Abstract
Background
Potent anti‐inflammatory rheumatoid arthritis (RA) treatments are associated with reduced cardiovascular risk as well as increases in low‐density lipoprotein (LDL) cholesterol. This apparent paradox may be explained by favorable changes in other lipid measurements. The objective of this study was to determine the longitudinal association between changes in inflammation with advanced lipoprotein measurements and high‐density lipoprotein (HDL) cholesterol efflux capacity.
Methods and Results
We conducted this study in a longitudinal RA cohort from a large academic center, including subjects with high‐sensitivity C‐reactive protein (hs‐CRP) reduction ≥10 mg/L at 2 time points 1 year apart. Subjects receiving statins during the study period or preceding 6 months were excluded. We compared total cholesterol, LDL cholesterol, HDL cholesterol, apolipoprotein B, and apolipoprotein A1 levels and HDL cholesterol efflux capacity at baseline and 1‐year follow‐up by using the paired t test. We also assessed the correlations between reductions in hs‐CRP with percentage change in lipid parameters. We studied 90 RA subjects (mean age 57 years, 89% female), all of whom were receiving disease‐modifying antirheumatic drugs. We observed a 7.2% increase in LDL cholesterol levels (P=0.02) and improvement in efflux capacity by 5.7% (P=0.002) between baseline and follow‐up, with a median hs‐CRP reduction of 23.5 mg/L. We observed significant correlations between reductions in hs‐CRP with increases in apolipoprotein A1 (r=0.27, P=0.01) and HDL cholesterol efflux capacity (r=0.24, P=0.02).
Conclusion
Among RA subjects experiencing reductions in hs‐CRP, we observed increased LDL cholesterol levels and concomitant improvements in HDL cholesterol efflux capacity. These findings provide further insight into lipid modulation and the beneficial effect of reduction in inflammation on lipids in vivo.
Keywords: inflammation, lipids, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) patients are at a 2‐fold risk for cardiovascular (CV) disease compared with the general population.1–2 This elevated risk is largely attributed to inflammation rather than to an increased prevalence of traditional CV risk factors such as hyperlipidemia. In fact, RA patients have lower total cholesterol (TC) and low‐density lipoprotein cholesterol (LDL‐C) levels than those in the general population.3–6 In RA, lower levels of TC and LDL‐C were found to be associated with higher CV risk, termed the “lipid paradox.”7–8 Large observational studies of the relationship between lipids and CV risk have also yielded seemingly paradoxical results. Tumor necrosis factor inhibitor (TNFi) therapy was associated with elevations in TC and LDL‐C by as much as 30%.9–10 However, in separate observational studies, TNFi use was associated with significantly lower CV risk compared with RA patients receiving other nonbiologic disease‐modifying anti‐rheumatic drugs (DMARDs).11–13 Together, the evidence suggests that treatments aimed at reducing inflammation are associated with an overall reduction in CV risk, despite increases in LDL‐C levels. Few studies have directly examined the association between longitudinal changes in serum inflammatory markers (eg, C‐reactive protein [CRP]) and changes in routinely measured lipid levels such as LDL‐C.
While routinely measured lipids may not associate well with CV risk due to fluctuations in inflammation, other measurements such as high‐density lipoprotein (HDL) cholesterol efflux capacity, which is the ability of HDL particles to extract cholesterol from lipid‐laden macrophages, may be better correlated. Few studies have evaluated inflammation with longitudinal changes in HDL efflux capacity, because both inflammation and HDL efflux capacity are considered relatively stable in the general population in the absence of treatment.14 In RA, there is evidence that level of inflammation is associated with HDL cholesterol efflux capacity. A cross‐sectional study (n=25) found that RA patients with a high level of disease activity had impaired HDL function compared with subjects with low disease activity.15 HDL cholesterol efflux capacity is associated with an increased risk of CV disease independent of HDL‐cholesterol (HDL‐C) levels14,16 and may be a more useful marker for CV risk prediction for patients with RA and other inflammatory diseases compared with the use of LDL‐C as a marker.
Current CV risk estimators such as the Framingham Risk and Reynolds’ Risk Score underestimate CV risk in RA by 2‐fold in women with RA.17 Understanding the relationship between inflammation and changes in lipid parameters is a key step for informing CV risk management in RA. The objective of this study was to provide an overview of changes in routine lipids including TC, LDL‐C, and HDL‐C, as well as advanced lipoprotein measurements including apolipoprotein (apo)B, apoA1, and HDL cholesterol efflux capacity in RA patients who experience a significant reduction in inflammation as measured with high‐sensitivity CRP. Our second objective was to quantify the correlation between longitudinal changes in CRP with changes in these lipid parameters. We hypothesized that a reduction in CRP would be associated with increases in LDL‐C and concomitant improvements in HDL cholesterol efflux capacity.
