Abstract
Objectives
It has been hypothesised that ectopy may be associated with increased susceptibility to sexually transmitted infections (STIs). In this cross-sectional study, we wanted to explore the association between STIs (including HIV) and cervical ectopy.
Methods
We included 700 sexually active young women attending randomly selected high schools in a rural district in KwaZulu-Natal, South Africa. The district is endemic of HIV and has a high prevalence of STIs. We did computer-assisted measurements of the ectocervical area covered by columnar epithelium (ectopy) in colposcopic images and STI analyses on cervicovaginal lavage and serum samples. All participating women answered a questionnaire about sexual behaviour and use of contraceptives.
Results
The mean age was 19.1 years. Ectopy was found in 27.2%, HIV in 27.8%, chlamydia in 25.3% and gonorrhoea in 15.6%. We found that age, parity, chlamydia and gonorrhoea, years since menarche, years since sexual debut and number of sexual partners were associated with ectopy. In multivariate analysis with chlamydia infection as the dependent variable, women with ectopy had increased odds of having chlamydia infection (adjusted OR 1.78, p=0.033). In women under 19 years of age, we found twofold higher odds of being HIV-positive for those with ectopy (OR 2.19, p=0.014).
Conclusions
In conclusion, cervical ectopy is associated with Chlamydia trachomatis infection and HIV in the youngest women.
Keywords: AFRICA, ADOLESCENT, HIV, ECTOPY, CHLAMYDIA INFECTION
Introduction
Young women carry a disproportionally high risk of contracting sexually transmitted infections (STI) including HIV.1 2 A complex relationship between sexual behaviour and biological risk factors contribute to this tendency.3 Cervical ectopy, the presence of columnar epithelium on the ectocervix, has been suggested to influence women’s susceptibility to STIs.4
Ectopy occurs when the columnar epithelium, normally found in the endocervical channel, extends onto the ectocervix. This epithelium consists of a single layer of columnar cells in contrast with the stratified (multilayer) squamous epithelium covering the rest of the ectocervical surface.5 Ectopy is commonly seen in young women.5 6 During and after puberty, the process of metaplasia transforms the columnar epithelium into squamous epithelium and the cervical ectopy decreases.5
Epidemiological studies have found an association between cervical ectopy and infection with Chlamydia trachomatis.4 7–9 This intracellular bacterium resides in columnar epithelial cells, making cervical ectopy a favourable condition for infection.7 It has been hypothesised that hormonal contraceptives may further enhance this susceptibility, as they have been shown to be associated with both increased ectopy and chlamydia infection.9–11 Several studies have also found an association between ectopy and HIV infection, but the topic remains controversial.4 6 12 13 Female genital schistosomiasis (FGS) has been shown to be associated with HIV in cross-sectional studies, 14 but to our knowledge, there have been no studies on the association with cervical ectopy. The ectocervical area covered by columnar epithelium is usually estimated subjectively by the investigating clinician, whereas computer assisted measurement of the area on colposcopic images has been shown to be a more reliable method.15 16
In this study of young women, we investigated the relationship between objectively measured ectopy and common reproductive tract infections. Due to the cross-sectional design of the study we could not explore causality. However, it seems biologically plausible that cervical ectopy may be associated with increased susceptibility to STIs. To our knowledge, this is the first study investigating ectopy in young women in an area with high prevalence of STIs including HIV.
Materials and methods
Enrolment
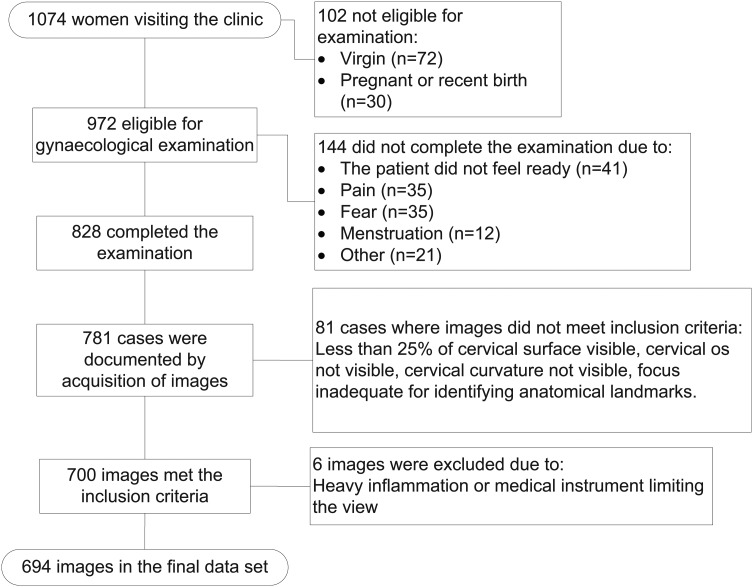
The participants were recruited in 2010–2012 from an ongoing study in coastal KwaZulu-Natal, South Africa, focusing on various aspects of FGS and STIs. Of the women (n=1074, figure 1) who visited the research clinic during this time period, all who had an image taken during the gynaecological exam that met the inclusion criteria (n=700) were included in this cross-sectional study. Young women from 31 randomly selected high schools were invited. All included women were sexually active and in their final years of high school. Women were not recruited based on symptoms.
