Abstract
Background
In spite of robust knowledge about underlying ischemic myocardial damage, acute coronary syndromes (ACS) with culprit-free angiograms raise diagnostic concerns. The present study aimed to evaluate the additional value of cardiac magnetic resonance (CMR) over commonly available non-CMR standard tests, for the differentiation of myocardial injury in patients with ACS and non-obstructed coronary arteries.
Material/Methods
Patients with ACS, elevated hs-TnT, and a culprit-free angiogram were prospectively enrolled into the study between January 2009 and July 2013. After initial evaluation with standard tests (ECG, echocardiography, hs-TnT) and provisional exclusion of acute myocardial infarction (AMI) in coronary angiogram, patients were referred for CMR with the suspicion of myocarditis or Takotsubo cardiomyopathy (TTC). According to the result of CMR, patients were reclassified as having myocarditis, AMI, TTC, or non-injured myocardium as assessed by late gadolinium enhancement.
Results
Out of 5110 patients admitted with ACS, 75 had normal coronary angiograms and entered the study; 69 of them (92%) were suspected for myocarditis and 6 (8%) for TTC. After CMR, 49 patients were finally diagnosed with myocarditis (65%), 3 with TTC (4%), 7 with AMI (9%), and 16 (21%) with non-injured myocardium. The provisional diagnosis was changed or excluded in 23 patients (31%), with a 9% rate of unrecognized AMI.
Conclusions
The study results suggest that the evaluation of patients with ACS and culprit-free angiogram should be complemented by a CMR examination, if available, because the initial work-up with non-CMR tests leads to a significant proportion of misdiagnosed AMI.
MeSH Keywords: Acute Coronary Syndrome, Coronary Angiography, Magnetic Resonance Imaging, Myocarditis, Troponin
Background
Acute coronary syndromes (ACS) are a manifestation of ischemic heart disease with high morbidity and are a leading cause of mortality in industrialized countries [1,2]. According to the universal definition of myocardial Infarction, elevated cardiac troponin is one of the key diagnostic components for the diagnosis of acute myocardial infarction (AMI) [3]. High-sensitivity troponin T (hs-TnT) assay does not provide information on the mechanism of cell damage despite its high sensitivity in the detection of myocardial necrosis. Accordingly, patients with chest pain, elevated markers of myocardial cell damage, and ECG changes are typically referred to invasive coronary angiography. However, a proportion of coronary angiograms, ranging from 4% to 7%, are revealed to be culprit-free, angiographically normal, epicardial coronary arteries [4,5]. Acute chest pain in non-acute coronary syndrome (non-ACS) patients may be the clinical manifestation of a variety of pathologies, including myocarditis, pulmonary or coronary artery embolism, stress cardiomyopathy (Takotsubo cardiomyopathy, TTC), and myocardial ischemia originating from microvascular dysfunction. In these patients the prognosis can range from benign to the development of severe heart failure. Therefore, an accurate diagnosis in this group is crucial to achieve accurate risk stratification and to choose the best treatment strategy. Cardiac magnetic resonance (CMR) has been shown to be ideally suited for myocardial tissue characterization and is able to detect and differentiate pathologic changes at the level of myocardium [6,7]. However, CMR is of limited availability across Europe.
The present study aimed at the evaluation of the additional value of CMR over commonly available non-CMR standard tests, including hs-TnT assay, for the differentiation of myocardial injury in patients with ACS and non-obstructed coronary arteries.
Material and Methods
Study protocol
Patients admitted with an initial diagnosis of ACS and having normal coronary arteries at coronary angiogram were prospectively enrolled in this multicenter, observational study. Enrollment was performed in 2 tertiary cardiology centers, the 2nd Department of Cardiology, Zabrze, Silesian Medical University of Katowice (SUM), Poland and the Department of Cardiology, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland between January 2009 and July 2013. The study did not require the approval of the local Ethics Committee.
Inclusion criteria were: 1) acute retrosternal chest pain during the last 24 h, 2) elevated hs-TnT level (>14 ng/l) at admission or after 6 h, and 3) nonsignificant coronary stenosis on coronary angiogram (stenosis <30%) as analyzed by 2 independent invasive cardiologists. Exclusion criteria were: 1) history of coronary revascularization, 2) past myocardial infarction, 3) hypertensive heart disease, 4) tachyarrhythmia, 5) peripheral artery disease, 6) history of stroke, 7) coronary vasospasm, 8) renal insufficiency (creatinine >200μmol/l), and 9) recent septicemia.
During hospitalization a clinical examination, ECG, and echocardiography were obtained for every patient. Baseline blood samples were analyzed for full blood count, C-reactive protein (CRP), hs-TnT, creatine kinase myocardial bound (CK-MB), and lipid profile. The measurement of hs-TnT was conducted on the Cobas e 411 immunoanalyzer, which is based on electrochemiluminescence technology (with detection limit of 3–10000 ng/l, 99th percentile level of 14 ng/l, and 10% coefficient of variation level of 13 ng/l) according to the manufacturer instructions (Roche Diagnostics, Mannheim Germany).
