Abstract
Objective
To study the impact of national economic and human development status on patient profiles and outcomes in the setting of acute coronary syndrome (ACS).
Methods
We conducted a retrospective analysis of the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial (TRILOGY ACS) population (51 countries; 9301 patients). Outcome measures compared baseline characteristics and clinical outcomes through 30 months by 2010 country-level United Nations Human Development Indices (HDIs) and per-capita gross national income.
Results
TRILOGY ACS enrolled 3659 patients from 27 very-high HDI countries, 3744 from 18 high-HDI countries and 1898 from 6 medium-HDI countries. Baseline characteristics of groups varied significantly, with the medium-HDI group having a lower mean age (63.0 years, vs 65.0 and 68.0 years for high-HDI and very-high HDI, respectively; p<0.001), lower baseline Global Registry of Acute Coronary Events risk score and lower rate of non-ST-segment elevation myocardial infarction (58.0%, vs 62.2% and 83.9% among high-HDI and very-high HDI, respectively). Medium-HDI and high-HDI patients had lower unadjusted 30-month rates for the composite of cardiovascular death/myocardial infarction/stroke (17.6%, 16.9% and 23.1% for medium-HDI, high-HDI and very-high HDI, respectively); this difference disappeared after adjusting for baseline characteristics. Adjusted HRs for the composite endpoint were lower in lower-income/middle-income countries vs upper-income/middle-income (0.791(95% CI 0.632 to 0.990)) and high-income countries (0.756 (95% CI 0.616 to 0.928)), with differences largely attributable to myocardial infarction rates.
Conclusions
Clinical patient profiles differed substantially by country HDI groupings. Lower unadjusted event rates in medium-HDI countries may be explained by younger age and lower comorbidity burden among these countries’ patients. This heterogeneity in patient recruitment across country HDI groupings may have important implications for future global ACS trial design.
Trial registration number
Introduction
Randomised controlled trials in cardiovascular diseases increasingly recruit patients on a global scale.1–4 Such global enrolment helps to facilitate participant recruitment and improves the validity and applicability of trial results worldwide.5 However, the characteristics and management of patients with acute coronary syndrome (ACS) have been reported to vary substantially among countries with developed economies.6–8 Limited data from countries with developing economies suggest higher event rates among clinical trial participants in these countries compared with those from countries with developed economies.9 10 Thus, the patient characteristics and outcomes of global ACS trials may be influenced by the developmental status of individual countries.
The United Nations Development Programme's (UNDP) Human Development Index (HDI)11 provides a standardised measure of the social and economic development of an individual country. HDI combines a country's per-capita gross national income (GNI) as determined by The World Bank, mean life expectancy of the population and mean or expected years of schooling of that country's citizens.
We analysed data from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial (TRILOGY ACS; ClinicalTrials.gov identifier NCT00699998), which recruited patients in 51 countries, to assess the effect of the development and economic statuses of countries, using HDI and per-capita GNI, on patient characteristics and outcomes in medically managed patients with ACS.
Methods
Trial details
The present study is a secondary data analysis of the TRILOGY ACS trial database. Details of the study design and results have been published.12 13 In brief, TRILOGY ACS was a double-blinded, randomised controlled trial that recruited patients with unstable angina or non-ST-segment elevation myocardial infarction (NSTEMI) who were planned to undergo medical management without revascularisation for their index ACS event. Study participants were randomly assigned to receive either prasugrel or clopidogrel against a background of low-dose aspirin therapy. Patients were recruited from 27 June 2008 to 12 September 2011 and were followed up to the completion of the trial in March 2012.
Study patients
Patients were recruited within 10 days of experiencing their index ACS event. Patients with NSTEMI were recruited if they had elevated cardiac markers, while potential participants with unstable angina were required to have >1 mm ST-segment depression on an ECG. In addition, all patients were required to have at least one of the following risk factors: age >60 years, diabetes mellitus, previous myocardial infarction (MI), or previous coronary revascularisation. Coronary angiography was not mandated; however, if performed, it was required to be done prior to randomisation and to show evidence of coronary artery disease (CAD) with a >30% lesion in native arteries or previous revascularisation. Major exclusion criteria included transient ischaemic attack/stroke or coronary revascularisation within the previous 30 days, renal failure requiring dialysis and concomitant anticoagulant use.
Study sites
This study analysed data collected from 9301 study participants enrolled at research sites in 51 countries. A total of 25 study participants enrolled in Taiwan were excluded from the present study due to lack of an officially reported HDI for Taiwan by the UNDP.
Study groups
We compared patient baseline clinical characteristics and clinical outcomes through 30 months by country-level HDI from 2010 (the midpoint of the trial) as determined by the UNDP11 and country-level, per-capita GNI from 2010 as determined by The World Bank.14 The 51 countries were categorised by HDI values into three groups (very high, high and medium) according to the definition provided by UNDP. Continuous per-capita GNI values were categorised into three groups (high, upper middle and lower middle) according to the definition provided by The World Bank.
