Abstract
Background
Endothelial dysfunction is a key factor in the development of atherosclerosis. Commonly, endothelial function is determined in the brachial artery, whereas patients with peripheral artery disease (PAD) present with lower limb atherosclerosis. We hypothesized that in PAD, a segmental or local association exists between endothelial dysfunction and atherosclerotic structural changes.
Methods and Results
We used ultrasound to study endothelial function as flow‐mediated vasodilation, intima media thickness, and local stiffness of the superficial femoral artery (SFA) and brachial artery (BA). PAD patients with symptomatic SFA or below‐the‐knee disease were compared with age‐matched patients without PAD and young healthy controls. PAD patients with SFA or below‐the‐knee disease exhibited endothelial dysfunction of the proximal SFA (flow‐mediated vasodilation: 3.9±0.6%, 3.7±0.6%) compared with healthy controls (7.4±1.0%) and patients without PAD (5.4±0.6%). Brachial artery flow‐mediated vasodilation values were not different in PAD patients with SFA or below‐the‐knee disease compared with patients without PAD, but they were significantly lower than those of healthy controls. Endothelial dysfunction correlated with increased intima media thickness or plaque thickness at the site of flow‐mediated vasodilation measurement across vascular sites. In PAD patients with SFA disease, SFA flow‐mediated vasodilation was further impaired within and distal to stenosis (prestenosis 3.9±0.6%, intrastenosis 2.3±0.7%, poststenosis 2.5±0.6%) and recovered within 24 hours after SFA balloon angioplasty to prestenotic values but not to the brachial artery or SFA values in patients without PAD or controls.
Conclusion
A close association exists between local endothelial function and atherosclerotic structural remodeling, suggesting that in PAD, local and segmental factors—in addition to systemic factors—influence local endothelial function. Our data point toward a pathophysiological role for lower extremity endothelial dysfunction in PAD.
Keywords: atherosclerosis, endothelial function, peripheral artery disease, vasodilation
Introduction
Atherosclerotic peripheral arterial disease (PAD) is a major public health burden that affects >27 million people across Europe and Northern America, equating to 16% of the population aged >55 years.1 Those with lower extremity PAD have an increased risk of ischemic cardiovascular events.2–5 Although the pathophysiological mechanisms for coronary artery disease (CAD) and PAD are similar, it is unknown why some patients with CAD develop PAD and some do not.
In the development and progression of atherosclerosis, endothelial dysfunction represents the pathophysiological key event.6 Cardiovascular risk factors including smoking, diabetes, hypertension, and dyslipidemia cause endothelial injury, depending on different vulnerability secondary to genetic and environmental backgrounds. Dysfunctional endothelium triggers a self‐perpetuating downward spiral involving inflammation and platelet and smooth muscle cell activation, leading to degenerative vascular processes with vascular wall thickening and stiffening. Consistent with this paradigm, the measurement of brachial artery (BA) flow‐mediated vasodilation (FMD) can be viewed as the noninvasive gold standard to determine endothelial function and is a prospectively validated surrogate parameter for predicting cardiovascular disease progression and outcome.7 In addition, BA vasodilator function can be understood and used as an in vivo system for the detection of the presence of systemic pathophysiological factors affecting the vascular health of the patient. Conceptually, there may be local or segmental factors that lead to atherosclerosis of individual or isolated vascular segments (eg, carotid, coronary, or lower limb). The spatial association of endothelial function with respect to localization of intimal hyperplasia and plaque development is not well studied.
We studied the association between endothelial function and intima media thickness (IMT) when measured at the same arterial location, namely, the BA and the proximal superficial femoral artery (SFA). We hypothesized that in PAD, a segmental or local association exists between endothelial dysfunction and atherosclerotic structural changes.
Methods
We studied whether local endothelial function is associated with local IMT at a non–atherosclerosis‐prone vascular segment (ie, the BA) and at an atherosclerosis‐prone segment (ie, the proximal SFA).
In patients with PAD of the SFA (SFA‐PAD), we determined local endothelial function; IMT or plaque thickness; and vascular stiffness proximal to a flow‐limiting stenosis (prestenosis), within stenosis (intrastenosis), and distal to stenosis (poststenosis). Furthermore, we investigated the response of these lesions after localized balloon angioplasty.
Study Population
We recruited 4 groups of study subjects. For the SFA‐PAD group with isolated <5‐cm‐long hemodynamically relevant SFA stenoses, inclusion criteria were claudication with <200 m pain‐free walking distance; ankle‐brachial index (ABI) of <0.9; and isolated hemodynamically relevant >70% stenosis of SFA, as measured by intrastenotic peak systolic velocity ratio of >3.4 and characteristic poststenotic Doppler spectrum lacking a dip. The other groups comprised patients with clinically relevant below‐the‐knee PAD (BTK‐PAD) without relevant stenosis of the iliac, femoral, or popliteal segment; age‐matched patients without PAD (No‐PAD); and young healthy volunteers (healthy controls) without PAD, as defined by absence of claudication and normal ABI. Patients with BTK‐PAD exhibited significant stenosis of fibular, posterior, or anterior tibial artery, as determined by >70% stenosis with an intrastenotic peak velocity ratio of >3.4 or characteristic poststenotic Doppler spectrum lacking dip without hemodynamically relevant stenoses of aorta, iliac, femoral, or popliteal arteries. Because patients both with and without PAD had diagnoses of stable CAD, both groups were on standard optimal medical therapy including statin, β‐blocker, angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, aspirin, or clopidogrel. Exclusion criteria were significant stenosis of iliac arteries or aorta, dyspnea limiting walking distance (New York Heart Association class III to IV), malignancies, terminal renal failure, severe cardiac arrhythmias, and acute inflammation (C‐reactive protein >0.5 mg/dL). The study protocol was approved by the ethics committee of the Heinrich Heine University Düsseldorf, and all volunteers gave written informed consent.