All patients were referred to the hospital with a suspicion of AMI and underwent an initial work-up with past medical history, current symptoms, clinical, electrocardiographic and echocardiographic examination, hs-TnT level, and coronary angiogram. Baseline left ventricular ejection fraction (LVEF) was measured with echocardiography. The infection was defined as symptoms of recent (<14 days) respiratory or gastro-intestinal infection with or without fever. After exclusion of AMI with a culprit-free coronary angiogram, patients were reclassified and referred for CMR with a suspicion of either TTC (based on Mayo Clinic criteria [8]) or myocarditis (based on European Society of Cardiology position statement [9]). All procedures were conducted according to current clinical standards of care and no additional consent was obtained regarding ethical concerns. Final diagnosis was based on the distribution pattern of myocardial injury as identified by the late gadolinium enhancement (LGE) in CMR.
Cardiac magnetic resonance
CMR studies were performed using a GE Signa MR scanner (SUM, Poland), a Siemens Magnetom Symphony (until 2009), or a Magnetom Aera (starting in 2009) MR scanner (CHUV, Switzerland). The CMR protocol included sequences for left ventricular (LV) volumes, function, and mass. LV function and mass were assessed using a stack of standard ECG-gated, steady-state, free precession (SSFP), short axis images covering the LV from base to apex. Recommended major diagnostic CMR criteria for myocarditis were primarily based on the findings on LGE, a marker of incomplete washout of contrast medium from myocardium, late after the injection (10–20 min after the intravenous administration of conventional extracellular gadolinium chelate at a dose of 0.15–0.2 mmol/kg) [10,11]. In a subset of patients (see below), edema-sensitive T2-weighted images (T2-ratio) (using a triple inversion-recovery sequence with inversion pulses, including blood flow and fat suppression) and global T1 enhancement (early gadolinium enhancement [EGE] obtained from spin echo imaging techniques and defined as an increased normalized uptake of gadolinium chelate early [~3 min] after its intravenous administration) were also assessed [1,12]. To efficiently null the signal of viable myocardium, the optimal inversion time was adjusted. Abnormalities were analyzed from short axis view, 2-, 3-, and 4-chamber views, and localized based on the American Heart Association 17-segment model [13]. AMI was diagnosed if subendocardial signal enhancement in the distribution of a coronary artery was detected on LGE sequences (with or without a corresponding positive finding on T2-weighted images and/or MVO) (Figure 1). Myocarditis was diagnosed if subepicardial and/or patchy LGE lesions were detected in the LV myocardium according to previously published studies [10,11] (Figure 2). When scan logistics were favorable, T2-weighted and T1-weighted sequences were also acquired to calculate T2-ratio and T1-global enhancement as described elsewhere (n=24, SUM, Poland). CMR was considered normal if there were no LGE, MVO, or T2 intensity abnormalities, and if left and right ventricular function were normal. In the patients with suspected TTC (based on normal coronary arteries in coronary angiogram and typical wall motion abnormalities on baseline echocardiography), the diagnosis of TTC was maintained after the CMR study, if myocarditis and typical infarct pattern in LGE was excluded by CMR, even when regional wall motion abnormalities were no longer present.
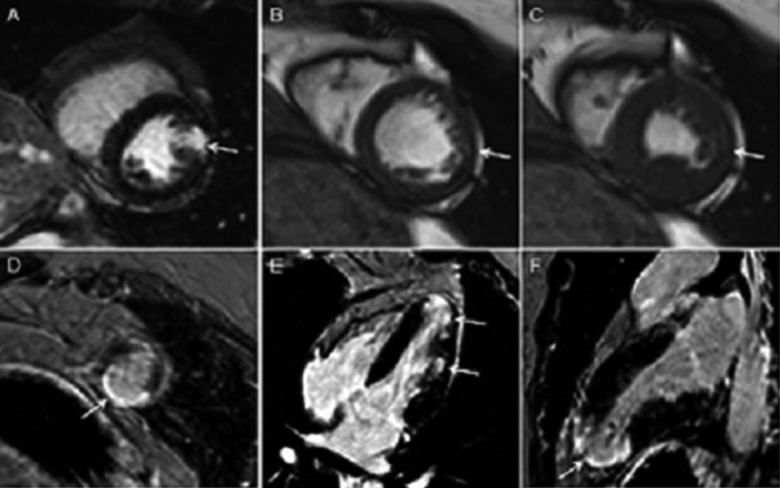
Figure 1.
Cardiac magnetic resonance – myocardial infarction. A – Localized, transmural zone of late gadolinium enhancement (LGE) within lateral wall (A) and apex (D) in short axis view (SAX) and 4-chamber (E) and 2-chamber (F) view (arrows). Cine MR images in SAX (B – diastole, C – systole) demonstrate corresponding wall motion abnormalities of the lateral wall (arrows in B and C).
Figure 2.
