metrics | NH‐White | NH‐Black | Hispanic | Men | Women | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate* (SE) | P Value | Estimate* (SE) | P Value | Estimate* (SE) | P Value | Estimate* (SE) | P Value | Estimate* (SE) | P Value | |
| Year 5 of follow‐up | ||||||||||
| No. of ideal CVH metrics | ||||||||||
| 0 to 1 vs 2 | −2.09 (1.43) | 0.145 | −0.72 (1.29) | 0.573 | −1.22 (0.70) | 0.083 | −1.01 (0.81) | 0.216 | −1.54 (0.76) | 0.043 |
| 0 to 1 vs 3 | −4.90 (1.39) | 0.0004 | −1.74 (1.31) | 0.185 | −0.16 (0.73) | 0.832 | −1.18 (0.82) | 0.148 | −1.95 (0.79) | 0.013 |
| 0 to 1 vs 4 | −3.76 (1.50) | 0.012 | 0.13 (1.59) | 0.937 | −2.67 (0.97) | 0.006 | −1.20 (0.93) | 0.198 | −2.68 (1.00) | 0.008 |
| 0 to 1 vs 5 to 7 | −4.71 (1.96) | 0.016 | −0.73 (2.38) | 0.759 | −1.42 (1.53) | 0.355 | −1.67 (1.25) | 0.182 | −2.91 (1.65) | 0.078 |
| Score of CVH metrics | ||||||||||
| 0 to 5 vs 6 | −0.41 (1.65) | 0.805 | −0.47 (1.48) | 0.751 | −1.50 (0.79) | 0.058 | −2.23 (0.95) | 0.019 | −0.79 (0.85) | 0.355 |
| 0 to 5 vs 7 | −3.56 (1.54) | 0.021 | −1.72 (1.40) | 0.219 | −0.58 (0.77) | 0.455 | −1.75 (0.86) | 0.042 | −1.72 (0.84) | 0.042 |
| 0 to 5 vs 8 | −4.23 (1.55) | 0.006 | −0.85 (1.46) | 0.563 | −2.24 (0.81) | 0.006 | −3.17 (0.88) | 0.0003 | −2.13 (0.88) | 0.016 |
| 0 to 5 vs 9 to 13 | −4.76 (1.35) | 0.0004 | −1.53 (1.32) | 0.246 | −2.56 (0.79) | 0.001 | −2.83 (0.81) | 0.0005 | −3.03 (0.82) | 0.0002 |
| Year 10 of follow‐up | ||||||||||
| No. of ideal CVH metrics | ||||||||||
| 0 to 1 vs 2 | −5.10 (1.51) | 0.0007 | −1.68 (1.36) | 0.218 | −1.73 (0.72) | 0.017 | −2.03 (0.86) | 0.018 | −2.48 (0.79) | 0.002 |
| 0 to 1 vs 3 | −7.03 (1.46) | <0.0001 | −1.10 (1.38) | 0.427 | −1.34 (0.76) | 0.075 | −2.61 (0.86) | 0.002 | −2.26 (0.81) | 0.006 |
| 0 to 1 vs 4 | −3.78 (1.58) | 0.017 | −1.40 (1.68) | 0.403 | −3.56 (0.98) | 0.0003 | −0.91 (0.98) | 0.351 | −3.21 (1.03) | 0.002 |
| 0 to 1 vs 5 to 7 | −7.05 (2.03) | 0.0005 | −3.01 (2.43) | 0.215 | −3.76 (1.57) | 0.016 | −3.01 (1.29) | 0.020 | −4.14 (1.68) | 0.014 |
| Score of CVH metrics | ||||||||||
| 0 to 5 vs 6 | −1.69 (1.75) | 0.334 | 0.42 (1.57) | 0.790 | −1.51 (0.81) | 0.064 | −3.80 (1.01) | 0.0002 | −0.21 (0.88) | 0.807 |
| 0 to 5 vs 7 | −4.49 (1.63) | 0.006 | −1.29 (1.48) | 0.383 | −1.51 (0.79) | 0.058 | −3.50 (0.91) | 0.0001 | −1.30 (0.87) | 0.136 |
| 0 to 5 vs 8 | −4.48 (1.64) | 0.006 | −0.22 (1.53) | 0.886 | −3.98 (0.83) | <0.0001 | −5.07 (0.93) | <0.0001 | −2.24 (0.91) | 0.014 |
| 0 to 5 vs 9 to 13 | −5.10 (1.43) | 0.0003 | −1.48 (1.38) | 0.285 | −3.84 (0.81) | <0.0001 | −3.41 (0.86) | <0.0001 | −3.18 (0.85) | 0.0002 |
BI indicates Barthel index; CVH, cardiovascular health; NH, non‐Hispanic.