Study outcomes
The efficacy outcomes evaluated for this study were (1) the primary efficacy composite endpoint of cardiovascular death, MI, or stroke, and (2) individual component endpoints of cardiovascular death, all MI events, all stroke events and all-cause death. All outcomes were ascertained through 30 months.
Statistical analysis
Baseline characteristics were compared among the three HDI groups within the intention-to-treat population. Continuous variables are presented as medians (25th, 75th centiles), and differences were compared using the Kruskal–Wallis test. Categorical variables are presented as counts (percentages). Differences were compared using the χ2 test when cell frequencies were sufficient; otherwise, an exact test was used. Event counts and unadjusted Kaplan–Meier event rates at 30 months after randomisation were presented for the same groups and compared using the log-rank test. The same analysis was repeated for the three per-capita GNI groups.
The extent of CAD in patients who underwent prerandomisation angiography was also assessed across the three HDI groups. The number of diseased vessels and types of vessels with ≥50% diameter stenosis are presented as counts (percentages). Differences were compared using the χ2 test when cell frequencies were sufficient; otherwise, an exact test was used. p Values are reported for each of the four vessel types (left anterior descending, left circumflex, left main and right coronary artery) because patients could have more than one diseased vessel.
To determine the relationship between HDI (per-capita GNI) and the risk of an ischaemic event, a Cox model was fitted using two separate approaches. The first approach was an unadjusted analysis in which time to first event was regressed on only HDI (per-capita GNI). The second approach was an adjusted analysis in which time to first event was regressed on HDI (per-capita GNI), including a collection of prespecified ‘adjustment’ covariates inherent to the TRILOGY ACS trial (see online supplementary appendix for further details regarding the adjusted modelling approach). The highest level of each measure was used as the reference level in the model (very high for HDI and high for per-capita GNI). The relationship between HDI (per-capita GNI) and clinical outcomes was assessed by performing a global test of association across all three levels and then reporting HRs and 95% CIs for each pairwise comparison among the levels of HDI (per-capita GNI). To account for correlation within countries, each model used a robust sandwich estimate for the covariance matrix that was then used in the Wald tests for examining the global null hypothesis and null hypotheses of individual parameters.
Results
Country distribution
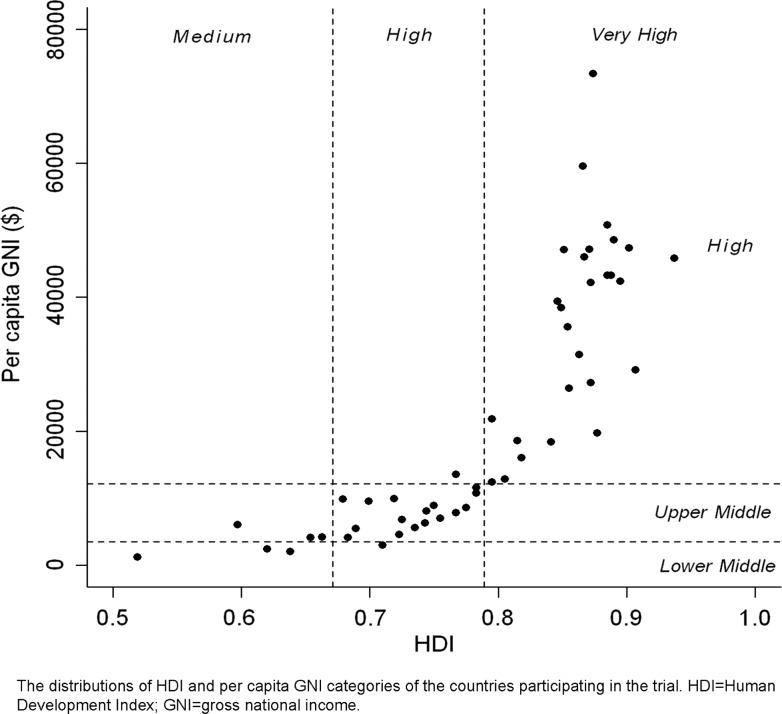
The distributions of HDI and per-capita GNI of the countries participating in the TRILOGY ACS trial are shown in figure 1. The distributions of the participating countries by their respective HDI and per-capita GNI, and the numbers of participants from each country are shown in the online supplementary appendix. No low-HDI or low-GNI countries participated in the trial.
Figure 1.
Distributions of Human Development Index (HDI) and per-capita gross national income (GNI) categories of the countries participating in the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial.
Patient characteristics
The baseline characteristics of study participants by HDI classification are shown in table 1. There were significant differences among groups for all reported baseline characteristics except for Killip class. Patients from medium-HDI countries were younger and more often weighed <60 kg. They were less likely to have hypertension, hyperlipidaemia or family history of CAD and overall had a lower median Global Registry of Acute Coronary Events (GRACE) risk score. However, they had a higher prevalence of diabetes, and fewer of them underwent prerandomisation angiography. They were also less likely to be receiving angiotensin-converting enzyme inhibitors (ACE-Is)/angiotensin receptor blockers (ARBs), β-blockers or statins. Among those participants who underwent angiography, the extent and distribution of CAD varied significantly by HDI grouping (table 2).