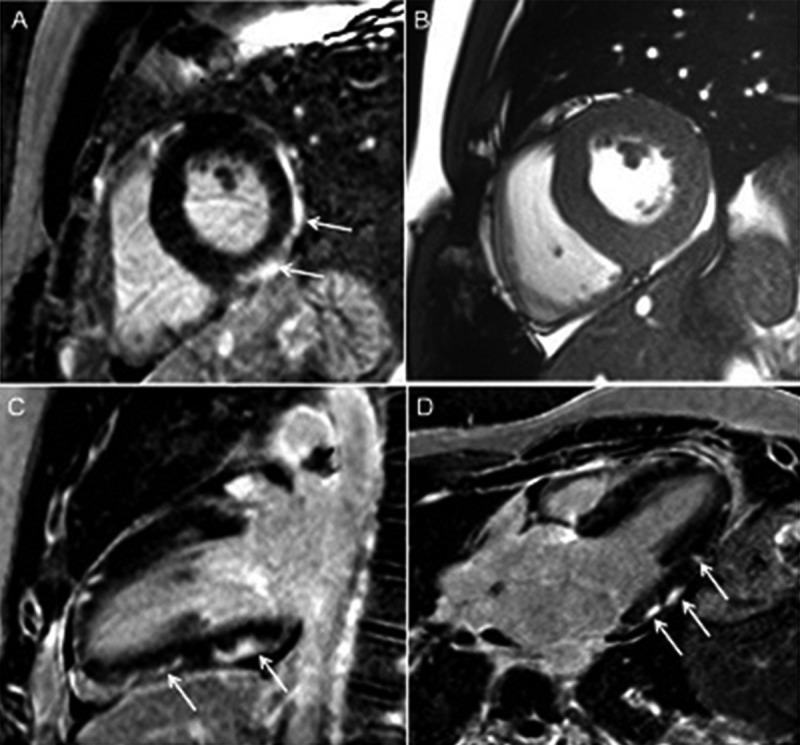
Cardiac magnetic resonance – myocarditis. Typical subepicardial and patchy zones of late gadolinium enhancement (LGE) in short axis (SAX) (A), 2-chamber (C) and 3-chamber (D) views (arrows) with a corresponding SAX cine image (B).
Outcomes
Patients were followed up at 30 days and 6 months. Data at 30 days were obtained by phone calls or were retrieved from medical records. Data at 6 months were gathered during a control visit in the outpatient unit, which included basic physical examination, echocardiographic examination, and a review of medical treatment.
A combined clinical end-point included death of cardiovascular origin, non-fatal AMI, heart failure (de novo according to Framingham criteria [14] or decompensation of existing heart failure), and electrocardiographically documented ventricular tachyarrhythmia.
Statistical analysis
Values are presented as means ±standard deviation or median (25th; 75th percentile) as appropriate. The comparison of variables between groups was performed using t-test or Mann-Whitney U test and Kruskal-Wallis test for continuous variables and chi-square test for categorical variables. A p value <0.05 was considered statistically significant. All analyses were performed using Statistica software, version 9PL (StatSoft Inc., Tulsa, OK, USA) and GraphPad Prism software version 6.00 (GraphPad, La Jolla, California, USA).
Results
From January 2009 to July 2013, 5110 patients were admitted to the Emergency Departments with chest pain and were identified with elevated levels of cardiac biomarkers. Of these, 75 patients (1.5%) had a normal coronary angiogram and were eligible for the study.
Population characteristics
The mean age was 40.0±14.6 years, 31 patients (79%) were male, and 21 patients (28%) presented with a recent history of infection. Baseline clinical characteristics are summarized in Table 1.
Table 1.
Characteristics of the population and subgroups according to the initial diagnosis.
| Characteristics | Total population n=75 | Myocarditis n=69 | Takotsubo n=6 | p value* |
|---|---|---|---|---|
| Clinical | ||||
| Age (years) | 44.9±16.2 | 43.6±15.8 | 59±14.2 | 0.03 |
| Male | 54 (72) | 54 (72) | 0 (0) | <0.001 |
| BMI (kg/m2) | 26.9 (24.5; 30.1) | 27.1 (24.6; 30.4) | 21.9 (20.3; 28.4) | 0.12 |
| Familial history of coronary artery disease (%) | 11 (15) | 11 (16) | 0 (0) | 0.58 |
| Hypertension (%) | 22 (29) | 21 (30) | 1 (17) | 0.66 |
| Dyslipidemia (%) | 16 (21) | 14 (20) | 2 (33) | 0.60 |
| Diabetes (%) | 5 (7) | 4 (6) | 1 (17) | 0.35 |
| Current smoking (%) | 25 (3) | 23 (33) | 2 (33) | >0.9 |
| Former smoking (%) (>1 year abstinence) | 11 (15) | 11 (16) | 0 (0) | 0.58 |
| Recent infection (%) | 23 (31) | 23 (33) | 0 (0) | 0.17 |
| Laboratory | ||||
| NT-proBNP (pg/ml) | 233.3 (95.3;693) | 229.3 (88.7;593.3) | 3905 | – |
| C-reactive protein (mg/l) | 10 (5;28.5) | 10 (5;29.9) | 6 (2.8; 9.3) | 0.27 |
| Peak creatine kinase – MB (IU/l) | 47 (30.5;93) | 49 (31,99) | 44 (26;62) | 0.51 |
| Peak hs-TnT (ng/ml) | 527.6 (139.8;3024) | 650.8 (108.2;3060) | 464.5 (365;2998) | 0.67 |
| Leukocytes (g/l) | 9.4 (6.7; 11.7) | 9.4 (6.8;11.7) | 8.6 (6.5; 14.7) | 0.82 |
| Erythrocytes (g/l) | 4.89 (4.68;5.2) | 4.9 (4.72; 5.2) | 4.48 (4.29; 4.76) | 0.004 |
| Hemoglobin (g/l) | 148 (139; 153) | 150 (140; 154) | 135 (128; 142) | 0.005 |
| Hematocrite (%) | 44 (41.6; 46.6) | 44 (42; 46.7) | 40.8 (38.8; 43.1) | 0.02 |
| Platelets (g/l) | 231 (195.5;270.8) | 230.5 (196;265.8) | 304.5 (184.4;340) | 0.14 |
| Na (mmol/l) | 139 (136;142) | 139 (136;142) | 140.5 (135.5;142.3) | 0.76 |
| Cholesterol total (mg/dl) | 173 (139; 212) | 172 (139; 207.5) | 226 (110.8;270.5) | 0.21 |
| HDL (mg/dl) | 47 (35;60.4) | 46 (34.8; 58) | 131 (59.9; 136.7) | 0.008 |