Study Protocol
We studied the association between local endothelial function and IMT at the non–atherosclerosis‐prone BA and the atherosclerosis‐prone SFA in patients with and without PAD along with young healthy controls. Vascular ultrasound examinations included measurements of FMD, nitroglycerin‐mediated vasodilation (NMD), and IMT of the proximal SFA at areas without visible plaques (defined as a local intimal protrusion with thickness >150% of adjacent IMT) compared with the nondiseased BA (intraindividually) and disease‐free SFA of control subjects (interindividually). In patients with SFA‐PAD, we measured vascular properties proximal to, within, and distal to hemodynamically relevant atherosclerotic SFA stenoses. The latter measurements were repeated at 6 and 24 hours after balloon angioplasty of the stenosis segment in patients with SFA‐PAD who did not require stent placement (n=6).
Ankle‐Brachial Index
The BA systolic and diastolic blood pressures were obtained automatically (Dinamap Vital Signs Monitor) in both arms. Systolic blood pressure in the dorsalis pedis arteries of both legs was measured using a nondirectional Doppler flow detector (Schabert Instrumente Röttenbach) with a pencil probe (8 MHz). The arm and the leg yielding the higher systolic pressures were used to calculate ABI.
Assessment of Pain‐Free Walking Distance by Treadmill
The pain‐free walking distance was assessed in all participants with reduced ABI. The pain‐free walking distance was tested with a standardized protocol (slope: 12%, velocity: 3.2 km/h). The investigation was stopped when patients were not able to walk further because of ischemic leg pain or at 200 m.
Ultrasound Measurements of Arterial Function, IMT, and Local Stiffness
FMD was measured, as described previously.8 Briefly, the diameter and the angle‐corrected flow velocity of the BA and the SFA were measured using a 12‐MHz transducer (Vivid I; General Electric) and automatic edge‐detection software (brachial analyzer; Medical Imaging Applications), yielding SDs of mean differences between repeated measurements of <1%. BA diameter was measured 2 cm proximal to the elbow, and proximal SFA was measured 2 to 4 cm distal to femoral bifurcation in a vessel section without visible intima media protrusions of >50% compared with adjacent IMT. Reactive hyperemia was induced by 5 minutes of distal lower arm or above‐knee occlusion with a sphygmomanometric cuff inflated to 250 mm Hg. After cuff deflation, at 20, 40, 60, and 80 seconds, the diameter was assessed, and FMD was calculated as maximal relative diameter gain relative to baseline. Finally, sublingual nitroglycerin (0.4 mg) was administered, and endothelium‐independent NMD was assessed at 4 minutes in the BA and immediately thereafter in the SFA.
IMT was measured with an automatic contour‐detection software between the intimal and adventitial layers (Vivid I; General Electric). The IMT was measured at identical sites used for FMD measurements in the BA and SFA of all investigated participants, using the same software as described for FMD measurements. Within SFA stenoses, we determined the maximal plaque thickness at the near and far walls. For better comparability with IMT measurements, which take only the far‐wall IMT into account, and because of asymmetry of plaques with regard to near and far walls, we determined both maximal plaque thicknesses and present the average side by side with pre‐ and poststenosis IMT. Plaque thickness is presented as maximal near plaque thickness+maximal far plaque thickness/2. Local arterial stiffness was determined at sites used for FMD and IMT measurements as fractional diameter change during the heart cycle and calculated as ![]() .
.
Balloon Angioplasty
To evaluate the impact of localized balloon angioplasty on local arterial function, we evaluated FMD and fractional diameter change in the SFA‐PAD group 6 and 24 hours after a single balloon angioplasty of the respective segments. Briefly, SFA‐PAD patients received a retrograde arterial sheath placement (Terumo Destination 6F) in the contralateral common femoral artery using the standard Seldinger puncture technique. Using an internal mammary artery catheter and hydrophilic 0.035‐in wire (Terumo Advantage) for cross‐over maneuver, the tip of the sheath was advanced into the common femoral artery proximal to the SFA stenosis, and a 5000‐IU heparin bolus was applied. After acquisition of a diagnostic angiogram using digital subtraction angiography (Philips Alura), the wire was passed through the stenosis, and a 20‐ to 40‐mm balloon (Biotronic Passeo 35) was placed in the lesion and inflated for 2 minutes, and a final angiogram was taken. No flow‐limiting dissections or recoils were observed in the patients.