Methods
We conducted this study in the Brigham and Women's Hospital Rheumatoid Arthritis Sequential Study (BRASS),18 a prospective observational cohort study. All subjects were age 18 or older and had a rheumatologist diagnosis of RA. Subjects in BRASS had annual clinical assessments including measurements of RA disease activity, assessments of CV risk factors, and CRP. Details of this cohort were previously reported.18 We included subjects in BRASS who experienced a CRP reduction ≥10 mg/L at 2 consecutive time points, 1 year apart, and with blood samples available for analysis. Samples were measured for TC, LDL‐C, HDL‐C, apoA1, apoB, and HDL cholesterol efflux capacity. Because statins are potent LDL‐C–lowering agents, we included only patients who did not receive statins 6 months prior to the baseline blood collection date and during the 1‐year follow‐up period.
High‐sensitivity CRP was measured by using a standard immunoturbidimetric assay on the Roche P Modular system (Roche Diagnostics) with reagents and calibrators from Roche. In this assay, an antigen–antibody reaction occurs between CRP in the sample and an anti‐CRP antibody sensitized to latex particles resulting in agglutination. The antigen–antibody complex is detected spectrophotometrically with the magnitude of change proportional to the concentration of CRP in the sample.19 TC, LDL‐C, HDL‐C, apoA1, and apoB measurements were performed according to standardized techniques in the clinical laboratories.20–21 We performed HDL cholesterol efflux capacity per published methods by using J774 cells derived from a murine macrophage cell line.14 Briefly, the J774 cells were plated and radiolabeled with 2 mCi of 3H‐cholesterol/mL. ATP‐binding cassette transporter A1 (ABCA1) was up‐regulated by means of a 16‐hour incubation with 0.3 mmol/L 8‐(4‐chlorophenylthio)‐cAMP. We added 2.8% apoB‐depleted serum to the efflux medium for 4 hours. To quantify the efflux of radioactive cholesterol from the cells, we used liquid scintillation counting. Efflux was calculated by using the following formula: ([microcuries of 3H‐cholesterol in media containing 2.8% apoB‐depleted serum‐microcuries of 3H‐cholesterol in serum‐free media]/[microcuries of 3H‐cholesterol in cells extracted before the efflux step]). All assays were performed in duplicate.
We tested for significant differences in TC, LDL‐C, HDL‐C, apoA1, apoB, and HDL cholesterol efflux capacity at baseline and 1‐year follow‐up by using the paired t test. For the primary analyses, we determined the correlations between changes in CRP and the percentage change in each lipid parameter [(lipidfollow‐up−lipidbaseline))/lipidbaseline] by using the Pearson correlation test. Because of the non‐normal distribution of CRP, we performed all correlation and association studies by using the natural log of the change (reduction) in CRP (CRPbaseline−CRPfollow‐up). We performed a sensitivity analysis by using a Spearman rank correlation test between the change in CRP (CRPbaseline−CRPfollow‐up) and percentage change in the lipid parameters.
As a secondary analysis, we tested for confounding of the association between changes in CRP and lipids by age and sex. Additionally, we tested the association between the change in CRP and HDL cholesterol efflux capacity adjusted by change in HDL‐C levels in one model and change in apoA1 levels in a second model. To determine whether the changes in the lipid parameters may be more specific to serum markers of inflammation compared with other clinical measures of RA disease activity, we tested the association between changes in the RA Disease Activity Score 28 (DAS28‐CRP) and changes in HDL cholesterol efflux capacity. The DAS28 is an index score that includes objective and subjective clinical signs and symptoms of RA: the swollen and tender joint count of 28 joints, CRP level, and the patient global score of arthritis disease activity over the past week (scale of 0 to 10, with 0 being no disease activity and 10 being the worst disease activity).22 High disease activity as defined by DAS28‐CRP is a score of >5.1, moderate disease activity is >3.2 and ≤5.1, low disease activity is ≥2.6 to ≤3.2, and remission is <2.6.23
All aspects of this study were approved by the Partners Healthcare Institutional Review Board, and the subjects gave informed consent. Analyses were performed by using SAS 9.2 (SAS Institute).