Mean difference, adjusted for age, education, health insurance, marital status, number of friends, moderate alcohol drinking, depression, history of coronary artery disease, history of peripheral vascular disease, and sex and race‐ethnicity if applicable.
Discussion
In this large, urban, multiethnic population‐based cohort study with long‐term follow‐up, we found a gradient of improved function with increasing numbers of ideal CVH metrics and higher CVH metric scores. This gradient was seen in fully adjusted models and was sustained even after adjusting for stroke and MI occurring during follow‐up, although the absolute values of the differences were reduced. This suggests that even when accounting for the predominant vascular events that cause reduced function, CVH predicts long‐term disability. CVH was associated with both mobility and nonmobility BI domains, suggesting an effect not only on gross motor function but also fine motor and cognitive function. In a fully adjusted model, the mean BI score at 10 years of follow‐up was 4.21 points higher among individuals with 5 to 7 ideal CVH metrics compared to those with 0 to 1. This mean difference is approximately equivalent to 1 level of function on the BI; for example, a shift from needing help with stairs to being independent, or from being dependent in bathing to being independent. Put another way, this magnitude of difference is approximately equivalent to the effect on function of being 7 years younger. When stroke and MI were adjusted for, the magnitude of difference was reduced but still significant at 2.32 points. For an individual, this represents about half of 1 level of function on the BI, or the equivalent of being 3 to 4 years younger. Such a magnitude, although potentially small for an individual, would translate to a large effect on disability in the entire population. Even with a dichotomous definition of the BI,18 there remained a gradient such that increasing numbers of ideal CVH metrics, and higher scores of CVH metrics, were associated with improved functional status. Specifically, those with 5 to 7 ideal CVH metrics were approximately twice as likely to be independent over time as those with 0 to 1 metrics.
The strong gradient of function and predictive ability of the ideal CVH metric suggest that vascular functional impairment may be a useful construct. Analogous to the concept of vascular cognitive impairment, vascular functional impairment posits that vascular risk factors, which are encapsulated in the ideal CVH metric, lead to cerebrovascular disease, both clinical and subclinical. The effects of clinical cerebrovascular disease on disability have been well studied. Subclinical effects of vascular risk factors include subclinical infarcts,13,36 subclinical cardiac disease, white matter disease,37–40 and vascular dysfunction,41 which may cause cognitive impairment, gait disorders, parkinsonism, and incontinence, and which have other direct effects on function. These effects may become apparent even before clinical events are appreciated and hence would be independent of vascular events such as clinical stroke and MI. Also, the pathways causing vascular functional impairment would likely be independent of arthritis and pain, 2 major causes of disability that were not systematically measured in this study. However, future research would clarify the relationships between vascular risk factors and these nonvascular causes of disability.