Statistical Analyses
Results are expressed as mean±SD. Linear relationships between continuous variables were expressed as Pearson's r. Statistical significance was assumed at P≤0.05. Baseline characteristics were analyzed with ANOVA and consecutive post hoc analysis (Tukey). Baseline vascular measurements in BA and SFA including baseline diameter, FMD, NMD, and IMT were analyzed by 2‐way repeated measures ANOVA with the between‐subject factors of group (control, No‐PAD, SFA‐PAD, BTK‐PAD) and the within‐subject factor of BA versus SFA with consecutive Tukey's multiple comparisons test. Vascular measurements taken at the site of stenosis before and after percutaneous transluminal angioplasty (PTA) were analyzed with a 1‐way repeated measures ANOVA with consecutive Tukey's multiple comparisons test. Analyses were performed with Prism 6 for Mac OSX version 6.0a (GraphPad Software Inc).
Results
Baseline Characteristics of Study Groups
See Table 1 for baseline characteristics. In the healthy control and No‐PAD groups, claudication was absent and the ABI was normal (healthy control: 1.1±0.1; No‐PAD: 1.0±0.1). All patients had a concomitant diagnosis of CAD. ABI was <0.9 in SFA‐PAD (0.6±0.1) and BTK‐PAD (0.6±0.1). The mean pain‐free walking distance in patients with PAD was 130±60 m for SFA‐PAD and 150±50 m for BTK‐PAD (P=0.29 versus SFA‐PAD, t test). There were no significant differences in blood pressure, cholesterol, and glucose levels among the groups.
Table 1.
Clinical Characteristics of Study Population
| Healthy Control | No PAD | SFA‐PAD | BTK‐PAD | P Value | |
|---|---|---|---|---|---|
| n (female/male) | 10 (4/6) | 10 (5/5) | 10 (3/7) | 10 (5/5) | |
| Pain‐free walking distance, m | Not limited | Not limited | 130±60 | 150±50 | 0.29, t‐test |
| ABI | 1.1±0.1 | 1.0±0.2 | 0.7±0.1*† | 0.6±0.1*† | 0.04 |
| CAD, n | 0 | 10 | 10 | 10 | |
| Age, y | 48±18 | 68±6* | 68±9* | 71±13* | 0.01 |
| Systolic blood pressure, mm Hg | 120±8 | 131±9 | 139±6 | 129±7 | 0.06 |
| Diastolic blood pressure, mm Hg | 80±4 | 73±4 | 99±3 | 90±7 | 0.09 |
| Smoking, n | 2 | 5 | 6 | 4 | |
| Arterial hypertension, n | 2 | 10 | 10 | 9 | |
| Diabetes mellitus, n | 0 | 2 | 6 | 6 | |
| Creatinine, mg/dL | 0.8±0.1 | 1.2±0.5 | 1.3±0.6 | 1.3±0.6 | 0.61 |
| Triglyceride, mg/dL | 88±46 | 129±61*† | 136±62*† | 123±54*† | 0.03 |
| Cholesterol, mg/dL | 195±23 | 171±32 | 164±40 | 164±23 | 0.23 |
| CRP, mg/dL | 0.3±0.1 | 0.3±0.1 | 0.3±0.1 | 0.3±0.1 | 0.12 |
| Plasma glucose, mg/dL | 107±29 | 110±28 | 120±31 | 118±32 | 0.66 |
| HbA1c, % | 5.4±0.2 | 5.8±0.1 | 6.1±0.1 | 6.2±0.1 | 0.12 |
P value refers to overall 1‐way ANOVA. ABI indicates ankle‐brachial index; BTK, below‐the‐knee; CAD, coronary artery disease; CRP, C‐reactive protein; HbA1c, glycated hemoglobin; PAD, peripheral artery disease; SFA, superficial femoral artery.
*P<0.05 vs healthy control. †P<0.05 vs no PAD.
Lower Extremity Endothelial Dysfunction and Intimal Thickening in PAD
The results from vascular measurements along with statistical analyses are summarized in Table 2. In healthy controls, the endothelium‐dependent vasodilation (BA‐FMD: 7.4±1.2% versus SFA‐FMD: 7.1±1.1%) and endothelial‐independent vasodilation (BA‐NMD: 11.1±0.6% versus SFA‐NMD: 10.7±0.6%) did not differ between the SFA and BA. Similarly, measurements in the No‐PAD group showed that BA‐ and SFA‐FMD were comparable (BA‐FMD: 5.9±0.7%, SFA‐FMD: 5.4±0.6%; BA‐NMD: 11.0±1.0%, SFA‐NMD: 10.4±0.5%) but significantly impaired compared with healthy controls, indicating systemic endothelial dysfunction in the No‐PAD group in nondiseased peripheral arteries, which may be explained by the pre‐existing CAD in these patients. SFA‐IMT was significantly greater than BA‐IMT in all groups. Along with decreased FMD, the SFA‐IMT was increased in the No‐PAD group (SFA‐IMT 0.80±0.09 mm) with a diagnosis of CAD compared with healthy controls (SFA‐IMT 0.66±0.04 mm), indicating the systemic presence of early structural arteriosclerosis changes of the arterial wall at sites of endothelial dysfunction, as determined by impaired FMD, described above.