| Triglycerides (mg/dl) | 91.5 (63.5;131.5) | 93 (67;133) | 64 (35; 112) | 0.23 |
| LDL (mg/dl) | 102.1 (77.2;131.1) | 101.8 (71.8;130) | 104.9 (85; 217.3) | 0.48 |
| Echocardiogram transthoracic | ||||
| Left ventricular ejection fraction (%) | 57 (50; 60) | 59 (50; 60) | 48 (23.6; 49.2) | <0.001 |
| LVEDV (ml) | 96 (78; 110) | 99 (78; 111.5) | 87 (73.3; 92.5) | 0.2 |
| LVESV (ml) | 44.5 (28.5; 50) | 44 (27.5; 52) | 45 (35; 48) | 0.9 |
| Wall motion abnormalities | 29 (39) | 25 (36) | 4 (67) | 0.16 |
| Pericardial effusion | 13 (17) | 13 (19) | 0 (0) | 0.37 |
| Cardiac arrest/shock | 0 (0) | 0 (0) | 0 (0) | – |
Data are presented as mean ±SD, median (25th; 75th percentile) or n (%);
between the myocarditis group and TTC group.
Initial evaluation
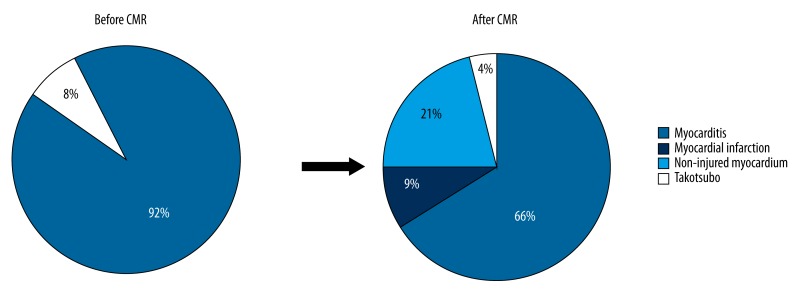
After initial clinical assessment using standard tests and provisional exclusion of AMI by coronary angiogram, 69 patients (92%) had suspected myocarditis and 6 patients (8%) had suspected TTC (Figure 3). Patients in these 2 diagnostic categories did not differ regarding cardiovascular risk factors or baseline ECG. Patients in the TTC group were older (p=0.03) and all were female (p<0.001). Median LVEF was lower for TTC patients than in myocarditis patients (48% vs. 59%, respectively, p<0.001). No differences were found regarding wall motion abnormalities (WMA), chamber diameters, or pericardial effusion. The biomarkers of myocardial necrosis for the total cohort were elevated but did not differ between groups (p=0.67 for hs-TnT, p=0.51 for CK-MB). In patients in the TTC group, lower values of erythrocytes count, hemoglobin and hematocrit and higher values of high-density lipoprotein (HDL) were observed (Table 1).
Figure 3.
The evolution of the diagnosis. CMR – cardiac magnetic resonance.
Cardiac magnetic resonance
CMR was performed in all 75 patients, with a mean delay of 12.8 days after the onset of cardiac symptoms. CMR showed diagnostic changes in 79% of cases (59 patients) and was normal in 21% (16 patients) (Figure 3).
Out of the 16 patients with negative LGE findings, in 6 patients T2-ratio and T1 global enhancement were also determined. In only 1 patient out of those (1/6 patients), a borderline positive T1 global enhancement of 3.4 was found (normal <2.9 [15]), while the T2 ratio of 1.3 was also within normal limits (normal <2.0 [11,16]). Therefore, this patient was categorized as normal, i.e. non-injured myocardium as assessed by LGE (= myocarditis negative). Out of the 49 patients with LGE findings positive for myocarditis, in 18 patients T2-ratio and T1 global enhancement were also determined. Out of these 18 LGE-positive patients, T2-ratio and T1 global enhancement were positive in 8 and 7 patients, respectively.
In the patients included into the study (i.e., patients with acute chest pain, elevated markers of myocardial necrosis, and normal coronary arteries), the diagnosis of the standard diagnostic tests (clinical examination, ECG, echocardiography, biomarkers, and coronary angiogram) was correct in only 66% of patients (50 patients) based on the final results provided by CMR as the gold standard (47 out of 69 suspected and 3 TTC out of 6 suspected). With the initial assessment, the final diagnosis was missed in 25 patients (33%). Specifically, the diagnosis was changed in 9 patients (12%), and suspected myocardial disease was excluded in another 16 patients (21%). Out of 69 patients with initially suspected myocarditis, the diagnosis was confirmed in 47 patients (68%), reclassified to AMI in 6 patients (9%) and to non-injured myocardium in 16 patients (23%). Among 6 patients with suspected TTC, the diagnosis was confirmed in 3 (50%), reclassified to myocarditis in 2 (33%), and to AMI in 1 (17%).