Results
We studied 90 RA cases with a mean age of 57.0; 89% were female, 78% were anti‐CCP positive, and they had a mean RA disease duration of 16.5 years (Table 1). The median CRP at baseline was 28.6 mg/L, and the mean DAS28 was 5.0 (moderate disease activity). The median CRP at 1‐year follow‐up was 4.3 (IQR 5.6), an absolute reduction of 23.5 mg/L (IQR 54.0, P<0.0001), and the mean reduction in DAS28‐CRP was 1.6 (SD 1.5, P<0.0001). This represented an overall 85% reduction in median CRP and a significant change in the DAS28‐CRP from the higher end of moderate to low disease activity. All subjects were receiving ≥1 DMARD with the majority of subjects receiving methotrexate (53%) or a TNFi (48%); 27% were receiving combination MTX and TNFi at baseline.
Table 1.
Baseline Clinical Characteristics of RA Cohort
| Characteristics | N=90 |
|---|---|
| Age, mean (SD) y | 57.0 (12.0) |
| Female, % | 88.9 |
| RA disease duration, mean (SD) y | 16.5 (11.5) |
| Anti‐CCP positive, n (%) | 70 (77.8) |
| RF positive, n (%) | 67 (74.4) |
| BMI, mean (SD) kg/m2 | 27.0 (5.5) |
| Diabetes mellitus, n (%) | 11 (12.2) |
| Hyperlipidemia, n (%) | 19 (21.0) |
| Hypertension, n (%) | 31 (34.4) |
| History of ischemic heart disease, n (%) | 6 (6.7) |
| Hs‐CRP, median (IQR), mg/L | 28.6 (21.7) |
| DAS28, mean (SD) | 5.0 (1.6) |
| RA treatment, n (%) | |
| Methotrexate | 44 (53.1) |
| Tumor necrosis factor inhibitor | 40 (48.2) |
| Other DMARDs | 13 (14.4) |
Anti‐CCP indicates anti‐cyclic citrullinated peptide; RA, rheumatoid arthritis; RF, rheumatoid factor; BMI, body mass index; hs‐CRP, high‐sensitivity C‐reactive protein; DAS28, Disease Activity Score 28; DMARD, disease‐modifying anti‐rheumatic drug; .
Compared with baseline, we observed a significant increase in LDL‐C by 7.2% (P=0.02) and improvements in HDL cholesterol efflux capacity by 5.7% (P=0.002) (Table 2). There was a trend toward increased apoA1 of 4.1% (P=0.05). We observed no significant changes in HDL‐C, apoB, and 2 measures of the atherogenic index TC/HDL‐C or apoB/apoA1 between baseline and 1‐year follow‐up.
Table 2.
Mean Change in Lipids, Lipoproteins, and HDL Cholesterol Efflux Capacity Between Baseline and 1‐Year Follow‐up (N=90*)
| Measurement | Baseline (SD) | Follow‐up (SD) | Mean ∆ (SD) | P Value |
|---|---|---|---|---|
| Total cholesterol, mg/dL | 187.2 (43.1) | 183.2 (37.0) | −4.4 (35.2) | 0.24 |
| LDL‐C, mg/dL | 102.0 (32.4) | 109.0 (34.6) | +7.0 (3.0) | 0.02* |
| HDL‐C, mg/dL | 65.3 (20.0) | 66.3 (20.5) | +0.99 (12.8) | 0.50 |
| apoB, mg/dL | 92.1 (25.1) | 95.1 (26.2) | +3.1 (19.3) | 0.14 |
| apoA1, mg/dL | 183.8 (45.0) | 191.4 (44.0) | +7.6 (35.8) | 0.05 |
| HDL cholesterol efflux capacity | 1.05 (0.17) | 1.11 (0.16) | +0.06 (0.16) | 0.002* |
| Atherogenic indices | ||||
| Total cholesterol/HDL‐C | 3.2 (1.2) | 3.0 (1.2) | −0.11 (0.88) | 0.21 |
| apoB/apoA1 | 0.53 (0.19) | 0.52 (0.2) | −0.006 (0.10) | 0.72 |
Median change in CRP was a reduction of 23.5 mg/L (IQR 54.0) between baseline and follow‐up. HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; apoB, apolipoprotein B; apoA1, apolipoprotein A1; CRP, C‐reactive protein.
Significant, P<0.05
N=87 for LDL‐C, HDL‐C, apoB, and apoA1 due to insufficient sample volumes in 3 subjects.