Although the original definition of ideal CVH1 was the simultaneous presence of all 7 ideal CVH metrics, this rarely occurs;2,8,42 in the Northern Manhattan Study, no subject had all 7 ideal metrics and only 0.5% of the cohort had 6. Hence, ideal CVH may be considered an ideal that is currently rarely attained, although the hope is that it becomes more prevalent over time. Hence, to operationalize the construct of ideal CVH and analyze its predictive utility, we used 2 definitions. The first was a sum score of the number of ideal CVH metrics for each individual, which has been used in previous research in this cohort and others.3,11 The second definition was a sum score of an individual's scores on each CVH metric, with 0 assigned to the poor category, 1 to intermediate, and 2 to ideal.3 With both of these definitions, we found a gradient in functional outcomes such that higher scores on each metric were associated with improved functional ability. This suggests that the recognition not only of ideal CVH status but also poor and intermediate status is informative for functional ability as well as vascular events, as seen in previous research.3
In secondary analyses, we found significant differences in function among categories of each CVH metric. Specifically, the mean BI score for poor physical activity was 3.37 points lower compared to ideal physical activity, and the mean BI score was 4.57 points higher with ideal FG levels compared to poor. These findings confirm and extend prior research in this cohort, which found a strong effect of diabetes on physical function, even when censoring vascular events such as stroke and MI.15 Also, increased physical activity has been shown to promote functional status.43 We also found significant race‐ethnic and sex‐related differences in associations between ideal CVH metrics and disability, with a clearer significant gradient seen among non‐Hispanic whites, Hispanics, and women, but not among non‐Hispanic blacks and men. It is possible that vascular disease, as represented by the ideal CVH metrics, is a predominant cause of disability among non‐Hispanic whites, Hispanics, and women, while other nonvascular causes of decreased function play a larger role among non‐Hispanic blacks and men.
Strengths of this study include large sample size, long‐term follow‐up, minimal loss to follow‐up, annual functional status assessments with a validated activities of daily living measure, and representation of an urban, multiethnic underlying population. Limitations include lack of information about conditions such as arthritis and pain that have an impact on functional status. Also, CVH measures were assessed at study entry, and we do not have data in the entire cohort on status of CVH metrics during follow‐up. However, the purpose of this study was to determine the predictive ability of the ideal CVH construct to assess long‐term disability, and for this baseline measurements of CVH status are most relevant.
In conclusion, we found a gradient of improved functional status with improved CVH, as measured by the American Heart Association/American Stroke Association's 7 ideal CVH metrics. The fact that this gradient was maintained even after accounting for incident stroke and MI suggests that vascular functional impairment may cause a significant proportion of disability in a population, independent of clinical events. Vascular functional impairment is a patient‐centered outcome whose impact may be reduced by optimizing cardiovascular health. Further study will refine this concept, and future interventions to improve ideal CVH would likely have an impact not only on vascular events but also disability.
Sources of Funding
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (R01 NS48134, Elkind; R37 29993, Sacco/Elkind; K23NS079422, Dhamoon).
Disclosures
None.
References
- 1.Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010; 121:586-613. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Chi HJ, Cui LF, Yang XC, Wu YT, Huang Z, Zhao HY, Gao JS, Wu SL, Cai J. The ideal cardiovascular health metrics associated inversely with mortality from all causes and from cardiovascular diseases among adults in a Northern Chinese industrial city. PLoS One. 2014; 9:e89161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2012; 125:2975-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q, Zhou Y, Gao X, Wang C, Zhang S, Wang A, Li N, Bian L, Wu J, Jia Q, Wu S, Zhao X. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke. 2013; 44:2451-2456. [DOI] [PubMed] [Google Scholar]
