Abstract
Background
Rehospitalizations for acute coronary syndromes (ACS) and coronary revascularization after an acute myocardial infarction (AMI) are not only common and costly but can also impact patients’ quality of life. In contrast to mortality and all‐cause readmissions, little insight is available into risk factors associated with ACS and revascularization after AMI.
Methods and Results
In a multicenter AMI registry, we examined the rates and predictors of rehospitalizations for ACS and revascularization within the year after AMI among 3283 patients. Staged revascularization procedures were excluded. Kaplan–Meier estimated rates of rehospitalization due to ACS and revascularization were 6.8% and 4.1%, respectively. In hierarchical, multivariable models, the strongest predictors of rehospitalization for ACS were coronary artery bypass graft prior to AMI hospitalization (hazard ratio [HR] 2.12, 95% CI 1.45 to 3.10), female sex (HR 1.67, 95% CI 1.23 to 2.25), and in‐hospital PCI (HR 1.85, 95% CI 1.28 to 2.69). The strongest predictors of subsequent revascularization were multivessel disease (HR 2.89, 95% CI 1.90 to 4.39) and in‐hospital percutaneous coronary intervention with a bare metal stent (HR 2.08, 95% CI 1.19 to 3.63). The Global Registry of Acute Coronary Events mortality risk score was not associated with the risk of rehospitalization for ACS or revascularization.
Conclusions
Unique characteristics are associated with admissions for ACS and revascularization, as compared with survival. These multivariable risk predictors may help identify patients at high risk for ACS and revascularization, in whom intensification of secondary prevention therapies or closer post‐AMI follow‐up may be warranted.
Keywords: myocardial infarction, rehospitalization, revascularization, unstable angina
Introduction
While the mortality associated with an acute myocardial infarction (AMI) has been steadily declining,1 this trend has been accompanied by a growing need to better manage patients’ postdischarge and chronic care after an AMI. In particular, rehospitalizations for acute coronary syndromes (ACS) and coronary revascularization continue to occur commonly after an AMI,2 impacting patients’ quality of life and increasing healthcare costs. Although several risk models have identified clinical factors associated with higher risk of mortality,3–4 all‐cause rehospitalization,5 or a variety of composite clinical end points,6 there are no studies, which we are aware of, to describe the risk factors associated specifically with ACS rehospitalization or coronary revascularization after AMI. Analyses examining predictors of composite events, such as major adverse cardiac events, are reasonable if the individual end points of that composite have similar predictors. However, if the risk factors for different facets of the combined outcome are different, then different interventions may be necessary to prevent their occurrence. As such, more insight into the risk factors for ACS and coronary revascularization is needed (in particular, if these vary from the risk factors for mortality after AMI).
To address this gap in knowledge, we examined patients from a prospective multicenter registry of AMI patients in whom validated hospitalizations for ACS and coronary revascularization procedures over the year after discharge were collected. These analyses could lay the foundation for better transitional care in high‐risk patients and help identify a cohort of patients at high risk for ACS or revascularization to be studied in future clinical trials seeking to improve these outcomes.
Methods
Study Population and Protocol
The study design, patient selection, site characteristics, and follow‐up assessments of the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status (TRIUMPH) study have been previously published.7 Briefly, consecutive patients with AMI admitted to 24 US hospitals were screened for enrollment into the TRIUMPH registry between April 2005 and December 2008. Eligible patients were required to have biomarker evidence of myocardial necrosis and additional clinical evidence supporting the diagnosis of an AMI, including ischemic signs/symptoms or electrocardiographic ST changes during the initial 24 hours of admission. Eligible patients were also required to either initially present to an enrolling institution or be transferred to that hospital within 24 hours of presentation.
Baseline sociodemographic and clinical data were obtained through chart abstraction and a detailed structured interview within 24 to 72 hours after admission. As part of study enrollment, patients were asked to provide permission for TRIUMPH study personnel to obtain and adjudicate medical records from any subsequent hospitalizations that took place in the year following their AMI. Only patients who provided consent for medical record collection were included in these analyses. Each participating hospital obtained Institutional Research Board approval, and all patients provided written informed consent.