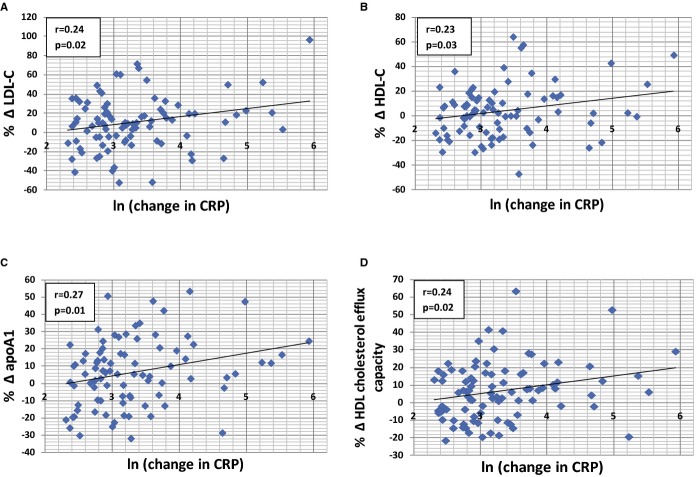
We observed significant correlations between a reduction in CRP with increases in LDL‐C, HDL‐C, and apoA1 and improvements in HDL cholesterol efflux capacity (Figure). No significant correlation was observed between change in CRP and apoB levels (r=0.14, P=0.20) and the atherogenic indices (TC/HDL‐C, r=−0.21, P=0.05 and apoB/apoA1, r=−0.10, P=0.33). In the sensitivity analysis, the significant correlation between a reduction in CRP and apoA1 and HDL cholesterol efflux capacity remained (apoA1, r=0.27, P=0.009; HDL cholesterol efflux capacity, r=0.24, P=0.03). However, the correlations between reduction in CRP with increases in LDL‐C (r=0.18, P=0.10) and HDL‐C (r=0.17, P=0.10) had the same trend as the primary analysis but did not reach statistical significance.
Figure 1.
Correlations between magnitude of reduction in CRP (natural log transformed) and the percentage change in (A) LDL‐C, (B) HDL‐C, (C) apoA1, and (D) HDL cholesterol efflux capacity between baseline and 1‐year follow‐up. apoA1 indicates apolipoprotein A1; CRP, C‐reactive protein; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
We examined the association between change in CRP with the lipid parameters adjusted by age and sex and found that neither age or sex was significant in the models. The reduction in CRP remained significantly associated with all lipid measurements after adjustment for age and sex: LDL‐C (P=0.02), HDL‐C (P=0.04), apoA1 (P=0.02), and HDL cholesterol efflux capacity (P=0.03).
In a secondary analysis, we investigated the association between reduction in CRP and HDL cholesterol efflux when adjusting for changes in HDL‐C and apoA1 levels. The association was attenuated after adjusting for changes in HDL‐C levels (P=0.06) and apoA1 levels (P=0.14). While we found no significant correlation between changes in HDL‐C levels and HDL cholesterol efflux capacity (r=0.17, P=0.12), we observed a significant correlation between changes in apoA1 levels with improvements in HDL cholesterol efflux capacity (r=0.33, P=0.002).
None of the changes in DAS28‐CRP were significantly correlated with lipid parameters; however, the trends were similar to those observed with changes in CRP and lipid parameters (Table 3).
Table 3.
Correlation Between Reduction in DAS28 and Percentage Change in Lipid and Lipoprotein Parameters
| Measurements | Correlation | P Value |
|---|---|---|
| Total cholesterol | 0.06 | 0.56 |
| LDL‐C | 0.10 | 0.35 |
| HDL‐C | 0.09 | 0.42 |
| apoB | 0.03 | 0.76 |
| apoA1 | 0.01 | 0.88 |
| HDL cholesterol efflux capacity | 0.12 | 0.29 |
| Atherogenic indices | ||
| TC/HDL‐C | 0.02 | 0.86 |
| apoB/apoA1 | 0.03 | 0.76 |
LDL‐C indicates low‐density lipoprotein cholesterol; HDL‐C, high‐density lipoprotein cholesterol; apoB, apolipoprotein B; apoA1, apolipoprotein A1; DAS28, Disease Activity Score 28; TC, total cholesterol.