In summary, the final diagnosis after CMR was unrecognized AMI in 7 patients (9%), myocarditis in 49 patients (65%), TTC in 3 patients (4%), and non-injured myocardium in 16 patients (21%) (Figure 3). No differences in ECG, echocardiographic characteristics, and biomarkers were found between those groups (Table 2). Groups differed regarding age (older patients in the TTC group and younger patients in the myocarditis group, p=0.002) and sex (with 86% of males in the myocarditis group and no males in the TTC group, p<0.001).
Table 2.
Clinical characteristics of the population and subgroups based on CMR result.
| Characteristics | Myocarditis n=49 | Ischemia n=7 | TTC n=3 | Non-injured myocardium n=16 | p value* |
|---|---|---|---|---|---|
| Clinical | |||||
| Age (years) | 37 (28; 51) | 58 (50;61) | 66 (54; 82) | 52.5 (40.5;65.8) | 0.002 |
| Male (%) | 42 (86) | 2 (28) | 0 (0) | 10 (63) | <0.001 |
| BMI (kg/m2) | 26.8 (24.8;30.2) | 28.3 (19.9;39.8) | 21.2 (20.4;21.9) | 27.8 (24.6;29.9) | 0.27 |
| Familial history of coronary artery disease (%) | 7 (14) | 0 (0) | 0 (0) | 4 (25) | 0.39 |
| Hypertension (%) | 13 (27) | 3 (43) | 0 (0) | 6 (38) | 0.49 |
| Dyslipidemia (%) | 10 (20) | 1 (14) | 0 (0) | 5 (31) | 0.59 |
| Diabetes (%) | 2 (4) | 1 (14) | 1 (33) | 1 (6) | 0.22 |
| Current smoking (%) | 18 (37) | 3 (43) | 1 (33) | 3 (19) | 0.54 |
| Former smoking (%) (1 year abstinence) | 6 (12) | 0 (0) | 0 (0) | 5 (31) | 0.15 |
| Recent infection (%) | 17 (35) | 0 (0) | 0 (0) | 6 (38) | 0.16 |
| Laboratory | |||||
| NT-proBNP (pg/ml) | 233.7 (151;332.4) | 821.4 (79.8;1563) | - | 224.8 (22.4;1083) | – |
| C-reactive protein (mg/l) | 10 (5; 32) | 20.4 (4; 60.2) | 7 (7;7) | 5.8 (4.3;16.4) | 0.51 |
| Peak creatin kinase - MB (IU/l) | 50 (34; 103) | 59 (21; 77) | 35.5 (24; 47) | 34 (24;88) | 0.47 |
| Peak hs-TnT (ng/ml) | 774.3 (258.8;3091) | 1850 (410;7180) | 700 (449;9890) | 165 (40.6;811.1) | 0.09 |
| Leukocytes (g/l) | 9.8 (6.7;11.7) | 10.7 (6; 11.9) | 9.7 (7.5;14.3) | 8.8 (7; 9.9) | 0.84 |
| Erythrocytes (g/l) | 4.9 (4.8; 5.2) | 4.7 (4.3; 5.0) | 4.3 (4.2; 4.8) | 4.8 (4.5; 5.1) | 0.03 |
| Hemoglobin (g/l) | 150 (143;154) | 147 (129;154) | 133 (125;140) | 144 (136;152) | 0.02 |
| Hematocrite (%) | 44.4 (42;46.9) | 44 (40; 45.6) | 39 (38; 42) | 43 (40.5;46.6) | 0.05 |
| Platelets (g/l) | 230 (196;265.8) | 231 (170;269) | 336 (185;352) | 243 (197.5;283.8) | 0.63 |
| Na (mmol/l) | 139 (136;142) | 141 (136;143) | 141.1 (140;142) | 139 (137;142) | 0.57 |
| Cholesterol total (mg/dl) | 163.5 (129;204.8) | 208 (111;216) | 224 (146;228) | 197 (158;212) | 0.42 |
| HDL (mg/dl) | 46 (34; 57.5) | 44 (35.6;70.3) | 131 | 54 (39.5;69.5) | 0.28 |
| Triglycerides (mg/dl) | 85.5 (69.5;118.3) | 88.5 (49.8;119.8) | 35 | 138 (44; 220) | 0.44 |
| LDL (mg/dl) | 102.1 (69.3;135) | 109.5 (71.1;131.6) | 85 | 110.8 (75.4;128.1) | 0.980 |
| Echocardiogram transthoracic | |||||
| Left ventricular ejection fraction (%) | 58 (50; 60) | 60 (55; 60) | 25 (20; 50) | 55 (54; 60) | 0.06 |
| LVEDV (ml) | 90 (90; 93) | 97.5 (75.5;110) | 83 (83; 83) | 110 (78; 120) | – |
| LVESV (ml) | 37.5 (27; 50) | 52 (48; 56) | 45 (45; 45) | 50 (46; 56) | – |
| Wall motion abnormalities | 20 (41) | 4 (57) | 2 (67) | 3 (19) | 0.14 |
| Pericardial effusion (%) | 10 (20) | 0 (0) | 0 (0) | 3 (19) | 0.49 |
| Cardiac arrest/shock | 0 | 0 | 0 | 0 | – |
Data are presented as mean ±SD, median (25th; 75th percentile) or n (%);
between all groups.