Rehospitalization Data
Detailed follow‐up interviews were attempted on all survivors at 1, 6, and 12 months after AMI. All patients were asked to report interval events (eg, procedures, diagnostic tests, hospitalizations, and outpatient visits) since their last study contact. If a patient reported being hospitalized since the previous interview, records of that hospitalization were obtained to adjudicate cardiovascular events, including ACS (ie, ST‐elevation and non‐ST‐elevation AMI and unstable angina [UA]) and revascularization procedures. Rehospitalization for AMI was defined in the same manner as per the incident event in TRIUMPH, as described above. UA was defined, per guidelines, as a hospitalization due to symptoms suggestive of ischemia that occurred at rest, was of new onset, or was increasing in severity (ie, more frequent, longer in duration, or lower in threshold).8 Revascularization procedures included percutaneous coronary interventions (PCI) and coronary artery bypass graft (CABG) surgeries that were performed for elective, urgent, or emergent indications over the year following AMI. To assure we were not just examining predictors of recurrent AMI, revascularizations in the setting of a repeat AMI were excluded. In addition, to exclude revascularizations that were planned during the index AMI, staged PCI (determined through adjudication) and elective CABGs within the 4 weeks of AMI hospitalization were excluded. Chart abstractions were sent to 2 cardiologists who independently classified the reason for hospitalization. If there was disagreement between the 2 cardiologists, the record was adjudicated by a third senior cardiologist and, if disagreement persisted, up to 5 cardiologists independently reviewed the charts until consensus was obtained. To ensure the validity of the ACS and revascularization hospitalizations, patients who reported hospitalizations for which their data were not available for adjudication were excluded.
Statistical Analysis
We first examined the unadjusted incidence of ACS and revascularization hospitalizations with Kaplan–Meier curves. To examine for potential biases due to attrition from mortality, we considered death as a competing event to compare the cumulative incidence rates with the Kaplan–Meier estimated rate of rehospitalization. We then constructed a series of multivariable Cox regression models (1) to identify whether the predictors of mortality differed from the predictors of ACS and revascularization hospitalizations; and (2) to identify specific predictors of ACS and revascularization. All models were adjusted for site using stratified proportional hazards models that allowed for site‐specific baseline hazards.
To examine whether the predictors of mortality differed from the predictors of ACS and revascularization hospitalizations, we first constructed 2 models including only the Global Registry of Acute Coronary Events (GRACE) score,3 which incorporates several prognostically important factors including age, creatinine, heart failure, and in‐hospital revascularization procedures. Next, we constructed separate multivariable Cox regression models to (1) identify the predictors of ACS and (2) identify the predictors of revascularization. Candidate covariates for the model were selected a priori, based on clinical judgment, and included age, female sex, white race, self‐reported avoidance of care due to cost, lack of medical insurance, currently working, prior MI, prior PCI, prior CABG, prior heart failure, diabetes mellitus, prior stroke or transient ischemic attack, peripheral artery disease, depression, multivessel disease (2 or more epicardial stenoses ≥70% or left main stenosis ≥50%), ST‐elevation AMI (versus non‐ST‐elevation), discharge heart rate, discharge systolic blood pressure, serum creatinine, in‐hospital PCI, in‐hospital CABG, and quality‐of‐care indicators (defined as receiving all quality‐of‐care eligible treatments during AMI [aspirin and β‐blocker within 24 hours; aspirin, β‐blocker, and angiotensin‐converting enzyme/angiotensin II receptor blocker at discharge; smoking cessation instructions; and timely reperfusion for ST‐elevation AMI]). For in‐hospital PCI, we tested whether there was any significant difference between type of stent (drug‐eluting stent versus bare metal stent or balloon angioplasty only), and if this was significant, included stent type in the model.
Harrell's backward selection strategy was used to identify factors significantly associated with UA and revascularization.9 Each covariate was ranked by its contribution to the multivariable model (as assessed by F‐value), and the variables with the smallest contribution to the model were sequentially eliminated until further variable elimination led to a >10% loss in model prediction, as compared with the initial model. The remaining covariates comprised the final parsimonious model and explained >90% of the variance of the full model. As an additional analysis, this approach was repeated with UA alone as the ACS outcome of interest, given its common inclusion as a component of major adverse cardiac events and the absence of any existing model specifically focusing on this outcome.