Discussion
Among RA subjects who experienced a reduction in CRP, we observed a significant increase in LDL‐C levels and concomitant improvement in HDL cholesterol efflux capacity. The magnitude of CRP reduction was significantly correlated with larger reductions in CRP were correlated with larger increases in ApoA1 and larger improvements in HDL cholesterol efflux capacity. A trend for correlation also existed between the magnitude of CRP reduction and increases in LDL‐C and HDL‐C. These findings provide a potential explanation for the apparent paradox observed in the associations between lipid levels and CV risk in RA.7–8 Concurrent with increases in LDL‐C were favorable lipid changes that may counterbalance the elevated CV risk associated with higher LDL‐C levels. These changes included improvements in the ability of HDL to efflux cholesterol from lipid laden plaques, alongside stable apoB levels, considered a more direct measure of atherogenic non–HDL‐C particles than LDL‐C.24
The relation between a reduction in CRP and changes in lipid parameters was present despite patients receiving various RA treatment modalities, suggesting that the association is not treatment specific. The trend for an inverse relation between inflammation and HDL‐C levels observed in this present study corroborate findings from a cross‐sectional study of 11,437 subjects with simultaneous CRP and HDL‐C levels from 4 large biochemistry laboratories.25 In this heterogeneous population, higher levels of CRP, defined as levels >5 mg/L, were associated with lower levels of HDL‐C. While we believe our findings support a link between reduction in inflammation and reduction in CV risk, our results cannot account for whether specific RA treatments may have additional beneficial or deleterious influences on CV risk beyond modifying inflammation.
Changes in lipid levels were more closely associated with changes in CRP than the DAS28, a composite measure of RA disease activity that includes objective (eg, swollen joint count) and subjective (eg, patient reported global health) measures. Although the study subjects experienced significant improvements in DAS28, suggesting a response to therapy,26 we did not observe a significant correlation between DAS28 reduction and changes in the lipid parameters. This lack of correlation was not surprising. The DAS28 is a composite index that includes subjective measures such as the patient's assessment of arthritis activity over the past week. DAS28 scores can remain high in the absence of inflammation, particularly in subjects with persistent pain secondary to structural joint damage from RA.27 Thus, CRP and potentially other serum inflammatory markers may be more optimal biomarkers to interpret lipid changes than global RA disease activity indices.
We believe this study provides convincing evidence showing that reduction in inflammation is associated with improvements in HDL cholesterol efflux capacity, consistent with the limited human studies to date. In cross‐sectional studies, HDL cholesterol efflux capacity is impaired in RA subjects with high disease activity compared with those with low disease activity,15 as well as other inflammatory diseases such as systemic lupus erythematosus28 and psoriasis,29 compared with controls. Evidence for a longitudinal relationship between inflammation and HDL cholesterol efflux capacity was demonstrated in a study that induced low‐grade endotoxemia, a state of heightened inflammation, in 20 healthy adults and found impairment in HDL cholesterol efflux function when comparing HDL function before and after endotoxemia.30 A study of 15 subjects found that anti‐psoriatic treatment was associated with a significant improvement in HDL cholesterol efflux capacity.31 In addition, a recent study in mice linked myeloperoxidase‐mediated oxidation of apoA1 to impairment of reverse cholesterol transport in vivo and the ability of apoA1 to reduce lipid and macrophage content in atherosclerotic lesions.32 Increased myeloperoxidase activity is associated with higher CRP levels in RA.15
In RA, knowledge of the inflammatory state may have additional importance for interpretation of lipid levels and CV risk. An RA patient with a low LDL‐C and high CRP may be at elevated CV risk, based on other lipid parameters such as impaired HDL cholesterol efflux function, compared with a patient with a higher LDL‐C level but well‐controlled disease and normal HDL cholesterol efflux function. Whether the effect of reducing inflammation in the general population will have a significant impact on HDL cholesterol efflux capacity is not clear. The CV risk reduction associated with lowering inflammation as measured by CRP in the general population was demonstrated in several randomized controlled trials by using statin therapy.33–35 However, the magnitude of inflammation and, by definition, the amount of reduction that can occur in an individual without an underlying inflammatory disease are smaller compared with an individual with RA. As an example, an individual with high‐sensitivity CRP >3 mg/L in the general population is considered to have elevated CV risk,36–37 while the mean high‐sensitivity CRP in a treated cohort of RA patients is 9.7 mg/L.18
Interestingly, while LDL‐C levels increased with reduction in inflammation, the levels of apoB that typically mirror LDL‐C levels in the general population remained stable. The mechanism underlying discrepant changes in LDL‐C and apoB in this study is unclear. The Friedewald equation used to calculate LDL‐C may be inaccurate when triglyceride levels are high38 but would not explain the consistent increases in LDL‐C observed across different studies associated with RA biologic therapy.9–10,9–44 DMARDs are not known to be associated with significant increases in triglyceride levels.41,45–46 This finding requires careful follow‐up studies using LDL‐particle number and size to provide further mechanistic insight.