- 5.Aatola H, Hutri‐Kahonen N, Juonala M, Laitinen TT, Pahkala K, Mikkila V, Telama R, Koivistoinen T, Lehtimaki T, Viikari JS, Raitakari OT, Kahonen M. Prospective relationship of change in ideal cardiovascular health status and arterial stiffness: the Cardiovascular Risk in Young Finns Study. J Am Heart Assoc. 2014; 3:e00053210.1161/JAHA.113.000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulshreshtha A, Goyal A, Veledar E, McClellan W, Judd S, Eufinger SC, Bremner JD, Goldberg J, Vaccarino V. Association between ideal cardiovascular health and carotid intima‐media thickness: a twin study. J Am Heart Assoc. 2014; 3:e00028210.1161/JAHA.113.000282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogagarue ER, Lutsey PL, Klein R, Klein BE, Folsom AR. Association of ideal cardiovascular health metrics and retinal microvascular findings: the Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2013; 2:e00043010.1161/JAHA.113.000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alman AC, Maahs DM, Rewers MJ, Snell‐Bergeon JK. Ideal cardiovascular health and the prevalence and progression of coronary artery calcification in adults with and without type 1 diabetes. Diabetes Care. 2014; 37:521-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q, Zhang S, Wang C, Gao X, Zhou Y, Zhou H, Wang A, Wu J, Bian L, Wu S, Zhao X. Ideal cardiovascular health metrics on the prevalence of asymptomatic intracranial artery stenosis: a cross‐sectional study. PLoS One. 2013; 8:e58923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espana‐Romero V, Artero EG, Lee DC, Sui X, Baruth M, Ruiz JR, Pate RR, Blair SN. A prospective study of ideal cardiovascular health and depressive symptoms. Psychosomatics. 2013; 54:525-535. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen‐Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk in Communities Study. Circulation. 2013; 127:1270-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014; 129:e28-e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007; 6:611-619. [DOI] [PubMed] [Google Scholar]
- 14.Murray CJ, Lopez AD. On the comparable quantification of health risks: lessons from the Global Burden of Disease Study. Epidemiology. 1999; 10:594-605. [PubMed] [Google Scholar]
- 15.Dhamoon MS, Moon YP, Paik MC, Sacco RL, Elkind MS. Diabetes predicts long‐term disability in an elderly urban cohort: the Northern Manhattan Study. Ann Epidemiol. 2014; 24:362-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacco RL, Anand K, Lee HS, Boden‐Albala B, Stabler S, Allen R, Paik MC. Homocysteine and the risk of ischemic stroke in a triethnic cohort: the Northern Manhattan Study. Stroke. 2004; 35:2263-2269. [DOI] [PubMed] [Google Scholar]
- 17.Elkind MS, Cheng J, Boden‐Albala B, Paik MC, Sacco RL. Elevated white blood cell count and carotid plaque thickness: the Northern Manhattan Stroke Study. Stroke. 2001; 32:842-849. [DOI] [PubMed] [Google Scholar]
- 18.Dhamoon MS, Moon YP, Paik MC, Boden‐Albala B, Rundek T, Sacco RL, Elkind MS. Long‐term functional recovery after first ischemic stroke: the Northern Manhattan Study. Stroke. 2009; 40:2805-2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boden‐Albala B, Elkind MS, White H, Szumski A, Paik MC, Sacco RL. Dietary total fat intake and ischemic stroke risk: the Northern Manhattan Study. Neuroepidemiology. 2009; 32:296-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bibbins‐Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007; 357:2371-2379. [DOI] [PubMed] [Google Scholar]
- 21.Ford ES, Capewell S. Proportion of the decline in cardiovascular mortality disease due to prevention versus treatment: public health versus clinical care. Annu Rev Public Health. 2011; 32:5-22. [DOI] [PubMed] [Google Scholar]
- 22.Moss AJ, Parsons VL. Current estimates from the National Health Interview Survey. United States, 1985. Vital Health Stat 10. 1986; 160:i-iv. [PubMed] [Google Scholar]
- 23.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965; 14:61-65. [PubMed] [Google Scholar]