Outcomes
No clinical endpoints were reported for the 30-day and 6-month follow-up. At the 6-month follow-up, no significant echocardiographic changes were observed when compared with the initial examination. The median change in LVEF as assessed by echocardiography was – 2% (range – 22;20) for the total population and – 3% (range – 22;20), – 1% (range – 16;8) and – 4% (range – 3;7) for the myocarditis, AMI, and non-injured myocardium group, respectively, p=0.91.
Discussion
Patients with chest pain, elevated troponin, and normal coronary arteries constitute a heterogeneous group with unclear prognosis [17]. In several studies these patients had similar or even worse prognosis than patients with AMI receiving revascularization therapy [18–20] whereas other authors reported an evolution without adverse events [21,22]. Establishing the correct diagnosis is therefore crucial to ensure that patients are correctly risk-stratified and receive adequate treatment.
Our data suggest that the standard diagnostic workup with ECG, biomarkers, and echocardiography is insufficient in this setting, and that a large proportion of patients presenting with acute coronary syndrome, elevated troponin, and normal coronary angiogram have probable myocarditis [23]. The sensitivity of CMR to detect myocarditis ranges from 60% to 90% [24–26], depending on localization of the pathological process and LGE in the myocardium, the CMR sequence used, wall thickness, the challenging comparison between histological segments with CMR locations, the technique of histopathological analysis used, and patient selection. Endomyocardial biopsy is the gold standard test to diagnose myocarditis, with a recent position paper of the ESC favoring the systematic use of endomyocardial biopsy in this particular setting [9,27], but its use is currently restricted to specific clinical situations [28] because of its invasive nature and the potential complications associated with this procedure.
In the present study, CMR was performed according to the current recommendations [7,11]. The enrollment criteria were consistent with appropriate indications for CMR imaging. In the cohort, 65% of patients received the final diagnosis of myocarditis and 9% of the study population was diagnosed with unrecognized AMI. This is in contrast to previous data in which CMR yielded a higher rate of AMI in patients with ACS presentation and culprit-free coronary angiogram [29], but it is in line with other reports showing that most patients admitted with chest pain, high troponin, and normal coronary angiogram were finally diagnosed with myocarditis [30]. Of notice, the diagnosis of myocarditis was based on LGE assessment only in the majority of cases with 32% ratio of co-evaluation with T1 global enhancement and T2-ratio, which should be mentioned in the context of assessing the rate of myocarditis/AMI in the studied population.
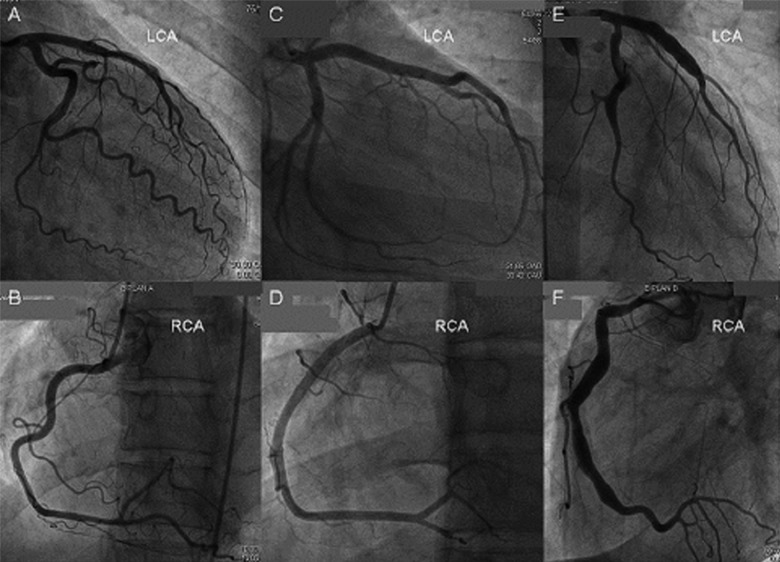
One might have expected to find a higher proportion of ischemia-induced myocardial damage (LGE positive areas) with CMR in the population diagnosed with elevated hs-TnT as the marker for myocardial damage. Previous studies reported a higher incidence of myocardial necrosis despite the absence of significant (>30%) epicardial coronary stenosis [31]. However, these studies also included patients with minimal atheromatosis in coronary angiogram and a positive history of coronary artery disease (CAD). Conversely, in our cohort, patients with a positive history of CAD were excluded and the majority of patients had completely smooth coronary arteries in angiography (Figure 4) with no case showing diffuse CAD (mild pathological changes were found in only 8 patients). This is reflected in the low (1.5%) incidence of fulfilling the criteria for culprit-free angiograms. Thus, in the earlier studies CAD was more advanced than in our study, most likely explaining the lower incidence of necrotic lesions detected by CMR in our study. Out of the 8 patients with mild pathological changes in the coronary angiogram, 1 was diagnosed with AMI. A retrospective analysis of the angiogram in this patient with AMI revealed an occluded thin diagonal branch (<1 mm), which was missed at first, possibly responsible for the localized transmural zone of LGE on CMR.
Figure 4.