Table 1.
Baseline characteristics of the study patients
| HDI (2010) classification | ||||
|---|---|---|---|---|
| Characteristic | Very high (N=3659) | High (N=3744) | Medium (N=1898) | p Value |
| Demographics | ||||
| Age (years) | 68.0 (60.0, 76.0) | 65.0 (59.0, 73.0) | 63.0 (56.0, 70.0) | <0.001 |
| Age ≥75 (%) | 1078/3659 (29.5%) | 712/3744 (19.0%) | 278/1898 (14.6%) | <0.001 |
| Female sex (%) | 1405/3659 (38.4%) | 1552/3744 (41.5%) | 684/1898 (36.0%) | <0.001 |
| Weight (kg) | 80.0 (69.0,92.0) | 77.0 (68.0,86.5) | 64.0 (56.0,73.0) | <0.001 |
| Weight <60 kg (%) | 339/3654 (9.3%) | 388/3742 (10.4%) | 660/1898 (34.8%) | <0.001 |
| Presentation characteristics | ||||
| Disease classification (%) | <0.001 | |||
| Unstable angina | 588/3659 (16.1%) | 1417/3744 (37.8%) | 797/1898 (42.0%) | |
| NSTEMI | 3071/3659 (83.9%) | 2327/3744 (62.2%) | 1101/1898 (58.0%) | |
| Killip class II–IV on presentation (%) | 448/3659 (12.2%) | 461/3744 (12.3%) | 218/1898 (11.5%) | 0.638 |
| Cardiovascular risk factors | ||||
| Family history of CAD (%) | 1437/3139 (45.8%) | 841/3305 (25.4%) | 232/1817 (12.8%) | <0.001 |
| Hypertension (%) | 3070/3648 (84.2%) | 3301/3733 (88.4%) | 1231/1897 (64.9%) | <0.001 |
| Hyperlipidaemia (%) | 2700/3573 (75.6%) | 2076/3466 (59.9%) | 456/1807 (25.2%) | <0.001 |
| Diabetes mellitus (%) | 1426/3649 (39.1%) | 1292/3736 (34.6%) | 810/1896 (42.7%) | <0.001 |
| Current/recent smoking* (%) | 857/3595 (23.8%) | 596/3724 (16.0%) | 383/1884 (20.3%) | <0.001 |
| Baseline risk assessment | ||||
| GRACE risk score | 123.0 (106.0,143.0) | 123.0 (106.0,140.0) | 113.0 (99.0,128.0) | <0.001 |
| Prerandomisation treatments | ||||
| Angiography performed (%) | 2448/3658 (66.9%) | 966/3744 (25.8%) | 433/1898 (22.8%) | <0.001 |
| Concomitant medications at randomisation | ||||
| Aspirin (%) | ||||
| Daily dose <100 mg | 1438/3659 (39.3%) | 877/3744 (23.4%) | 793/1898 (41.8%) | <0.001 |
| Daily dose 100–250 mg | 1495/3659 (40.9%) | 2524/3744 (67.4%) | 916/1898 (48.3%) | <0.001 |
| Daily dose >250 mg | 467/3659 (12.8%) | 134/3744 (3.6%) | 71/1898 (3.7%) | <0.001 |
| β-blocker (%) | 3056/3659 (83.5%) | 2966/3744 (79.2%) | 1211/1898 (63.8%) | <0.001 |
| ACE-I/ARB (%) | 2857/3659 (78.1%) | 3023/3744 (80.7%) | 1132/1898 (59.6%) | <0.001 |
| Statin (%) | 3161/3659 (86.4%) | 3078/3744 (82.2%) | 1520/1898 (80.1%) | <0.001 |
Data presented as n/N (%) or as median (25th, 75th centiles).
*Smoking within 30 days of randomisation.
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; CAD, coronary artery disease; GRACE, Global Registry of Acute Coronary Events; NSTEMI, non-ST-segment elevation myocardial infarction.
Table 2.
Extent of CAD in patients who underwent prerandomisation angiography
| Human Development Index (2010) classification | p Value | |||
|---|---|---|---|---|
| Very high (n=2448) | High (n=966) | Medium (n=433) | ||
| Number of diseased vessels | <0.001 | |||
| 0 | 358/2448 (14.6%) | 158/966 (16.4%) | 73/433 (16.9%) | |
| 1 | 955/2448 (39.0%) | 328/966 (34.0%) | 186/433 (43.0%) | |
| 2 | 551/2448 (22.5%) | 193/966 (20.0%) | 72/433 (16.6%) | |
| 3 | 516/2448 (21.1%) | 242/966 (25.1%) | 70/433 (16.2%) | |
| Vessels ≥50% diameter stenosis* | ||||
| LAD | 1386/2380 (58.2%) | 537/921 (58.3%) | 229/401 (57.1%) | 0.907 |
| LCX | 942/2380 (39.6%) | 415/921 (45.1%) | 139/401 (34.7%) | 0.001 |
| LM | 205/2380 (8.6%) | 59/921 (6.4%) | 13/401 (3.2%) | <0.001 |
| RCA | 1141/2380 (47.9%) | 458/921 (49.7%) | 167/401 (41.6%) | 0.024 |
Data presented as n/N (%).