Baseline data were complete, with 7% of patients missing 1 baseline data item, 0.1% missing 2 items, and a mean number of missing items per patient of 0.08. Missing data were imputed with 5 imputation data sets using IVEware (Imputation and Variance Estimation Software; University of Michigan's Survey Research Center, Institute for Social Research, Ann Arbor, MI). All analyses were conducted using SAS v9.3 (SAS Institute, Inc, Cary, NC), and statistical significance was determined by a 2‐sided P<0.05.
Results
Patient Population
Among 4340 patients with AMI enrolled in TRIUMPH, 71 died within the first month and never had the opportunity for follow‐up. Of the 4269 patients eligible for follow‐up, 637 (14.9%) patients were lost to follow‐up and an additional 349 (8.2%) were excluded as they reported hospitalizations for which the records were unavailable for adjudication, either because the patient did not consent to a medical records release (n=77) or the admitting hospital refused to honor the medical records release (n=272). The final analytic cohort comprised the remaining 3283 patients (Figure 1). Excluded patients were more likely to be younger, nonwhite, conservatively managed during the index hospitalization, and to have more extensive baseline cardiovascular disease and risk factors (Table 1). Overall, the mean age of the patients included in the final analytic cohort was 59 years, one third were female, 70% were white, and 45% presented with ST‐elevation AMI. Clinical characteristics were typical of patients with AMI in contemporary studies, including 29% with diabetes, 20% with prior AMI, and 25% with prior coronary revascularization procedures.
Figure 1.
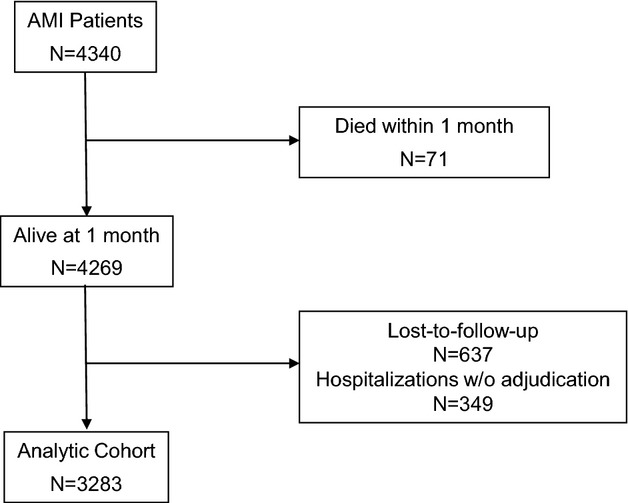
Flowchart of patients. AMI indicates acute myocardial infarction.
Table 1.
Baseline Characteristics of Patients Included in the Analytic Cohort Versus Those With Missing Data
| Analytic Cohort (n=3283) | Missing Data (n=986) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age | 59.4±12.0 | 57.5±13.2 | <0.001 |
| Female sex | 32.7% | 35.4% | 0.109 |
| White race | 69.9% | 59.2% | <0.001 |
| Married | 53.4% | 44.8% | <0.001 |
| High school education | 79.9% | 77.8% | 0.159 |
| No medical insurance | 19.3% | 25.0% | <0.001 |
| Currently working | 50.6% | 45.1% | 0.003 |
| Medical history | |||
| Dyslipidemia | 49.4% | 48.0% | 0.429 |
| Hypertension | 66.1% | 67.7% | 0.336 |
| Prior stroke/TIA | 6.7% | 7.7% | 0.275 |
| Peripheral vascular disease | 4.7% | 4.6% | 0.899 |
| Diabetes mellitus | 29.0% | 35.8% | <0.001 |
| Prior myocardial infarction | 19.6% | 24.8% | <0.001 |
| Prior coronary angioplasty | 19.1% | 21.4% | 0.116 |
| Prior coronary bypass surgery | 10.6% | 13.6% | 0.009 |
| Chronic kidney disease | 6.3% | 10.4% | <0.001 |
| Chronic lung disease | 7.0% | 7.8% | 0.392 |
| Chronic heart failure | 6.9% | 13.6% | <0.001 |
| Current smoker | 38.4% | 43.2% | 0.007 |