Coronary angiogram. Angiograms showing smooth coronary arteries in a patient finally diagnosed with myocardial infarction (A, B), with Takotsubo cardiomyopathy (C, D), and aneurismal coronary arteries in a patient finally diagnosed with myocarditis (E, F). Final diagnosis was established according to the result of cardiac magnetic resonance. LCA – left coronary artery, RCA – right coronary artery.
Early performance of CMR in patients with suspected myocarditis has been proposed [32]. Some authors suggest that CMR could be chosen even before coronary angiography as the primary investigation if supported by relevant clinical findings [33,34]. Others raised the problem of cardiovascular risk underestimation [35] and demonstrated that the incidence of AMI in young adults is not marginal [34]. In the present population, young patients (<40 years old) constituted 55% of the myocarditis group and 14% of the AMI group (median 58 vs. 37 years, respectively, p=0.02). Although young patients are being diagnosed with myocarditis 4 times more frequently than older patients, their 14% contribution to the AMI group is important. Therefore, also in young patients, CMR should not be preferred over coronary angiography, with the latter remaining the diagnostic method of choice for a definite exclusion of CAD in the setting of acute chest pain.
Considering the relatively weak diagnostic yield of standard tests, CMR should be strongly advised in cases of acute chest pain, elevated troponin, and normal coronary arteries. The real-world circumstances limit the possibilities of its introduction into clinical practice. The study hypothesis was built and driven by a threatening diversity in the statistics of CMR utilization across Europe. It is known that in Western European regions, CMR has a utilization rate of 13% [36] and the regional diversity in CMR availability is important. In Switzerland, a single university hospital (CHUV, Lausanne) reports approximately 1500 exams per year (per ~0.5–1 million inhabitants), while an annual rate of 250 CMR examinations is reported in the Silesian region of Poland for a population of over 4.6 million inhabitants (data from the Silesian Department of the National Health Insurance Service, 2013). And finally, in Romania, CMR is still rarely available in some regions (e.g., no CMR was available until 2013 in Jassy, with a population of >300 000). In the era of minimizing invasive diagnostic procedures and the tendency to perform evidence-based medicine, CMR emerges as the most appropriate diagnostic method, counterbalancing the financial, social, and personal costs of a misdiagnosed patient.
Limitations
The number of enrolled patients was rather low and was not sufficient to achieve statistical significance for the comparison of a large number of variables between groups. Although included patients mainly had smooth coronary arteries on coronary angiography, the lack of routine application of intravascular ultrasound or coronary optical coherence tomography has to be mentioned. Due to independent technical conditions, the time window of CMR was broad and the results provided could led to under-diagnosis of the characteristic transient pattern of TTC or lower frequency of abnormalities indicative of myocarditis (edema and LGE).
Conclusions
The study results suggest the high diagnostic yield of CMR in patients with an acute coronary syndrome and a culprit-free angiogram. The diagnostic performance of commonly available non-CMR-based tests in this setting is insufficient and leads to a 9% missed diagnosis of AMI if not verified by CMR. The evaluation of patients with chest pain, elevated troponin, and normal coronary arteries should be complemented by a CMR examination if available.
Footnotes
Conflict of interests
The authors declare that they have no conflict of interests.
Source of support: Departmental sources
References
- 1.Nichols M, Townsend N, Scarborough P, et al. European cardiovascular disease statistics. 2012 edition. European Heart Network and European Society of Cardiology; 2012. pp. 14–17.pp. 35 [Google Scholar]
- 2.World health statistics 2012. World Health Organization; 2012. pp. 80–81. [Google Scholar]
- 3.Thygesen K, Alpert JS, Jaffe AS, et al. Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial InfarctionThird universal definition of myocardial infarction. Circulation. 2012;126(16):2020–35. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 4.Stensaeth KH, Fossum E, Hoffmann P, et al. Clinical characteristics and role of early cardiac magnetic resonance imaging in patients with suspected ST-elevation myocardial infarction and normal coronary arteries. Int J Cardiovasc Imaging. 2011;27(3):355–65. doi: 10.1007/s10554-010-9671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agewall S, Daniel M, Eurenius L, et al. Risk factors for myocardial infarction with normal coronary arteries and myocarditis compared with myocardial infarction with coronary artery stenosis. Angiology. 2012;63(7):500–3. doi: 10.1177/0003319711429560. [DOI] [PubMed] [Google Scholar]
- 6.Mather AN, Fairbairn TA, Artis NJ, et al. Diagnostic value of CMR in patients with biomarker-positive acute chest pain and unobstructed coronary arteries. JACC Cardiovasc Imaging. 2010;3(6):661–64. doi: 10.1016/j.jcmg.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 8.Madhavan M, Prasad A. Proposed Mayo Clinic criteria for the diagnosis of Tako-Tsubo cardiomyopathy and long-term prognosis. Herz. 2010;35:240–44. doi: 10.1007/s00059-010-3339-x. [DOI] [PubMed] [Google Scholar]
- 9.Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–48. 2648a–48d. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 10.Mahrholdt H, Goedecke C, Wagner A, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–58. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 11.Schwitter J, editor. CMR Update. (2nd edition) Chapter 11:198–210. [Google Scholar]