*Sample sizes are smaller when examining vessel type due to missing values. Sample sizes are 2380, 921 and 401 for the very-high HDI, high-HDI and medium-HDI groups, respectively. Furthermore, the χ2 p value is reported for each vessel type since any given patient can have multiple diseased vessels.
CAD, coronary artery disease; HDI, Human Development Indices; LAD, left anterior descending; LCX, left circumflex artery; LM, left main; RCA, right coronary artery.
Study outcomes
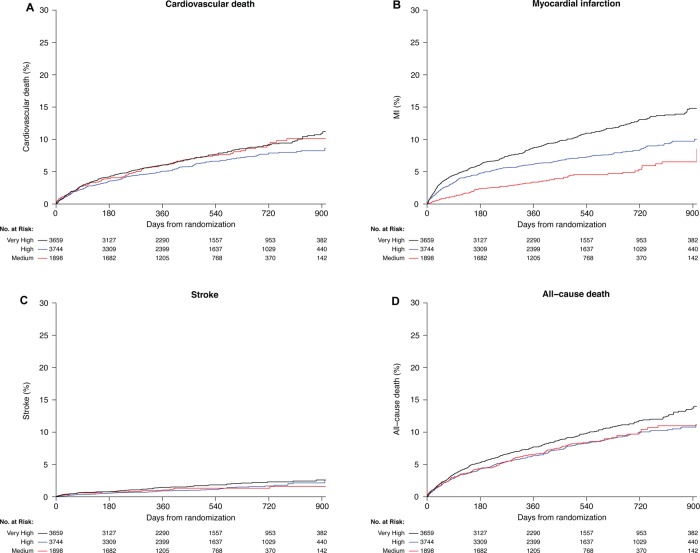
The event rates of study patients according to country-level HDI groupings are presented in table 3. Rates of occurrence of the composite endpoint and the individual component endpoint of MI differed significantly among groups, with the high-HDI country group having the lowest event rate for the composite endpoint and the medium-HDI country group having the lowest rate of MI. The unadjusted and adjusted HRs for the study outcomes are presented in table 4. The Kaplan–Meier curves for the unadjusted outcomes for CVD/MI/stroke are shown in figure 2 and other outcomes in figure 3. Whereas the unadjusted HRs for the composite endpoint and the MI component endpoint were lower for medium-HDI and high-HDI countries, the adjusted study outcomes were similar among the different HDI groups. In analysing data based on the per-capita income grouping of countries, event rates for the primary endpoint are significantly different in the unadjusted and adjusted analyses (table 5). The lower-middle-income group had a significantly lower rate of the composite endpoint compared with upper-middle-income and high-income countries; this difference was primarily driven by lower MI rates in lower-middle-income countries.
Table 3.
Efficacy outcomes through 30 months by Human Development Index classification
| Event | Human Development Index (2010) classification | p Value | ||
|---|---|---|---|---|
| Very high (n=3659) | High (n=3744) | Medium (n=1898) | ||
| Cardiovascular death, MI or stroke | <0.001 | |||
| No. of events | 599 | 452 | 209 | |
| Event rate at 30 months* | 23.1 (21.1 to 25.0) | 16.9 (15.1 to 18.7) | 17.6 (13.4 to 21.8) | |
| Cardiovascular death | 0.084 | |||
| No. of events | 270 | 231 | 134 | |
| Event rate at 30 months* | 11.3 (9.8 to 12.9) | 8.6 (7.3 to 10.0) | 10.1 (8.2 to 12.0) | |
| MI | <0.001 | |||
| No. of events | 382 | 270 | 79 | |
| Event rate at 30 months* | 14.7 (13.1 to 16.3) | 10.0 (8.6 to 11.4) | 8.5 (4.3 to 12.7) | |
| Stroke | 0.237 | |||
| No. of events | 61 | 47 | 22 | |
| Event rate at 30 months* | 2.6 (1.8 to 3.3) | 2.5 (1.5 to 3.5) | 1.6 (0.8 to 2.4) | |
| All-cause death | 0.069 | |||
| No. of events | 346 | 295 | 148 | |
| Event rate at 30 months* | 13.4 (11.9 to 15.0) | 10.7 (9.3 to 12.1) | 10.6 (8.7 to 12.5) | |
*Data presented as K-M rate at 30 months (95% CI).
MI, myocardial infarction.
Table 4.