| Body mass index, kg/m2 | 29.6±6.5 | 29.5±6.5 | 0.64 |
| Acute presentation | |||
| Left ventricular dysfunction | 17.1% | 22.3% | <0.001 |
| Diseased vessels | <0.001 | ||
| 0 | 7.7% | 9.7% | |
| 1 | 40.2% | 34.2% | |
| 2 | 24.6% | 23.1% | |
| 3 | 21.3% | 21.2% | |
| Unknown (no angiogram) | 6.2% | 11.8% | |
| ST‐elevations | 44.6% | 37.6% | <0.001 |
| Troponin peak, ng/dL | 29.3±75.6 | 25.7±61.4 | 0.174 |
| GRACE discharge score | 99.9±28.9 | 100.9±33.1 | 0.358 |
| Acute treatments | |||
| In‐hospital coronary angioplasty | 67.0% | 59.8% | <0.001 |
| In‐hospital coronary bypass surgery | 10.1% | 6.8% | 0.002 |
| Aspirin at discharge* | 95.0% | 92.3% | 0.001 |
| β‐Blocker at discharge* | 90.7% | 90.2% | 0.606 |
| Statin at discharge* | 88.5% | 87.1% | 0.255 |
| ACE inhibitor/ARB at discharge* | 74.6% | 74.7% | 0.909 |
| Smoking cessation counseling* | 38.8% | 42.0% | 0.07 |
| Cardiac rehabilitation referral | 58.8% | 35.7% | <0.001 |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; GRACE, Global Registry of Acute Coronary Events; TIA, transient ischemic attack.
Among patients who were eligible for treatments (eg, ACE inhibitor/ARB among those with ventricular dysfunction; smoking cessation counseling among current smokers, etc.).
Characteristics Associated With ACS Rehospitalizations
The Kaplan–Meier estimated rate of rehospitalization due to ACS was 6.8% (0.4% ST‐elevation AMI, 1.6% non‐ST‐elevation AMI, 5.0% UA; 49% of which were treated with revascularization), with a mean time to event of 4.9 months after hospital discharge. If we consider the competing risk of death, the cumulative incidence rate of readmission for ACS remained 6.8%, indicating no significant bias due to attrition from mortality (Figure 2A). In the model to determine whether the predictors of death differed from those of ACS, GRACE score (a score that estimates risk of long‐term mortality after AMI) was not associated with risk of ACS (per 10‐point increase: hazard ratio [HR] 0.96, 95% CI 0.92 to 1.01, P=0.17). In the multivariable model, the most significant predictors of rehospitalization for ACS (in terms of F‐value) were CABG prior to AMI hospitalization (HR 2.12, 95% CI 1.45 to 3.10), female sex (HR 1.67, 95% CI 1.23 to 2.25), and in‐hospital PCI (HR 1.85, 95% CI 1.28 to 2.69; Table 2). There was no significant difference between stent types, in terms of risk of ACS rehospitalization (P=0.10). Other significant predictors of ACS rehospitalization were PCI prior to AMI hospitalization, younger age, and if the patient was not currently working at the time of their AMI. There were minimal differences when predicting an admission of UA alone (versus the composite outcome of ACS) in the year after AMI (Table 3).
Figure 2.
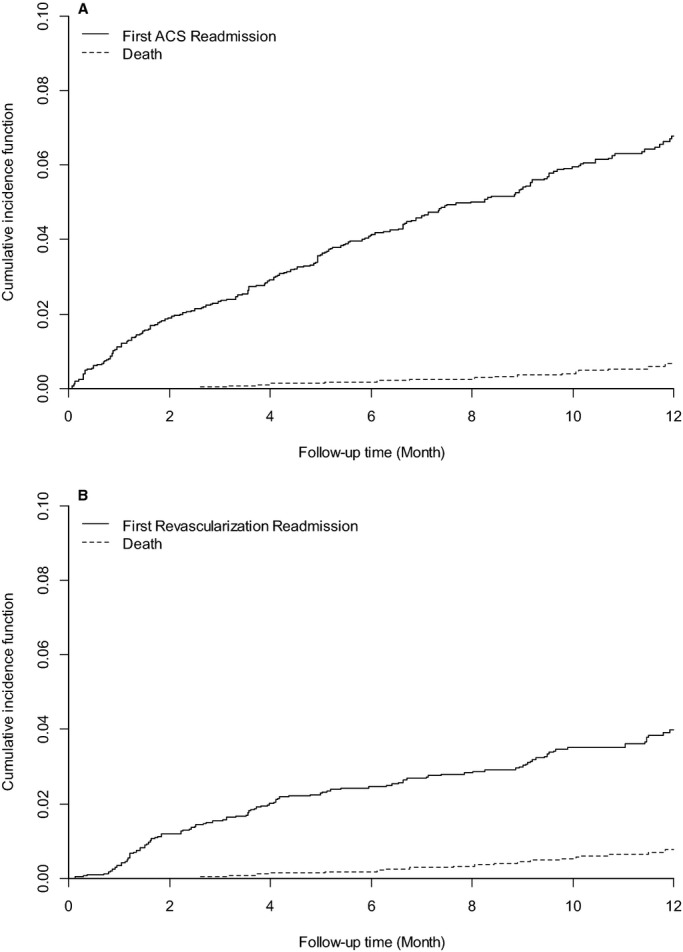
Kaplan–Meier curves of cumulative incidence of rehospitalization due to (A) acute coronary syndromes (ACS) and (B) revascularization, with the competing risk of death.