Table 2.
Summary of Baseline Vascular Measurements
| Healthy Control | No PAD | SFA‐PAD | BTK‐PAD | P Value | |
|---|---|---|---|---|---|
| BA diameter, mm | 4.5±0.5 | 4.4±0.5 | 4.3±0.5 | 4.3±0.4 | 0.2945 |
| SFA diameter, mm | 6.7±0.6§ | 7.0±1.4§ | 6.4±0.8§ | 6.8±0.7§ | |
| BA‐FMD, % | 7.4±1.2 | 5.9±0.7* | 5.8±1.1* | 5.3±0.4* | 0.0016 |
| SFA‐FMD, % | 7.1±1.1 | 5.4±0.6* | 3.7±0.6*†§ | 3.9±0.5*†§ | |
| BA‐NMD, % | 11.1±0.6 | 11.0±1.0 | 9.8±0.9*† | 9.7±0.7*† | 0.0448 |
| SFA‐NMD, % | 10.7±0.6 | 10.4±0.5§ | 9.9±0.8 | 9.8±0.9* | |
| BA‐FMD/NMD ratio | 0.67±0.11 | 0.54±0.06* | 0.59±0.10 | 0.55±0.8* | 0.0001 |
| SFA‐FMD/NMD ratio | 0.66±0.12 | 0.53±0.06*† | 0.38±0.07*†§ | 0.40±0.07*†§ | |
| BA‐IMT, mm | 0.27±0.04 | 0.31±0.03 | 0.31±0.04 | 0.33±0.4 | 0.0001 |
| SFA‐IMT/plaque thickness, mm | 0.66±0.04§ | 0.80±0.09*†§ | 1.44±0.20*†§ | 1.56±0.15*†‡§ |
P values refer to 2‐way repeated measures ANOVA interaction between BA vs SFA and group. BA indicates brachial artery; BTK, below‐the‐knee; FMD, flow‐mediated vasodilation; IMT, intima media thickness; NMD, nitroglycerin‐mediated vasodilation; PAD, peripheral artery disease; SFA, superficial femoral artery.
Symbols refer Tukey's multiple pairwise comparisons: *P<0.05 vs healthy control, †P<0.05 vs no PAD, ‡P<0.05 vs SFA‐PAD, §P<0.05 vs respective value measured in BA.
Similar to the No‐PAD group, we observed in patients with PAD that BA‐FMD was impaired (No‐PAD: 5.9±0.7%, SFA‐PAD: 5.8±1.1%, BTK‐PAD: 5.8±0.9%) compared with healthy controls (7.4±1.2%), yet there was no significant difference between the patient groups with or without PAD. In contrast, the SFA‐FMD was significantly lowered in patients with SFA‐PAD and BTK‐PAD compared with the No‐PAD group (No‐PAD: 5.4±0.6%, SFA‐PAD: 3.7±0.6%, BTK‐PAD: 3.9±0.5%) and healthy controls (7.1±1.1%), indicating the presence of pronounced endothelial dysfunction in segments of the arterial tree that suffer from clinically manifest atherosclerosis. Likewise, the endothelium‐independent vasodilation of the BA was not significantly different between patients with and without PAD (No‐PAD: 11.0±1.0%, SFA‐PAD: 9.8±0.9%, BTK‐PAD: 9.7±0.7%). Importantly, the SFA‐FMD/NMD ratio was impaired in patients with PAD compared with the No‐PAD group, indicating pronounced endothelial dysfunction (No‐PAD: 0.53±0.06, SFA‐PAD: 0.38±0.07, BTK‐PAD: 0.40±0.07). The baseline diameters of the BA and the SFA were comparable among all groups. Baseline flow was also not significantly different among the groups.
IMT, a parameter of early atherosclerotic structural changes, was increased in the SFA in patients with SFA‐PAD and BTK‐PAD compared with the No‐PAD group (No‐PAD: 0.8±0.09 mm, SFA‐PAD: 1.44±0.20 mm, BTK‐PAD: 1.56±0.15 mm) at the site of FMD measurements. Despite that fact that no local protrusions of >50% of surrounding IMT were noted in the studied segments, the absolute thickness in the PAD patients’ SFA was disproportionately bigger than the BA and, in some cases, even above the 1.5‐mm threshold, which defines a plaque according to the Mannheim criteria.9 No difference was observed in BA‐IMT among groups. For all groups, we observed highly significant linear inverse correlations between IMT or plaque thickness and FMD measurements as measured at the identical site in BA (r=−0.69, P<0.001) (Figure 1A) and SFA (r=−0.74, P<0.001) (Figure 1B). Due to the small group size (n=10), no within‐group correlations were performed.
Figure 1.