Unadjusted and adjusted HRs with 95% CIs, Human Development Index classification
| Human Development Index (2010) classification | p Value | |||
|---|---|---|---|---|
| Event | High vs very high | Medium vs very high | High vs medium | |
| Cardiovascular death, MI, stroke | ||||
| Unadjusted HR | 0.720 (0.578 to 0.898) | 0.677 (0.491 to 0.934) | 1.063 (0.734 to 1.541) | 0.002 |
| Adjusted HR | 0.939 (0.812 to 1.087) | 0.996 (0.771 to 1.287) | 0.943 (0.712 to 1.249) | 0.701 |
| Cardiovascular death | ||||
| Unadjusted HR | 0.833 (0.633 to 1.097) | 1.003 (0.814 to 1.236) | 0.831 (0.615 to 1.123) | 0.403 |
| Adjusted HR | 1.054 (0.855 to 1.299) | 1.233 (0.939 to 1.620) | 0.854 (0.629 to 1.161) | 0.317 |
| MI | ||||
| Unadjusted HR | 0.675 (0.530 to 0.859) | 0.400 (0.215 to 0.744) | 1.687 (0.888 to 3.206) | <0.001 |
| Adjusted HR | 0.867 (0.696 to 1.081) | 0.667 (0.417 to 1.067) | 1.300 (0.812 to 2.081) | 0.161 |
| Stroke | ||||
| Unadjusted HR | 0.748 (0.534 to 1.049) | 0.732 (0.331 to 1.619) | 1.021 (0.439 to 2.376) | 0.193 |
| Adjusted HR | 1.070 (0.716 to 1.598) | 1.814 (1.007 to 3.268) | 0.590 (0.302 to 1.152) | 0.140 |
| All-cause death | ||||
| Unadjusted HR | 0.836 (0.627 to 1.117) | 0.879 (0.703 to 1.099) | 0.952 (0.687 to 1.319) | 0.069 |
| Adjusted HR | 1.085 (0.868 to 1.356) | 1.089 (0.840 to 1.412) | 0.996 (0.746 to 1.330) | 0.320 |
MI, myocardial infarction.
Figure 2.
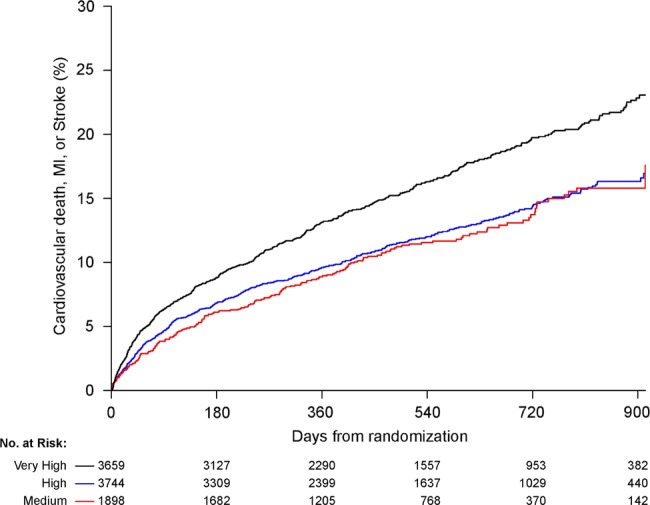
Cumulative Kaplan–Meier failure estimates of the composite study endpoint by Human Development Index (HDI) classification during the 30-month follow-up period. Black, very-high HDI; blue, high HDI; red, medium HDI.
Figure 3.
Cumulative Kaplan–Meier failure estimates of the individual component endpoints of (A) cardiovascular death, (B) all myocardial infarction (MI) events, (C) all stroke events and (D) all-cause death by Human Development Index (HDI) classification during the 30-month follow-up period. Black, very-high HDI; blue, high HDI; red, medium HDI.
Table 5.
Unadjusted and adjusted HRs with 95% CIs, per-capita gross national income classification
| Per-capita gross national income (2010) classification | ||||
|---|---|---|---|---|
| Event | Upper middle vs high | Lower middle vs high | Lower middle vs upper middle | p Value |
| Cardiovascular death, MI, stroke | ||||
| Unadjusted HR | 0.751 (0.626 to 0.902) | 0.548 (0.446 to 0.675) | 0.730 (0.569 to 0.935) | <0.001 |
| Adjusted HR | 0.956 (0.826 to 1.106) | 0.756 (0.616 to 0.928) | 0.791 (0.632 to 0.990) | 0.028 |
| Cardiovascular death | ||||
| Unadjusted HR | 0.870 (0.677 to 1.119) | 0.770 (0.557 to 1.064) | 0.855 (0.613 to 1.277) | 0.211 |
| Adjusted HR | 1.058 (0.864 to 1.294) | 0.905 (0.691 to 1.186) | 0.856 (0.636 to 1.150) | 0.585 |
| MI | ||||
| Unadjusted HR | 0.666 (0.533 to 0.831) | 0.370 (0.217 to 0.634) | 0.557 (0.319 to 0.970) | <0.001 |
| Adjusted HR | 0.851 (0.689 to 1.052) | 0.551 (0.382 to 0.796) | 0.648 (0.449 to 0.933) | 0.005 |
| Stroke | ||||
| Unadjusted HR | 0.843 (0.614 to 1.158) | 0.469 (0.292 to 0.753) | 0.556 (0.323 to 0.957) | 0.006 |
| Adjusted HR | 1.177 (0.763 to 1.815) | 1.027 (0.593 to 1.815) | 0.873 (0.468 to 1.626) | 0.761 |
| All-cause death | ||||
| Unadjusted HR | 0.903 (0.709 to 1.150) | 0.671 (0.485 to 0.930) | 0.743 (0.513 to 1.078) | 0.051 |
| Adjusted HR | 1.119 (0.917 to 1.366) | 0.828 (0.632 to 1.086) | 0.740 (0.551 to 0.995) | 0.134 |
MI, myocardial infarction.