Concurrent with a reduction in CRP and increases in LDL‐C, we observed increases apoA1 and a significant improvement in HDL cholesterol efflux capacity, pointing toward an improved lipid profile and reduced CV risk. While we did not observe a significant improvement in the atherogenic index, as has been observed in some RA treatment studies,47 we observed a trend toward reduced atherogenicity of the lipid profile as measured with TC/HDL‐C and apoB/apoA1, suggesting larger studies are needed to evaluate this relationship.
We acknowledge limitations to this study. Lipid measurements were not performed in the fasting state. However, there are data to suggest that the variation between fasting and nonfasting states for TC, LDL‐C, and HDL‐C would not significantly impact interpretation of CV risk. In a population‐based study, mean levels varied by <2% for TC and HDL‐C and <10% for LDL‐C.48 We could not account for patients who underwent lifestyle modifications that could result in lipid changes between baseline and 1‐year follow‐up. While we reviewed medical charts for statin use before and during the 1‐year follow‐up, it is possible that additional patients on statins were missed. Due to the methods used for sample collection and storage in the RA cohort, which were incompatible with NMR analyses, we were unable to further explore whether the discrepancy between the changes in LDL‐C and apoB levels were true changes or artifacts in assay sensitivity.
In summary, RA patients experiencing a reduction in inflammation may have an overall improved lipid profile despite increased LDL‐C levels. Reduction in CRP was correlated with increases in apoA1 and improvements in the HDL cholesterol efflux capacity. These findings highlight the importance of incorporating levels and changes in inflammation when considering lipid management and CV risk assessment in patients with RA and potentially other inflammatory diseases. Further, these data may provide insight into pathways for elevated CV risk in patients in the general population with higher levels of CRP. Future studies are planned to evaluate lipid composition by using NMR, which can provide information on the size and number of LDL and HDL particles with changes in levels of inflammation. Moreover, studies are needed to investigate the relationship between changes in inflammation and lipid levels and the impact of these changes on CV risk in RA.
Acknowledgments
We acknowledge Gary Bradwin and Dr Nader Rifai, Children's Hospital Boston, for their valuable suggestions on lipoprotein assays and expertise on methods for measurement.
Sources of Funding
Dr Liao is supported by National Institutes of Health (NIH) grant K08 AR060257 and the Harold and Duval Bowen Fund. Dr Mehta is funded by grant HL‐Z‐0000 from the Division of Intramural Research at the NIH.
Disclosures
None of the authors have conflicts of interest relevant to the topic of the manuscript. Dr Weinblatt receives research grant funding from Bristol Myers Squibb, UCB, Crescendo Bioscience; Dr Shadick receives research grant funding from Crescendo Bioscience, Amgen, UCB, Abbvie, BMS, and Genentech.
References
- Avina‐Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta‐analysis of observational studies. Ann Rheum Dis. 2012; 71:1524-1529. [DOI] [PubMed] [Google Scholar]
- Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, Stampfer MJ, Curhan GC. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003; 107:1303-1307. [DOI] [PubMed] [Google Scholar]
- Lazarevic MB, Vitic J, Mladenovic V, Myones BL, Skosey JL, Swedler WI. Dyslipoproteinemia in the course of active rheumatoid arthritis. Semin Arthritis Rheum. 1992; 22:172-178. [DOI] [PubMed] [Google Scholar]
- Liao KP, Cai T, Gainer VS, Cagan A, Murphy SN, Liu C, Churchill S, Shaw SY, Kohane I, Solomon DH, Plenge RM, Karlson EW. Lipid and lipoprotein levels and trends in rheumatoid arthritis compared with the general population. Arthritis Care Res. 2013; 65:2046-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myasoedova E, Crowson CS, Kremers HM, Fitz‐Gibbon PD, Therneau TM, Gabriel SE. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010; 69:1310-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semb AG, Kvien TK, Aastveit AH, Jungner I, Pedersen TR, Walldius G, Holme I. Lipids, myocardial infarction and ischaemic stroke in patients with rheumatoid arthritis in the Apolipoprotein‐related Mortality RISk (AMORIS) Study. Ann Rheum Dis. 2010; 69:1996-2001. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen L, Delzell E, Muntner P, Hillegass WB, Safford MM, Millan IY, Crowson CS, Curtis JR. The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis. 2014; 73:1301-1308. [DOI] [PubMed] [Google Scholar]
- Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz‐Gibbon PD, Therneau TM, Gabriel SE. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011; 70:482-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham BW, Wasko MC, Hsia EC, Fleischmann RM, Genovese MC, Matteson EL, Liu H, Rahman MU. Effects of golimumab, an anti‐tumour necrosis factor‐alpha human monoclonal antibody, on lipids and markers of inflammation. Ann Rheum Dis. 2013; 73:161-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro‐Millan I, Charles‐Schoeman C, Yang S, Bathon JM, Bridges SL, Jr, Chen L, Cofield SS, Dell'Italia LJ, Moreland LW, O'Dell JR, Paulus HE, Curtis JR. Changes in lipoproteins associated with methotrexate or combination therapy in early rheumatoid arthritis: results from the treatment of early rheumatoid arthritis trial. Arthritis Rheum. 2013; 65:1430-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WG, Watson KD, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti‐tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2007; 56:2905-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake SL, Colebatch AN, Baird J, Curzen N, Kiely P, Quinn M, Choy E, Ostor AJ, Edwards CJ. Tumour necrosis factor antagonists and the risk of cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology. 2011; 50:518-531. [DOI] [PubMed] [Google Scholar]
- Bili A, Tang X, Pranesh S, Bozaite R, Morris SJ, Antohe JL, Kirchner HL, Wasko MC. Tumor necrosis factor alpha inhibitor use and decreased risk for incident coronary events in rheumatoid arthritis. Arthritis Care Res. 2014; 66:355-363. [DOI] [PubMed] [Google Scholar]
- Khera AV, Cuchel M, de la Llera‐Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high‐density lipoprotein function, and atherosclerosis. N Engl J Med. 2011; 364:127-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles‐Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, Ranganath VK, Taylor M, McMahon M, Paulus HE, Reddy ST. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012; 71:1157-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014; 371:2383-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012; 110:420-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannaccone CK, Lee YC, Cui J, Frits ML, Glass RJ, Plenge RM, Solomon DH, Weinblatt ME, Shadick NA. Using genetic and clinical data to understand response to disease‐modifying anti‐rheumatic drug therapy: data from the Brigham and Women's Hospital Rheumatoid Arthritis Sequential Study. Rheumatology. 2011; 50:40-46. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non‐HDL cholesterol, apolipoproteins A‐I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005; 294:326-333. [DOI] [PubMed] [Google Scholar]
- Rifai N, Cole TG, Iannotti E, Law T, Macke M, Miller R, Dowd D, Wiebe DA. Assessment of interlaboratory performance in external proficiency testing programs with a direct HDL‐cholesterol assay. Clin Chem. 1998; 44:1452-1458. [PubMed] [Google Scholar]
- Rifai N, Iannotti E, DeAngelis K, Law T. Analytical and clinical performance of a homogeneous enzymatic LDL‐cholesterol assay compared with the ultracentrifugation‐dextran sulfate‐Mg2+ method. Clin Chem. 1998; 44:1242-1250. [PubMed] [Google Scholar]
- Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995; 38:44-48. [DOI] [PubMed] [Google Scholar]
- Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, Aletaha D, van Riel PL. Validation of the 28‐joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C‐reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009; 68:954-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Ginsberg HN, Schaefer EJ. Relative atherogenicity and predictive value of non‐high‐density lipoprotein cholesterol for coronary heart disease. Am J Cardiol. 2008; 101:1003-1008. [DOI] [PubMed] [Google Scholar]
- Johnsson H, Panarelli M, Cameron A, Sattar N. Analysis and modelling of cholesterol and high‐density lipoprotein cholesterol changes across the range of C‐reactive protein levels in clinical practice as an aid to better understanding of inflammation‐lipid interactions. Ann Rheum Dis. 2014; 73:1495-1499. [DOI] [PubMed] [Google Scholar]
- van Gestel AM, Prevoo ML, van't Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996; 39:34-40. [DOI] [PubMed] [Google Scholar]
- Lillegraven S, Prince FH, Shadick NA, Bykerk VP, Lu B, Frits ML, Iannaccone CK, Kvien TK, Haavardsholm EA, Weinblatt ME, Solomon DH. Remission and radiographic outcome in rheumatoid arthritis: application of the 2011 ACR/EULAR remission criteria in an observational cohort. Ann Rheum Dis. 2012; 71:681-686. [DOI] [PubMed] [Google Scholar]