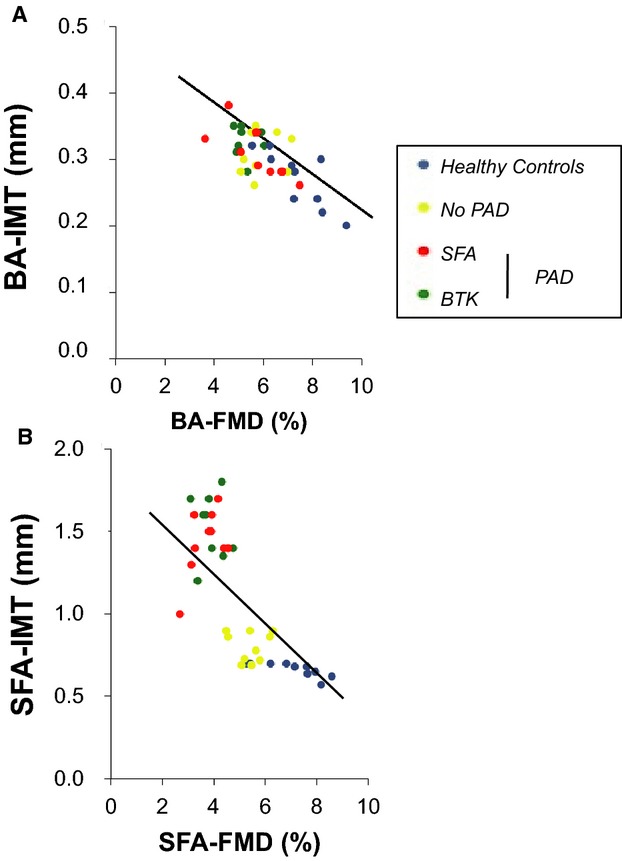
Correlation of FMD and IMT/plaque thickness in (A) the BA (r=−0.69, P<0.001) and (B) the SFA (r=−0.74, P<0.001). BA indicates brachial artery; BTK, below‐the‐knee; FMD, flow‐mediated vasodilation; IMT, intima media thickness; PAD, peripheral artery disease; SFA, superficial femoral artery.
Locally Impaired Vasodilation Within SFA Stenoses Associated With Local Stiffness
In a subgroup of patients with SFA‐PAD who received an isolated PTA of the studied segment (n=6), we investigated the FMD proximal to, within, and distal to a hemodynamically significant SFA stenosis resulting from an atherosclerotic plaque causing luminal narrowing (Figure 2). All stenoses showed an increase of Doppler signal within the atherosclerotic lesion (Vmax >350 cm/s), indicating a hemodynamically relevant stenosis. The IMT or plaque thickness was significantly increased not only at the site of stenosis (intrastenosis: 4.1±0.7 mm) but also downstream (poststenosis: 2.2±0.3 mm) compared with the proximal reference segment (prestenosis: 1.5±0.1 mm). This went along with a significant narrowing of the luminal diameter within but also distal to the stenosis relative to the proximal reference segment. We observed a further local reduction of FMD within and downstream from the stenosis (intrastenosis: 1.7±0.7%, poststenosis: 2.2±0.6%) compared with prestenosis (4.1±0.3%). NMD was decreased (prestenosis: 8.8±0.8%, intrastenosis: 4.6±0.9%, poststenosis: 8.0±0.8%) only inside the stenosis.
Figure 2.
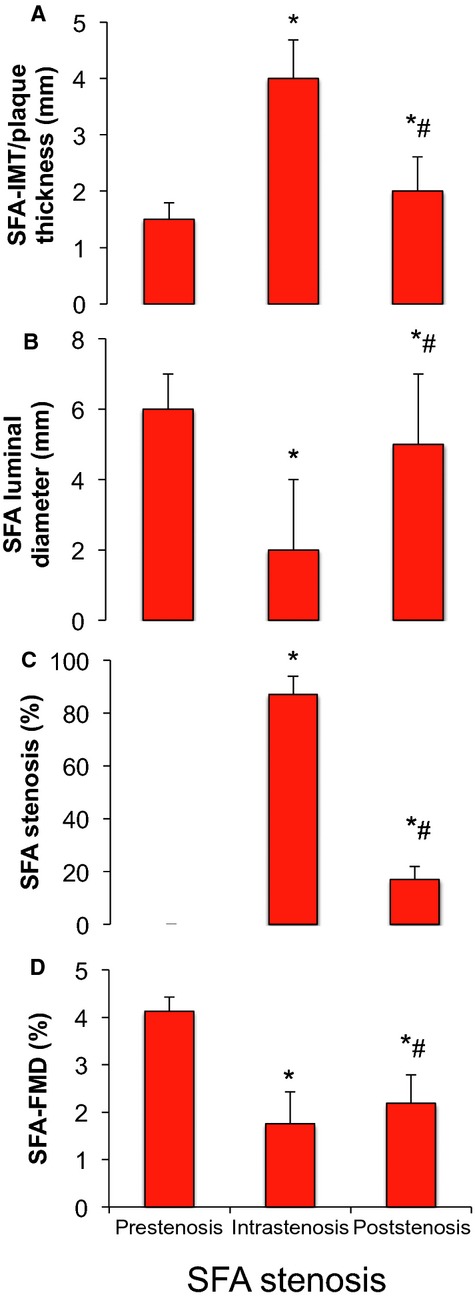
IMT or plaque thickness (A), luminal diameter (B), degree of stenosis (C), and FMD (D) of proximal to SFA stenosis (prestenosis), within SFA stenosis (intrastenosis), and distal to SFA stenosis (poststenosis). *P<0.05 vs prestenosis, #P<0.05 vs intrastenosis (1‐way repeated measures ANOVA). FMD indicates flow‐mediated vasodilation; IMT, intima media thickness; SFA, superficial femoral artery.