Table 2.
Factors Associated With Rehospitalization for Acute Coronary Syndrome and Revascularization
| Predictor (Ordered by F‐Value) | Acute Coronary Syndrome | Predictor (Ordered by F‐Value) | Revascularization | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | F‐Value | P Value | HR (95% CI) | F Value | P Value | ||
| Prior CABG | 2.12 (1.45 to 3.10) | 14.7 | <0.001 | Multivessel disease | 2.89 (1.90 to 4.39) | 24.7 | <0.001 |
| Female sex | 1.67 (1.23 to 2.25) | 11.2 | 0.001 | In‐hospital PCI (BMS) | 2.08 (1.19 to 3.63) | 6.5 | 0.011 |
| In‐hospital PCI | 1.85 (1.28 to 2.69) | 10.7 | 0.001 | In‐hospital PCI (DES) | 1.42 (0.81 to 2.48) | 1.5 | 0.318 |
| Prior PCI | 1.63 (1.17 to 2.25) | 8.5 | 0.004 | In‐hospital CABG | 0.22 (0.07 to 0.73) | 6.1 | 0.013 |
| Age | 0.98 (0.97 to 0.99) | 8.4 | 0.004 | Prior AMI | 0.60 (0.34 to 1.05) | 3.2 | 0.074 |
| Currently working | 0.69 (0.50 to 0.96) | 4.9 | 0.027 | Prior PCI | 1.48 (0.90 to 2.44) | 2.4 | 0.121 |
| Peripheral artery disease | 1.66 (0.98 to 2.80) | 3.6 | 0.057 | Female sex | 1.28 (0.86 to 1.90) | 1.4 | 0.229 |
| No medical insurance | 1.42 (0.97 to 2.10) | 3.2 | 0.073 | Prior heart failure | 0.68 (0.27 to 1.73) | 0.6 | 0.422 |
| Diabetes mellitus | 1.30 (0.95 to 1.75) | 2.8 | 0.096 | Currently working | 0.87 (0.59 to 1.28) | 0.5 | 0.483 |
| In‐hospital CABG | 0.85 (0.44 to 1.65) | 0.2 | 0.642 | ||||
C‐statistic for acute coronary syndrome model=0.662; for revascularization mode=0.687. AMI indicates acute myocardial infarction; BMS, bare metal stent; CABG, coronary artery bypass graft surgery; DES, drug‐eluting stent; HR, hazard ratio; PCI, percutaneous coronary intervention.
Table 3.
Factors Associated With Rehospitalization for Unstable Angina
| Predictor (Ordered by F Value) | Unstable Angina | ||
|---|---|---|---|
| HR (95% CI) | F‐Value | P Value | |
| In‐hospital PCI | 2.34 (1.48 to 3.71) | 13.0 | <0.001 |
| Female sex | 1.83 (1.30 to 2.59) | 11.7 | 0.001 |
| Prior CABG | 2.01 (1.27 to 3.19) | 9.0 | 0.003 |
| Age | 0.98 (0.96 to 1.00) | 6.4 | 0.012 |
| Prior PCI | 1.65 (1.12 to 2.44) | 6.4 | 0.011 |
| No medical insurance | 1.71 (1.11 to 2.64) | 5.8 | 0.016 |
| Peripheral artery disease | 1.76 (0.94 to 3.25) | 3.2 | 0.075 |
| Systolic BP (at discharge) | 0.99 (0.98 to 1.00) | 2.6 | 0.104 |
| Diabetes mellitus | 1.34 (0.94 to 1.92) | 2.6 | 0.108 |
| Currently working | 0.74 (0.51 to 1.08) | 2.4 | 0.118 |
| In‐hospital CABG | 0.59 (0.23 to 1.52) | 1.2 | 0.280 |
CABG indicates coronary artery bypass graft surgery; BP, blood pressure; HR, hazard ratio; PCI, percutaneous coronary intervention.