Discussion
The clinical profiles, management and clinical outcomes of patients differed substantially among countries by their HDI groupings in this global ACS trial. The higher unadjusted event rates in very-high HDI countries appear to be explained by the older age and higher risk profiles of these patients as these differences did not persist after adjustment.
With the increasing globalisation of clinical trials in ACS, variations in clinical outcomes by geographic region have been noticed and debated,15 16 reflecting the belief that treatment benefits may differ by region. However, the credibility and biological plausibility of such trials have been challenged,17 and an examination of trial outcomes grouped by the economic and social development of participating countries has not been previously performed. We therefore assessed study outcomes from the TRILOGY ACS trial according to HDI of participating countries because this index reflects country-level socioeconomic status, an important determinant of disease management and patient outcomes.17 The present study showed that patients from medium-HDI countries were younger and had fewer associated cardiovascular risk factors. This finding has been reported in earlier studies in ACS in which patients from developing countries were typically younger than those from developed countries.8 18 This finding would also account for the lower rates of hypertension and hyperlipidaemia seen among such patients. However, the rates of diabetes mellitus were highest in medium-HDI countries, possibly due to the large representation of patients from India, which has a very high prevalence of diabetes.19 Compared with patients from the very-high HDI group, the presentation characteristics of patients from the medium-HDI group were markedly different, with these patients also being more likely to present with unstable angina. The available angiographic characteristics of patients also differed by HDI group, with those from medium-HDI countries having an overall lower disease burden and substantially lower likelihood of undergoing angiography prior to randomisation. The reasons for these differences are not clear and could be due to variations among ACS patient populations or study recruitment patterns. Overall, the observed differences in baseline characteristics are responsible for these patients having lower ACS risk scores.
One intriguing finding of this study was that patients from lower-middle-income countries, the lowest-income group of countries, enrolled in TRILOGY ACS had better outcomes with regards to occurrence of the composite endpoint compared with patients from countries with higher per-capita GNI. Unlike the variations seen in HDI grouping, this difference persisted despite adjustment for standard covariates. This finding contradicts results from other multinational trials and registries that found higher event rates in lower-income countries.9 10 This may be due to the fact that TRILOGY ACS enrolled participants slated to undergo medical treatment for their ACS without planned coronary intervention; thus, a more equitable opportunity of management was available for all patients. Similarly, time of presentation and means of perfusion were less-important outcome determinants in this medical management trial compared with other ACS trials.10 The difference in endpoint occurrence was primarily driven by lower rates of MI in these patients and could be due to differences in medical care-seeking behaviour in lower-income countries or to the use of more sensitive and stringent tools to detect MI in higher-income countries. Another important study finding was the lower rate of use of drugs for secondary prevention (β-blockers, ACE-Is/ARBs and statins) in medium-HDI countries. This may reflect differences in practice patterns or could be due to the lower risk profiles of patients from these countries. Lower rates of use of secondary prevention drugs in developing countries were also observed in the Prospective Urban Rural Epidemiology study20 and in other trials.21 22
This study is the first of its kind to assess a global clinical trial population with ACS according to the HDI groupings of the participating countries, thereby revealing clinically important differences in the characteristics and management of patients with ACS across different groups. This study's strengths also include the availability of data drawn from a relatively large and globally distributed population that included patients who often have not been included in clinical trials examining ACS (ie, higher-risk patients slated for medical management of their index ACS events without revascularisation). The TRILOGY ACS dataset also incorporated long-term (up to 30 months postrandomisation) follow-up data.
We note several limitations to our study. HDI reported for a given country represents a standardised measure of the social and economic development of that country; however, there may be significant intra-country variations, especially in large medium-HDI countries such as India, where there are notable differences between rural and urban regions. An additional limitation is the lack of representation of low-HDI countries and the fact that the medium-HDI group is characterised by fewer countries and patients relative to the high-HDI and very-high HDI groups. However, this reflects the circumstances for much medical research, which largely remains concentrated in more developed countries, and thus this represents a good beginning for assessing patient characteristics and outcomes from less-developed countries in a global trial.23 We also note that the hospitals and the patients recruited in the trial in medium-HDI countries are mostly urban and not truly representative of their respective countries. Typically these patients are recruited from tertiary and academic institutions where the patient characteristics and management vary from those of other hospitals, especially in developing countries. Similarly, patient management and outcomes in a clinical trial may not reflect “real-world” clinical practice; however, these limitations are inherent to any clinical trial.