Improvement of Endothelial Function After Balloon Angioplasty
The patients with SFA‐PAD described in the previous paragraph underwent balloon angioplasty of the SFA stenosis. Measurements of FMD and local arterial stiffness were performed at 6 and 24 hours. For both FMD and fractional diameter change measurements, the treatment effects for overall 1‐way repeated measures ANOVA were highly significant (P<0.0001 each).
As depicted in Figure 3, FMD was significantly decreased at 6 hours after PTA compared with pre‐PTA values in the prestenotic segment (baseline: 4.1±0.3%, 6 hours: 1.5±0.7%) and the intrastenotic segment (baseline: 1.7±0.7%, 6 hours: 0.3±0.3%), likely because of temporal angioplasty‐related denudation.10 Interestingly, poststenotic FMD was significantly increased at 6 hours after PTA compared with baseline values measured at the same poststenotic location (baseline: 2.2±0.6%, 6 hours: 4.1±0.3%), consistent with re‐established unobstructed blood flow and no significant denudation. At 24 hours, the pre‐ and intrastenosis SFA‐FMD had increased to values observed before PTA (prestenosis: 4.1±0.5%, intrastenosis: 4.2±0.4%, poststenosis: 4.1±0.4%), and there was no significant difference between groups; however, the SFA‐FMD values did not increase to values observed in the BA or the SFA‐FMD values in the No‐PAD group (5.4±0.6%) or in healthy controls (7.1±1.1%).
Figure 3.
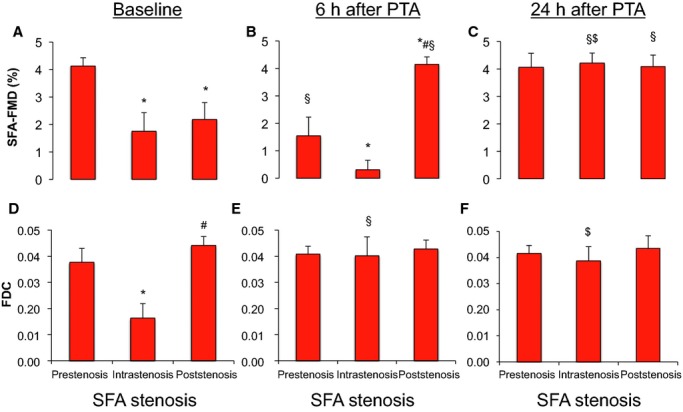
FMD (A–C) and FDC (D–F) of SFA pre‐, intra‐, and poststenosis at baseline before (A, D), at 6 hours (B, E), and 24 hours (C, F) after PTA. *P<0.05 vs prestenosis at same time, #P<0.05 vs intrastenosis at same time, §P<0.05 vs baseline at same location, $P<0.05 vs 6 hours after PTA at same location (1‐way repeated measures ANOVA). FDC indicates fractional diameter change; FMD, flow‐mediated vasodilation; PTA, percutaneous transluminal angioplasty; SFA, superficial femoral artery.
Importantly, we observed significantly increased local stiffness, as measured by a significantly lowered fractional diameter change intrastenosis (0.02±0.01) compared with pre‐ and poststenotic values (prestenosis: 0.04±0.01; poststenosis: 0.04±0.01). At 6 hours after PTA, the intrastenotic fractional diameter change increased to pre‐ and poststenosis values (prestenosis: 0.04±0.01, intrastenosis: 0.04±0.01, poststenosis: 0.04±0.01). BA‐FMD values were not affected by the interventions, ruling out systemic effects (baseline: 6.2±0.6%, 6 hours: 6.2±0.6%, 24 hours: 6.1±0.3%). This suggested that a structural restriction within the stenosis may physically limit vasodilation within stenoses in response to vasodilators limiting endothelium‐dependent vasodilation extending to segments downstream from the stenosis. The functional impairment at the poststenotic segment was rather due to an endothelial functional impairment as endothelium‐independent vasodilation was preserved and to similar local stiffness compared with prestenosis values.
Discussion
The key finding of the present study is that segmental and local endothelial dysfunction exists in the SFA of PAD patients. Local FMD responses showed a significant correlation with local IMT and plaque thickness. Further local impairment of FMD was noted within and downstream from SFA stenosis; this recovered after SFA balloon angioplasty and may be ascribed to structural and function impairments, respectively.