- Ronda N, Favari E, Borghi MO, Ingegnoli F, Gerosa M, Chighizola C, Zimetti F, Adorni MP, Bernini F, Meroni PL. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 2014; 73:609-615. [DOI] [PubMed] [Google Scholar]
- Mehta NN, Li R, Krishnamoorthy P, Yu Y, Farver W, Rodrigues A, Raper A, Wilcox M, Baer A, DerOhannesian S, Wolfe M, Reilly MP, Rader DJ, VanVoorhees A, Gelfand JM. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis. 2012; 224:218-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Llera Moya M, McGillicuddy FC, Hinkle CC, Byrne M, Joshi MR, Nguyen V, Tabita‐Martinez J, Wolfe ML, Badellino K, Pruscino L, Mehta NN, Asztalos BF, Reilly MP. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis. 2012; 222:390-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer M, Wolf P, Inzinger M, Trieb M, Curcic S, Pasterk L, Weger W, Heinemann A, Marsche G. Anti‐psoriatic therapy recovers high‐density lipoprotein composition and function. J Invest Dermatol. 2014; 134:635-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewing B, Parathath S, Barrett T, Chung WK, Astudillo YM, Hamada T, Ramkhelawon B, Tallant TC, Yusufishaq MS, Didonato JA, Huang Y, Buffa J, Berisha SZ, Smith JD, Hazen SL, Fisher EA. Effects of native and myeloperoxidase‐modified apolipoprotein A‐I on reverse cholesterol transport and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2014; 34:779-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, Orazem J, Magorien RD, O'Shaughnessy C, Ganz P. Statin therapy, LDL cholesterol, C‐reactive protein, and coronary artery disease. N Engl J Med. 2005; 352:29-38. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C‐reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005; 352:20-28. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008; 359:2195-2207. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003; 107:499-511. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007; 297:611-619. [DOI] [PubMed] [Google Scholar]
- Martin SS, Blaha MJ, Elshazly MB, Brinton EA, Toth PP, McEvoy JW, Joshi PH, Kulkarni KR, Mize PD, Kwiterovich PO, Defilippis AP, Blumenthal RS, Jones SR. Friedewald‐estimated versus directly measured low‐density lipoprotein cholesterol and treatment implications. J Am Coll Cardiol. 2013; 62:732-739. [DOI] [PubMed] [Google Scholar]
- Kiortsis DN, Mavridis AK, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005; 64:765-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes IB, McCarey DW, Sattar N. Do statins offer therapeutic potential in inflammatory arthritis? Ann Rheum Dis. 2004; 63:1535-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD, Codding CE, Carlson TH, Delles C, Lee JS, Sattar N. Effect of interleukin‐6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo‐controlled study. Ann Rheum Dis. 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa C, van den Hoogen FH, Radstake TR, Netea MG, Eijsbouts AE, den Heijer M, van der Meer JW, van Riel PL, Stalenhoef AF, Barrera P. Modulation of lipoprotein plasma concentrations during long‐term anti‐TNF therapy in patients with active rheumatoid arthritis. Ann Rheum Dis. 2007; 66:1503-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JA, Beg S, Lopez‐Olivo MA. Tocilizumab for rheumatoid arthritis: a Cochrane systematic review. J Rheumatol. 2011; 38:10-20. [DOI] [PubMed] [Google Scholar]
- Soubrier M, Jouanel P, Mathieu S, Poujol D, Claus D, Dubost JJ, Ristori JM. Effects of anti‐tumor necrosis factor therapy on lipid profile in patients with rheumatoid arthritis. Joint Bone Spine. 2008; 75:22-24. [DOI] [PubMed] [Google Scholar]
- Popa C, Netea MG, Radstake T, Van der Meer JW, Stalenhoef AF, van Riel PL, Barerra P. Influence of anti‐tumour necrosis factor therapy on cardiovascular risk factors in patients with active rheumatoid arthritis. Ann Rheum Dis. 2005; 64:303-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanakis E, Sidiropoulos P, Papadakis J, Ganotakis E, Katsikas G, Karvounaris S, Bizaki A, Kritikos H, Boumpas DT. Modest but sustained increase of serum high density lipoprotein cholesterol levels in patients with inflammatory arthritides treated with infliximab. J Rheumatol. 2006; 33:2440-2446. [PubMed] [Google Scholar]
- Jamnitski A, Visman IM, Peters MJ, Dijkmans BA, Voskuyl AE, Nurmohamed MT. Beneficial effect of 1‐year etanercept treatment on the lipid profile in responding patients with rheumatoid arthritis: the ETRA study. Ann Rheum Dis. 2010; 69:1929-1933. [DOI] [PubMed] [Google Scholar]
- Sidhu D, Naugler C. Fasting time and lipid levels in a community‐based population: a cross‐sectional study. Arch Intern Med. 2012; 172:1707-1710. [DOI] [PubMed] [Google Scholar]