In conclusion, our findings highlight the fact that despite the application of uniform study eligibility criteria, baseline characteristics were found to differ significantly among patients from different HDI-classified countries in this global study of patients with ACS. These baseline differences in turn lead to differential outcomes. This has important implications for clinical trial designs as such recruitment heterogeneity would affect sample size calculations and the statistical power of prospective studies. These findings may help in the conduct of future global ACS trials.
Key messages.
What is already known on this subject?
Randomised controlled trials increasingly recruit patients globally to facilitate recruitment and to improve validity and applicability.
Patient characteristics, management and outcomes of acute coronary syndrome (ACS) have been reported to vary significantly among countries. However, the influence of the developmental statuses of individual countries, as defined by the United Nations Development Programme's Human Development Index (HDI), on these factors has not been previously studied.
What might this study add?
This trial highlights the fact that despite the application of uniform study eligibility criteria, baseline characteristics differed significantly among patients from different HDI-classified countries in this global study of patients with ACS.
How might this impact on clinical practice?
This finding has important implications for the future design and conduct of global clinical trials in patients with ACS as such recruitment heterogeneity would affect sample size calculations and the statistical power of prospective studies.
Supplementary Material
Acknowledgments
The authors wish to thank Karen Pieper, MS, for expert coordination and management of the statistical analytic team. The authors also thank Morgan deBlecourt, Peter Hoffmann, and Jonathan McCall, MS, for expert editorial assistance with this manuscript, and Kerry Stenke for expert graphics assistance. Ms. Pieper, Ms. deBlecourt, Mr. Hoffmann, Mr. McCall and Ms. Stenke are employees of the Duke Clinical Research Institute, Duke University, Durham, North Carolina, USA, and received no compensation for their work on this manuscript other than their usual salaries.
Footnotes
Contributors: AR and DP designed the study. DDC and MLN performed the statistical analysis. AR created the first draft of the manuscript. All authors analysed and interpreted the data. All authors provided critical review and revision. All authors reviewed and approved the final manuscript for submission. All authors had full access to study data. MTR and EMO are guarantors for the study.
Funding: The TRILOGY ACS trial was funded by Daiichi Sankyo and Eli Lilly and Company. The study sponsors played no role in the conception, design and analysis of this study or in the initial drafting of this manuscript. An author employed by Daiichi Sankyo (DZ) contributed to subsequent drafts of the manuscript. All data analyses were performed by independent academic statisticians employed by the Duke Clinical Research Institute, Duke University, Durham, North Carolina, USA.
Competing interests: AR, DDC and MLN have no competing interests to declare. MTR receives consulting fees from Bristol-Myers Squibb, GlaxoSmithKline, Merck, Janssen Pharmaceuticals, KAI Pharmaceuticals, Sanofi-Aventis, Helsinn Pharmaceuticals, Regeneron, Novartis, AstraZeneca, Orexigen, Eli Lilly and Daiichi Sankyo; research grants from Bristol-Myers Squibb, Hoffmann-La Roche, Novartis, Schering-Plough, KAI Pharmaceuticals, Eli Lilly, AstraZeneca and Janssen Pharmaceuticals; and speakers’ bureau payments from AstraZeneca and Janssen. DZ is an employee of Daiichi Sankyo. KAAF receives grant funding from Bayer/Johnson & Johnson, Janssen, Eli Lilly and AstraZeneca; and personal fees from Bayer/Johnson & Johnson, Janssen, Eli Lilly, AstraZeneca and Sanofi-Aventis. HDW receives grant funding from Sanofi-Aventis, Eli Lilly, The Medicines Company, the National Institutes of Health, Roche, Merck Sharp & Dohme, AstraZeneca, GlaxoSmithKline and Daiichi Sankyo. PWA receives consulting fees from Eli Lilly, Hoffmann-La Roche, Regado Biosciences, Axio/Orexigen, Merck and Bayer; grant funding from Boehringer Ingelheim, Merck, GlaxoSmithKline, Amylin, Sanofi-Aventis and Regado; and payment for developing educational presentations from AstraZeneca. EMO receives grant funding and travel expenses from Daiichi Sankyo and Eli Lilly; consulting fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals, Liposcience, Merck, Pozen, Roche, Sanofi-Aventis, The Medicines Company and Web MD; grant funding from Gilead Sciences; and lecture fees from Gilead Sciences, Boehringer Ingelheim and The Medicines Company. DP receives research grants from Eli Lilly and the Medtronic Foundation, and honoraria from Eli Lilly.