Endothelial Dysfunction in PAD Patients
PAD patients are at increased risk of experiencing major adverse cardiovascular events, making detection and management of PAD an important public health priority.4 Several studies have demonstrated systemic endothelial dysfunction, as measured by impaired FMD of the BA—a nonatherosclerosis prone vessel—in patients with cardiovascular disease including CAD and PAD, predicting overall cardiovascular risk.11–14 Although some studies show that BA‐FMD values are not different in patients with PAD and patients with only CAD,15 others have reported in a large cohort that patients with PAD exhibit more severe impairment of vascular function (BA‐FMD) than age‐matched controls and patients with CAD but without PAD.16 This underscores a major limitation of the BA‐FMD approach, that only some factors causing endothelial dysfunction and consecutive accelerated intimal hyperplasia may act systemically and that other factors may act instead on 1 segment or location of the arterial tree (eg, carotid, femoral, coronary). BA‐FMD correlates only modestly with endothelial function of other vascular beds, including the coronary17 and lower limb arterial segment.18 In the current study, we observed that BA‐FMD was not different between CAD patients with and without PAD. This may be due to a major limitation of the present study, the relatively small sample size, together with a relatively small difference in the systemic factors causing endothelial dysfunction due to PAD with a background of pre‐existing CAD. Taken together, the present study clearly demonstrates a spatial association of endothelial function with respect to localization of intimal hyperplasia and plaque development that was not studied previously.
Segmental Endothelial Dysfunction in PAD Patients
Studies of the endothelial function in lower limb arteries are sparse, and SFA‐FMD measurements did not correlate well with BA‐FMD in an unselected population of supposedly healthy persons.18 To the best of our knowledge, our study is the first to study FMD in the SFA of patients with and without PAD and in patients with PAD locally at an SFA plaque before and after PTA. We measured the SFA‐FMD using methodologies similar to those described by others, and the SFA‐FMD values in our control subjects were comparable to results published by others.19–20 In the present study, we showed that an additional lowering of endothelial function in the SFA of patients with PAD compared with the BA and with matched controls. Furthermore, we showed intimal thickening even at proximal smooth SFA areas in patients with PAD. According to the Mannheim criteria (focal structure that encroaches into the arterial lumen of at least 0.5 mm or 50% of the surrounding IMT value or demonstrates a thickness >1.5 mm as measured from the media–adventitia interface to the intima–lumen interface),9 IMT values detected even at the visually smooth proximal segments of the SFA qualify as plaque or are close to it (plaque thickness 1.5 mm). Mechanistically, our current data provide correlative evidence that segmental lower extremity endothelial dysfunction may play a role in the pathophysiology of PAD; however, our data cannot answer the question of whether decreased FMD is secondary to the presence of atherosclerotic disease in the lower extremity or whether certain risk factors for PAD including smoking might have induced regional endothelial dysfunction and thereby predisposed to the development and progression of PAD.21 Our data on the response of local SFA‐FMD to PTA suggest that within a focal stenosis, mechanical factors lead to a restriction of vasodilator function, whereas downstream endothelial dysfunction is rather functional and recovers fast after reconstitution of blood flow. Further studies are needed to define molecular pathways specific to the lower extremity and potentially explain the increased vulnerability of certain patients’ peripheral arteries to develop PAD. Nevertheless, the measurement of SFA‐FMD is a promising technique that may be used to detect patients at increased risk for development of PAD and may serve as a therapeutic target parameter in the primary and secondary prevention of PAD. This needs to be shown in larger scale longitudinal studies addressing whether local endothelial dysfunction precedes plaque development.
Conclusion
Our data demonstrate a close association between local endothelial function and atherosclerotic structural remodeling and suggest that in PAD, systemic, segmental, and local factors that can be structural or functional in nature influence lower extremity endothelial function. Our data suggest that segmental lower extremity endothelial dysfunction may play a role in the pathophysiology of PAD. Furthermore, the results imply that even interventional treatment of PAD may help disrupt further development of PAD progression by targeting flow restrictions and re‐establishing better endothelial function downstream. Future studies should address whether endothelial heterogeneity exists with increased vulnerability of lower limb arterial endothelium toward certain environmental factors including smoking, kidney failure, or diabetes.
Acknowledgments
Technical assistance was provided by Daniel Boeing.
Sources of Funding
Heiss received research funding from the Forschungskommission of the Heinrich‐Heine University, Düsseldorf, Germany. Additional funding was provided by the Deutsche Forschungsgemeinschaft (KE405/5‐1, IRTG1902 TP9, and FOR809 TP7 to Kelm; RA969/7‐2 to Rassaf).
Disclosures
None.