Characteristics Associated With Coronary Revascularization Hospitalizations
The Kaplan–Meier estimated rate of rehospitalization for revascularization procedures that were not planned during the index AMI was 4.1% (48% of which were due to ACS), 89% of which were PCIs with a mean time to event of 4.9 months. In the competing risk analysis that included death, the cumulative incidence rate of readmission for revascularization remained 4.1%, indicating no significant bias due to attrition from mortality (Figure 2B). GRACE score was not significantly associated with risk for revascularization (per 10‐point increase: HR 0.95, 95% CI 0.89 to 1.02, P=0.132). In the multivariable model, the most significant predictor of rehospitalization for revascularization (in terms of F‐value) was the presence of multivessel disease, which was associated with a nearly 3‐fold increased hazard of revascularization in the year following AMI (HR 2.89, 95% CI 1.90 to 4.39; Table 2). Other significant predictors of revascularization were in‐hospital PCI and in‐hospital CABG (the latter predicting a decreased risk of revascularization). The type of stent received by the patient was associated with the risk for repeat revascularization over the year following AMI (P=0.011). In‐hospital PCI with a bare metal stent or angioplasty alone was associated with a 2.08 increased hazard of a subsequent revascularization rehospitalization (95% CI 1.19 to 3.63), while in‐hospital PCI with a drug‐eluting stent was not associated with a significantly increased hazard of repeat revascularization.
Discussion
In a large, prospective, multicenter US registry of patients hospitalized for AMI, we found that 6.8% of patients were rehospitalized for ACS and 4.1% are rehospitalized for revascularization procedures over the first year following AMI. Different risk factors were associated with these different types of admissions. Those with a prior CABG, female patients, those with prior or in‐hospital PCI, and patients not employed at the time of AMI were more likely to be hospitalized for ACS, while patients with multivessel disease and those who underwent PCI with a bare metal stent were more likely to be hospitalized for revascularization. Patients’ mortality risks, as measured with the GRACE mortality risk score, were not associated with the risk of either an ACS or a revascularization rehospitalization. These findings demonstrate that the predictors for rehospitalization for ACS and revascularization, which likely relate more to recurrence or progression of the underlying coronary disease, are different from those of mortality. Given the potential impact on patients’ quality of life and healthcare costs, targeted strategies are needed to reduce these events.
Many prior studies have investigated the frequency and predictors of death after AMI3–4 or composites of major adverse cardiac events (which include death).6 However, as mortality rates decline, additional focus is needed on other nonfatal outcomes such as rehospitalizations, procedures, health status,10 and cost. Rehospitalizations due to nonfatal coronary events not only consume healthcare resources,11 but also they can be stressful to patients and may adversely affect their quality of life.12 Understanding the predictors of these adverse events is important in order to create strategies of care to try to mitigate these hospitalizations. While some patient characteristics were differentially associated with the 2 events, many of the most important predictors that we identified related to the overall burden of cardiovascular disease, emphasizing the importance of aggressive secondary prevention efforts in high‐risk patients. Other characteristics associated with the outcomes, beyond risk factors for atherosclerotic disease, are more challenging to explain. For example, younger age and female sex would not be expected to be associated with greater cardiovascular disease progression, and yet are strongly associated with subsequent ACS admission, but less so with revascularization. This is congruent with prior studies that have shown that both female sex and younger age are associated with more angina13 and worse disease‐specific quality of life10 after an AMI, which may explain their association with ACS admissions, mostly in the absence of need for revascularization (ie, medically managed).