Ethics approval: The TRILOGY ACS study was approved by the national regulatory authority in each participating country and by the local ethics committee or institutional review board at each study centre.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Glickman SW, McHutchison JG, Peterson ED, et al. Ethical and scientific implications of the globalization of clinical research. N Engl J Med 2009;360:816–23. [DOI] [PubMed] [Google Scholar]
- 2.Thiers FA, Sinskey AJ, Berndt ER. Trends in the globalization of clinical trials. Nat Rev Drug Discov 2008;7:13–4. [Google Scholar]
- 3.Rowland C. Clinical trials seen shifting overseas. Int J Health Serv 2004;34:555–6. [DOI] [PubMed] [Google Scholar]
- 4.Getz KA. Global clinical trials activity in the details. Applied Clinical Trials Online. 2007. http://appliedclinicaltrialsonline.findpharma.com/appliedclinicaltrials/article/articleDetail.jsp?id=453243 (accessed 23 Sep 2013).
- 5.Lang T, Siribaddana S. Clinical trials have gone global: is this a good thing? PLoS Med 2012;9:e1001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox KA, Cokkinos DV, Deckers J, et al. The ENACT study: a pan-European survey of acute coronary syndromes. European Network for Acute Coronary Treatment. Eur Heart J 2000;21:1440–9. [DOI] [PubMed] [Google Scholar]
- 7.Fox KA, Goodman SG, Klein W, et al. Management of acute coronary syndromes. Variations in practice and outcome; findings from the Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2002;23:1177–89. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Flather M, Pogue J, et al. Variations between countries in invasive cardiac procedures and outcomes in patients with suspected unstable angina or myocardial infarction without initial ST elevation. OASIS (Organisation to Assess Strategies for Ischaemic Syndromes) Registry Investigators. Lancet 1998;352:507–14. [DOI] [PubMed] [Google Scholar]
- 9.Xavier D, Pais P, Devereaux PJ, et al. REATE registry investigators. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet 2008;371:1435–42. [DOI] [PubMed] [Google Scholar]
- 10.Orlandini A, Diaz R, Wojdyla D, et al. Outcomes of patients in clinical trials with ST-segment elevation myocardial infarction among countries with different gross national incomes. Eur Heart J 2006;27:527–33. [DOI] [PubMed] [Google Scholar]
- 11.United Nations Development Programme. Human Development Report 2010: the real wealth of nations: pathways to human development. http://hdr.undp.org/en/reports/global/hdr2010/ (accessed 23 Sep 2013).
- 12.Chin CT, Roe MT, Fox KA, et al. ; TRILOGY ACS Steering Committee . Study design and rationale of a comparison of prasugrel and clopidogrel in medically managed patients with unstable angina/non-ST-segment elevation myocardial infarction: the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. Am Heart J 2010;160:16–22. [DOI] [PubMed] [Google Scholar]
- 13.Roe MT, Armstrong PW, Fox KA, et al. ; TRILOGY ACS Investigators . Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 2012;367:1297–309. [DOI] [PubMed] [Google Scholar]
- 14.World Bank. World Development Indicators online database. 2010. http://data.worldbank.org/data-catalog/world-development-indicators/wdi-2010 (accessed 23 Sep 2013).
- 15.Serebruany VL. Viewpoint: paradoxical excess mortality in the PLATO trial should be independently verified. Thromb Haemost 2011;105:752–9. [DOI] [PubMed] [Google Scholar]
- 16.Pocock S, Calvo G, Marrugat J, et al. International differences in treatment effect: do they really exist and why? Eur Heart J 2013;34:1846–52. [DOI] [PubMed] [Google Scholar]
- 17.Alter DA, Venkatesh V, Chong A; SESAMI Study Group . Evaluating the performance of the Global Registry of Acute Coronary Events risk-adjustment index across socioeconomic strata among patients discharged from the hospital after acute myocardial infarction. Am Heart J 2006;151:323–31. [DOI] [PubMed] [Google Scholar]
- 18.Joshi P, Islam S, Pais P, et al. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA 2007;297:286–94. [DOI] [PubMed] [Google Scholar]
- 19.Joshi SR, Parikh RM. India--diabetes capital of the world: now heading towards hypertension. J Assoc Physicians India 2007;55:323–4. [PubMed] [Google Scholar]
- 20.Yusuf S, Islam S, Chow CK, et al.; Prospective Urban Rural Epidemiology (PURE) Study Investigators . Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet 2011;378:1231–43. [DOI] [PubMed] [Google Scholar]
- 21.Joshi R, Chow CK, Raju PK, et al. Fatal and nonfatal cardiovascular disease and the use of therapies for secondary prevention in a rural region of India. Circulation 2009;119:1950–5. [DOI] [PubMed] [Google Scholar]
- 22.Mendis S, Abegunde D, Yusuf S, et al. WHO study on prevention of REcurrences of Myocardial Infarction and Stroke (WHO-PREMISE). Bull World Health Organ 2005;83:820–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Prabhakaran P, Ajay VS, Prabhakaran D, et al. Global cardiovascular disease research survey. J Am Coll Cardiol 2007;50:2322–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.