- 24.Granger CV, Dewis LS, Peters NC, Sherwood CC, Barrett JE. Stroke rehabilitation: analysis of repeated Barthel index measures. Arch Phys Med Rehabil. 1979; 60:14-17. [PubMed] [Google Scholar]
- 25.de Morton NA, Keating JL, Davidson M. Rasch analysis of the Barthel index in the assessment of hospitalized older patients after admission for an acute medical condition. Arch Phys Med Rehabil. 2008; 89:641-647. [DOI] [PubMed] [Google Scholar]
- 26.Richards SH, Peters TJ, Coast J, Gunnell DJ, Darlow MA, Pounsford J. Inter‐rater reliability of the Barthel ADL index: how does a researcher compare to a nurse? Clin Rehabil. 2000; 14:72-78. [DOI] [PubMed] [Google Scholar]
- 27.Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999; 30:1538-1541. [DOI] [PubMed] [Google Scholar]
- 28.Shinar D, Gross CR, Bronstein KS, Licata‐Gehr EE, Eden DT, Cabrera AR, Fishman IG, Roth AA, Barwick JA, Kunitz SC. Reliability of the activities of daily living scale and its use in telephone interview. Arch Phys Med Rehabil. 1987; 68:723-728. [PubMed] [Google Scholar]
- 29.Dromerick AW, Edwards DF, Diringer MN. Sensitivity to changes in disability after stroke: a comparison of four scales useful in clinical trials. J Rehabil Res Dev. 2003; 40:1-8. [DOI] [PubMed] [Google Scholar]
- 30.Saver JL. Optimal end points for acute stroke therapy trials: best ways to measure treatment effects of drugs and devices. Stroke. 2011; 42:2356-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gan R, Sacco RL, Kargman DE, Roberts JK, Boden‐Albala B, Gu Q. Testing the validity of the lacunar hypothesis: the Northern Manhattan Stroke Study experience. Neurology. 1997; 48:1204-1211. [DOI] [PubMed] [Google Scholar]
- 32.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias‐Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, Huther ML, Richardson DWthe CAST Investigators. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991; 324:781-788. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer EJ, Lamon‐Fava S, Jenner JL, McNamara JR, Ordovas JM, Davis CE, Abolafia JM, Lippel K, Levy RI. Lipoprotein(a) levels and risk of coronary heart disease in men. The Lipid Research Clinics Coronary Primary Prevention Trial. JAMA. 1994; 271:999-1003. [DOI] [PubMed] [Google Scholar]
- 34.Dhamoon MS, Tai W, Boden‐Albala B, Rundek T, Paik MC, Sacco RL, Elkind MS. Risk of myocardial infarction or vascular death after first ischemic stroke: the Northern Manhattan Study. Stroke. 2007; 38:1752-1758. [DOI] [PubMed] [Google Scholar]
- 35.Willey JZ, Disla N, Moon YP, Paik MC, Sacco RL, Boden‐Albala B, Elkind MS, Wright CB. Early depressed mood after stroke predicts long‐term disability: the Northern Manhattan Stroke Study (NOMASS). Stroke. 2010; 41:1896-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high‐functioning older adults. J Am Geriatr Soc. 2005; 53:649-654. [DOI] [PubMed] [Google Scholar]
- 37.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, Manolio TA, Lefkowitz D, Jungreis C, Hirsch CH, O'Leary DH, Furberg CD. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005; 36:56-61. [DOI] [PubMed] [Google Scholar]
- 38.Baune BT, Schmidt WP, Roesler A, Berger K. Functional consequences of subcortical white matter lesions and MRI‐defined brain infarct in an elderly general population. J Geriatr Psychiatry Neurol. 2009; 22:266-273. [DOI] [PubMed] [Google Scholar]
- 39.Pohjasvaara TI, Jokinen H, Ylikoski R, Kalska H, Mantyla R, Kaste M, Erkinjuntti T. White matter lesions are related to impaired instrumental activities of daily living poststroke. J Stroke Cerebrovasc Dis. 2007; 16:251-258. [DOI] [PubMed] [Google Scholar]
- 40.Wolfson L, Wei X, Hall CB, Panzer V, Wakefield D, Benson RR, Schmidt JA, Warfield SK, Guttmann CR. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci. 2005; 232:23-27. [DOI] [PubMed] [Google Scholar]
- 41.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010; 67:181-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011; 57:1690-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah RC, Buchman AS, Leurgans S, Boyle PA, Bennett DA. Association of total daily physical activity with disability in community‐dwelling older persons: a prospective cohort study. BMC Geriatr. 2012; 12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]