Our finding that the predictors of ACS and revascularization rehospitalizations were disparate from those of mortality highlights the need to examine events individually, rather than as composite end points. Most prior studies that have explored the impact of recurrent hospitalizations after an AMI have focused on the first 30 days.14–16 This time frame has been of particular interest as 30‐day readmission has been put forth as a quality metric, is publicly reported, and has reimbursement implications for hospitals.17 In one of these studies, the rate of all‐cause readmission was 18.6% in the 30 days after AMI, and the rate of unplanned revascularization was 2.3%.14 While this prior study did not evaluate predictors of revascularization, patients who were medically managed or who had complications from invasive procedures were more likely to require rehospitalization in the 30 days after AMI.
In another study looking at readmissions after AMI, the rate of all‐cause readmission was 14.5% in the United States within the first 30 days after an ST‐elevation AMI, with multivessel disease being the strongest predictor of a readmission.15 While all‐cause readmissions are common and ought to be avoided, many of the reasons for such admissions are distinct from patients’ cardiovascular disease, as only 43% of rehospitalizations during the first 30 days were related to the index AMI in 1 study.14 In contrast to describing all‐cause readmissions, we focused on progression of coronary disease to capture progression of the underlying pathophysiological process that led to the initial AMI admission, which is why the observed rates in this study are so much lower than these previous reports. By evaluating ACS and revascularization hospitalizations in isolation, we sought to identify patients at higher risk for progression of their coronary disease, which may in turn encourage more aggressive secondary prevention or monitoring in such patients.
Furthermore, while these 30‐day studies are clearly important for hospital quality improvement, readmissions beyond this short time frame also impact both patients and the healthcare system, especially as accountable care organizations become more common. As traditional risk factors for mortality are not associated with a greater likelihood of rehospitalization for ACS or revascularization, treatments known to reduce mortality may not be as effective in reducing these rehospitalizations. Novel therapeutic strategies, such as aggressive antianginal medications at discharge or early postdischarge outpatient follow‐up visits, likely need to be developed and tested to reduce ACS and revascularization admissions after an AMI. Furthermore, while these hospitalizations are clearly an inconvenience to patients and consume healthcare resources, the long‐term implications of recurrent angina and revascularization, in terms of mortality and quality of life, are unknown and require further study.
There are a number of potential limitations to our study that merit discussion. First, we relied on patient reporting of hospitalization events to request records for adjudication. Due to both patient under‐reporting and loss to follow‐up, we may have underestimated the absolute rates of readmission. Nevertheless, being able to carefully adjudicate hospital admissions is a great strength of this study over using administrative codes for “chest pain” hospitalizations without further delineation into noncardiac chest pain, stable angina, or UA—particularly given the known limitations of using administrative data for clinical diagnoses.18 Second, due to the relatively small number of events, we were limited in our ability to investigate various demographic and clinical predictors of rehospitalization. While we did examine a wide range of demographic, socioeconomic, clinical, and treatment factors, there may be other factors that are prognostically important for ACS and revascularization hospitalizations that we did not consider or that were not collected in the TRIUMPH study. Future work to confirm the predictors identified here and to evaluate novel predictors of ACS and revascularization would be informative. Third, TRIUMPH was conducted when first‐generation drug‐eluting stents were used. How newer‐generation stenting would affect rehospitalizations is not known, but as drug‐eluting stents were not significantly associated with rehospitalizations for revascularizations, even in TRIUMPH, the results would be unlikely to be materially altered by use of newer stents. Finally, we have not described the impact of these hospitalizations in terms of future mortality and quality of life, and understanding the clinical importance of these hospitalizations is important.
In conclusion, we found that ≈7% of patients are rehospitalized for ACS and ≈4% are rehospitalized for revascularization procedures over the first year following AMI. The predictors for ACS and revascularization were distinct from those for mortality, as quantified by the GRACE score. As such, treatments known to reduce mortality may not be the best targets for reducing these rehospitalizations. Given the potential impact on patients’ quality of life and healthcare costs, novel therapeutic strategies are needed to reduce both the frequency and adverse impact of these hospitalizations in the post‐AMI period.
Sources of Funding
TRIUMPH was sponsored by a grant from the National Institutes of Health (National Heart, Lung, and Blood Institute): Washington University School of Medicine SCCOR Grant #P50HL077113‐01. This project was funded through a research grant from Eli Lilly
Disclosures
Dr Smolderen and Dr Spertus received research grant support from Eli Lilly. Dr Zhao is an employee of Eli Lilly.