References
- Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, Creager MA, Easton JD, Gavin JR, III, Greenland P, Hankey G, Hanrath P, Hirsch AT, Meyer J, Smith SC, Sullivan F, Weber MAPrevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. 2003; 163:884-892. [DOI] [PubMed] [Google Scholar]
- Yataco AR, Corretti MC, Gardner AW, Womack CJ, Katzel LI. Endothelial reactivity and cardiac risk factors in older patients with peripheral arterial disease. Am J Cardiol. 1999; 83:754-758. [DOI] [PubMed] [Google Scholar]
- Ouriel K. Peripheral arterial disease. Lancet. 2001; 358:1257-1264. [DOI] [PubMed] [Google Scholar]
- Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clement D, Collet JP, Cremonesi A, De Carlo M, Erbel R, Fowkes FG, Heras M, Kownator S, Minar E, Ostergren J, Poldermans D, Riambau V, Roffi M, Rother J, Sievert H, van Sambeek M, Zeller T. ESC guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the task force on the diagnosis and treatment of peripheral artery diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011; 32:2851-2906. [DOI] [PubMed] [Google Scholar]
- Stehouwer CD, Clement D, Davidson C, Diehm C, Elte JW, Lambert M, Sereni DEFIM Vascular Medicine Working Group. Peripheral arterial disease: a growing problem for the internist. Eur J Intern Med. 2009; 20:132-138. [DOI] [PubMed] [Google Scholar]
- Heiss C, Rodriguez‐Mateos A, Kelm M. Central role of eNOS in the maintenance of endothelial homeostasis. Antioxid Redox Signal. 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ras RT, Streppel MT, Draijer R, Zock PL. Flow‐mediated dilation and cardiovascular risk prediction: a systematic review with meta‐analysis. Int J Cardiol. 2013; 168:344-351. [DOI] [PubMed] [Google Scholar]
- Heiss C, Jahn S, Taylor M, Real WM, Angeli FS, Wong ML, Amabile N, Prasad M, Rassaf T, Ottaviani JI, Mihardja S, Keen CL, Springer ML, Boyle A, Grossman W, Glantz SA, Schroeter H, Yeghiazarians Y. Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease. J Am Coll Cardiol. 2010; 56:218-224. [DOI] [PubMed] [Google Scholar]
- Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima‐media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012; 34:290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss C, Balzer J, Hauffe T, Hamada S, Stegemann E, Koeppel TA, Merx M, Rassaf T, Kelm M, Lauer T. Vascular dysfunction of brachial artery following transradial access for coronary catheterization: impact of smoking and catheter changes. JACC Cardiovasc Interv. 2009; 2:1067-1073. [DOI] [PubMed] [Google Scholar]
- Perrone‐Filardi P, Cuocolo A, Brevetti G, Silvestro A, Storto G, Dellegrottaglie S, Corrado L, Cafiero M, Camerino R, Polimeno M, Zarrilli A, Caiazzo G, Maglione A, Petretta A, Chiariello M. Relation of brachial artery flow‐mediated vasodilation to significant coronary artery disease in patients with peripheral arterial disease. Am J Cardiol. 2005; 96:1337-1341. [DOI] [PubMed] [Google Scholar]
- Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long‐term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003; 41:1769-1775. [DOI] [PubMed] [Google Scholar]
- Silvestro A, Scopacasa F, Ruocco A, Oliva G, Schiano V, Zincarelli C, Brevetti G. Inflammatory status and endothelial function in asymptomatic and symptomatic peripheral arterial disease. Vasc Med. 2003; 8:225-232. [DOI] [PubMed] [Google Scholar]
- Pellegrino T, Storto G, Filardi PP, Sorrentino AR, Silvestro A, Petretta M, Brevetti G, Chiariello M, Salvatore M, Cuocolo A. Relationship between brachial artery flow‐mediated dilation and coronary flow reserve in patients with peripheral artery disease. J Nucl Med. 2005; 46:1997-2002. [PubMed] [Google Scholar]
- Maldonado FJ, Miralles Jde H, Aguilar EM, Gonzalez AF, Garcia JR, Garcia FA. Relationship between noninvasively measured endothelial function and peripheral arterial disease. Angiology. 2009; 60:725-731. [DOI] [PubMed] [Google Scholar]
- Kiani S, Aasen JG, Holbrook M, Khemka A, Sharmeen F, LeLeiko RM, Tabit CE, Farber A, Eberhardt RT, Gokce N, Vita JA, Hamburg NM. Peripheral artery disease is associated with severe impairment of vascular function. Vasc Med. 2013; 18:72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delegrange D, Lieberman EH, Ganz P, Creager A, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulation. J Am Coll Cardiol. 1995; 26:1235-1241. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Rowley N, Padilla J, Simmons GH, Laughlin MH, Whyte G, Cable NT, Green DJ. Relationship between upper and lower limb conduit artery vasodilator function in humans. J Appl Physiol. 2011; 111:244-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot PC, Poelkens F, Kooijman M, Hopman MT. Preserved flow‐mediated dilation in the inactive legs of spinal cord‐injured individuals. Am J Physiol Heart Circ Physiol. 2004; 287:H374-H380. [DOI] [PubMed] [Google Scholar]
- Gaenzer H, Neumayr G, Marschang P, Sturm W, Kirchmair R, Patsch JR. Flow‐mediated vasodilation of the femoral and brachial artery induced by exercise in healthy nonsmoking and smoking men. J Am Coll Cardiol. 2001; 38:1313-1319. [DOI] [PubMed] [Google Scholar]
- Yataco AR, Gardner AW. Acute reduction in ankle/brachial index following smoking in chronic smokers with peripheral arterial occlusive disease. Angiology. 1999; 50:355-360. [DOI] [PubMed] [Google Scholar]