- 12.Friedrich MG, Sechtem U, Schulz-Menger J, et al. for the International Consensus Group on Cardiovascular MR in Myocarditis. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475–87. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 14.McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441–46. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich MG, Strohm O, Schulz-Menger J, et al. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802–9. doi: 10.1161/01.cir.97.18.1802. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Aty H, Boyé P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–22. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 17.Kardasz I, De Caterina R. Myocardial infarction with normal coronary arteries: a conundrum with multiple aetiologies and variable prognosis: an update. J Intern Med. 2007;261:330–48. doi: 10.1111/j.1365-2796.2007.01788.x. [DOI] [PubMed] [Google Scholar]
- 18.Burgdorf C, Kurowski V, Bonnemeier H, et al. Long-term prognosis of the transient left ventricular dysfunction syndrome (Tako-Tsubo cardiomyopathy): focus on malignancies. Eur J Heart Fail. 2008;10:1015–19. doi: 10.1016/j.ejheart.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Dokainish H, Pillai M, Murphy SA, et al. TACTICS-TIMI-18 Investigators: Prognostic implications of elevated troponin in patients with suspected acute coronary syndrome but no critical epicardial coronary disease: a TACTICS-TIMI-18 substudy. J Am Coll Cardiol. 2005 Jan 4;45(1):19–24. doi: 10.1016/j.jacc.2004.09.056. Erratum in: J Am Coll Cardiol, 2005; 45(11):1911. [DOI] [PubMed] [Google Scholar]
- 20.Nakashima H, Umeyama Y, Minami K. Successive immunosuppressive treatment of fulminant myocarditis that is refrectory to mechanical circulatory support. Am J Case Rep. 2013;14:116–19. doi: 10.12659/AJCR.889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen AI, Galbraith PD, Ghali WA, et al. APPROACH Investigators. Characteristics and outcomes of patients with acute myocardial infarction and angiographically normal coronary arteries. Am J Cardiol. 2005;95(2):261–63. doi: 10.1016/j.amjcard.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Regnante RA, Zuzek RW, Weinsier SB, et al. Clinical characteristics and four-year outcomes of patients in the Rhode Island Takotsubo Cardiomyopathy Registry. Am J Cardiol. 2009;103:1015–19. doi: 10.1016/j.amjcard.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez-Castro CE, Alkhateeb H, Elfar A, et al. Recurrent myopericarditis as a complication of Marijuana use. Am J Case Rep. 2014;15:60–62. doi: 10.12659/AJCR.889808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korkusuz H, Esters P, Huebner F, et al. Accuracy of cardiovascular magnetic resonance in myocarditis: comparison of MR and histological findings in an animal model. J Cardiovasc Magn Reson. 2010;12:49. doi: 10.1186/1532-429X-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lurz P, Eitel I, Adam J, et al. Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. JACC Cardiovasc Imaging. 2012;5(5):513–24. doi: 10.1016/j.jcmg.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Mahrholdt H, Wagner A, Deluigi CC, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–90. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 27.Cooper LT, Baughman KL, Feldman AM, et al. American Heart Association, American College of Cardiology, European Society of Cardiology, Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007;50:1914–31. doi: 10.1016/j.jacc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Blohm JH, Blohm N, Hummel M, et al. Detection of clonal T-cell-receptor (TCR) Vbeta rearrangements in explanted dilated cardiomyopathy hearts by semi-nested PCR, GeneScan, and direct sequencing. Med Sci Monit Basic Res. 2013;19:111–17. doi: 10.12659/MSMBR.883851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerbaud E, Harcaut E, Coste P, et al. Cardiac magnetic resonance imaging for the diagnosis of patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Int J Cardiovasc Imaging. 2012;28:783–94. doi: 10.1007/s10554-011-9879-1. [DOI] [PubMed] [Google Scholar]
- 30.Laraudogoitia ZE, Perez-David E, Larena JA, et al. The value of cardiac magnetic resonance in patients with acute coronary syndrome and normal coronary arteries. Rev Esp Cardiol. 2009;62:976–83. doi: 10.1016/s1885-5857(09)73263-3. [DOI] [PubMed] [Google Scholar]
- 31.Collste O, Sörensson P, Frick M, et al. Myocardial infarction with normal coronary arteries is common and associated with normal findings on cardiovascular magnetic resonance imaging: results from the Stockholm Myocardial Infarction with Normal Coronaries study. J Intern Med. 2013;273(2):189–96. doi: 10.1111/j.1365-2796.2012.02567.x. [DOI] [PubMed] [Google Scholar]
- 32.Monney PA, Sekhri N, Burchell T, et al. Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart. 2011;97:1312–18. doi: 10.1136/hrt.2010.204818. [DOI] [PubMed] [Google Scholar]
- 33.Agewall S, Eurenius L, Hofman-Bang C, et al. Myocardial infarction with angiographically normal coronary arteries. Atherosclerosis. 2011;219(1):10–14. doi: 10.1016/j.atherosclerosis.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 34.Pellaton C, Monney P, Ludman AJ, et al. Clinical features of myocardial infarction and myocarditis in young adults: a retrospective study. BMJ Open. 2012;2:e001571. doi: 10.1136/bmjopen-2012-001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarich S, Luciano C, Hulford J, et al. Prevalence of metabolic syndrome in young patients with acute MI: does the Framingham Risk Score underestimate cardiovascular risk in this population? Diab Vasc Dis Res. 2006;3(2):103–7. doi: 10.3132/dvdr.2006.012. [DOI] [PubMed] [Google Scholar]
- 36.Carron S. Cardiac Imaging, technology market overview. Imaging Management. 2010;10(4):18. [Google Scholar]