References
- Krumholz HM, Wang Y, Chen J, Drye EE, Spertus JA, Ross JS, Curtis JP, Nallamothu BK, Lichtman JH, Havranek EP, Masoudi FA, Radford MJ, Han LF, Rapp MT, Straube BM, Normand SL. Reduction in acute myocardial infarction mortality in the United States: risk‐standardized mortality rates from 1995–2006. JAMA. 2009; 302:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013; 127:e6-e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6‐month postdischarge death in an international registry. JAMA. 2004; 291:2727-2733. [DOI] [PubMed] [Google Scholar]
- Boersma E, Pieper KS, Steyerberg EW, Wilcox RG, Chang WC, Lee KL, Akkerhuis KM, Harrington RA, Deckers JW, Armstrong PW, Lincoff AM, Califf RM, Topol EJ, Simoons ML. Predictors of outcome in patients with acute coronary syndromes without persistent ST‐segment elevation. Results from an international trial of 9461 patients. The PURSUIT investigators. Circulation. 2000; 101:2557-2567. [DOI] [PubMed] [Google Scholar]
- Bernheim SM, Grady JN, Lin Z, Wang Y, Savage SV, Bhat KR, Ross JS, Desai MM, Merrill AR, Han LF, Rapp MT, Drye EE, Normand SL, Krumholz HM. National patterns of risk‐standardized mortality and readmission for acute myocardial infarction and heart failure. Update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010; 3:459-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HW, Yoon CH, Kang SH, Choi DJ, Kim HS, Cho MC, Kim YJ, Chae SC, Yoon JH, Gwon HC, Ahn YK, Jeong MH. Early‐ and late‐term clinical outcome and their predictors in patients with ST‐segment elevation myocardial infarction and non‐ST‐segment elevation myocardial infarction. Int J Cardiol. 2013; 169:254-261. [DOI] [PubMed] [Google Scholar]
- Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA. Translational research investigating underlying disparities in acute myocardial infarction patients’ health status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011; 4:467-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the 2002 guidelines for the management of patients with unstable angina/non ST‐elevation myocardial infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007; 116:e148-e304. [DOI] [PubMed] [Google Scholar]
- Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. 2001New York, NY: Springer‐Verlag; 2001 [Google Scholar]
- Arnold SV, Masoudi FA, Rumsfeld JS, Li Y, Jones PG, Spertus JA. Derivation and validation of a risk standardization model for benchmarking hospital performance for health‐related quality of life outcomes after acute myocardial infarction. Circulation. 2014; 129:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SV, Morrow DA, Lei Y, Cohen DJ, Mahoney EM, Braunwald E, Chan PS. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN‐TIMI 36 trial. Circ Cardiovasc Qual Outcomes. 2009; 2:344-353. [DOI] [PubMed] [Google Scholar]
- Maddox TM, Reid KJ, Rumsfeld JS, Spertus JA. One‐year health status outcomes of unstable angina versus myocardial infarction: a prospective, observational cohort study of ACS survivors. BMC Cardiovasc Disord. 2007; 7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureshi F, Arnold SV, Li Y, Masoudi FA, Jones PG, Cresci S, Maddox TM, Ho PM, Spertus JA. Development of a 30‐day angina risk standardization model following an acute myocardial infarction. Circulation. 2013; 128:A14960 [Google Scholar]
- Dunlay SM, Weston SA, Killian JM, Bell MR, Jaffe AS, Roger VL. Thirty‐day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012; 157:11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kociol RD, Lopes RD, Clare R, Thomas L, Mehta RH, Kaul P, Pieper KS, Hochman JS, Weaver WD, Armstrong PW, Granger CB, Patel MR. International variation in and factors associated with hospital readmission after myocardial infarction. JAMA. 2012; 307:66-74. [DOI] [PubMed] [Google Scholar]
- Joynt KE, Orav EJ, Jha AK. Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011; 305:675-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services. Readmissions reduction program Available at: http://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Accessed October 9, 2013.
- Sarrazin MS, Rosenthal GE. Finding pure and simple truths with administrative data. JAMA. 2012; 307:1433-1435. [DOI] [PubMed] [Google Scholar]