Figure 1.
Flowchart showing inclusion of images.
The rural district on the east coast is one of the poorest in South Africa, endemic for urogenital schistosomiasis and has a high prevalence of HIV, chlamydia, gonorrhoea and trichomoniasis.
Clinical investigation and questionnaire
The study participants were interviewed by trained research assistants. The questionnaire was in the local language (isiZulu) and included questions on behavioural factors (including sexual behaviour, alcohol and drug use), contraceptives, obstetric history and urogenital symptoms.
Gynaecological examinations with colposcopy were performed by two female medical doctors (EK and KL). Pregnant women were not investigated. Cervicovaginal lavage (CVL) samples were collected by spraying 10 mL of saline on the cervix four times before drawing it back into a syringe and depositing it into tubes for frozen storage. Blood was collected into sterile acid-citrate-dextrose anticoagulated Vacutainer tubes. The examinations were documented using an Olympus OCS 500 colposcope with a mounted Olympus E 420 10 megapixel (Mpx) single lens reflex (SLR) device, or a Leisegang colposcope with a Canon EOS 40D 10 Mpx SLR. The image files were stored using high-quality JPEG compression along with data from the clinical investigation.
Image material
During the inclusion period, a total of 781 women underwent gynaecological examination with acquisition of colposcopic images. One image was selected per patient based on the following inclusion criteria: at least 25% of the ectocervical surface was visible, the cervical os was visible, a part of the cervical curvature was visible, and the focus was adequate for identifying anatomical landmarks. During the measurements, images were excluded if there was heavy inflammation rendering the evaluation impossible or if there was a non-cervical element rendering the extent of ectopy non-evaluable (such as blood, discharge or a medical instrument). The best image was chosen independent of whether it was taken before or after sampling for Pap smear, cervico-vaginal lavage or application of acetic acid.
Computer assisted measurement of cervical ectopy
All measurements were performed using the open source image analysis software ImageJ (US National Institutes of Health). It allows for a visually guided delimitation of structures and a subsequent measurement of the selected area (figure 2). We further calculated the ectopy as a fraction of the total ectocervical area. Finally, the images were saved with the measurement overlays for verification and adjustment by a senior gynaecologist (MO).
Figure 2.
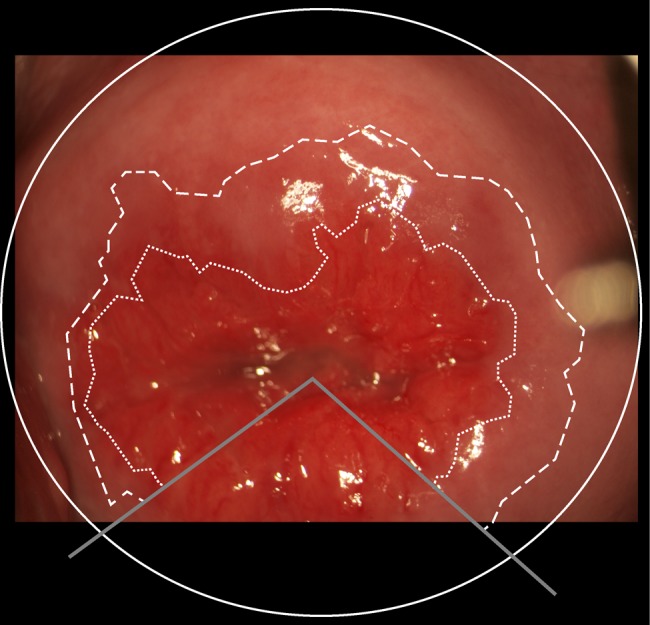
A photocolposcopic image of the ectocervix. Solid line: Delimiting the cervical area. Dashed line: Original squamocolumnar junction, outer delimitation of the transformation zone. Dotted line: Current squamocolumnar junction, represents the border between columnar epithelium and metaplastic squamous epithelium in the transformation zone.
The ectocervical area was delimited by using the cervical os as the centre of an elliptical shape that was manually fitted to conform to the visible cervical boundaries. The original squamocolumnar junction was defined as the outer delimitation of any of the following structures: glandular openings, Nabothian cysts and primary rugae extending from the cervical os. In cases where such anatomical landmarks were absent or too sparse, the original squamocolumnar junction was not measured. Finally, the current squamocolumnar junction (confining the area of ectopy) was defined as a distinct transition of colour from deep red to brighter red: The columnar epithelium is single layered and appears dense red whereas the transformation zone is covered in squamous epithelium, appearing brighter in colour.
In cases where the original or current squamocolumnar junctions were partially masked by medical instruments, blood or as a result of the angle of inspection or photographic section, the analysis of the ectocervix was divided into sectors. The largest sector(s) in which it was possible to demarcate the squamocolumnar junction was used as a basis for estimating the squamocolumnar junction that was impossible to measure.
The output of the measurements was in numbers of squared pixels. The focal distance of each image was unknown. In consequence, it was impossible to convert the computer measurements into absolute values of squared millimetres. All measurements were, therefore, converted to fractions of the total ectocervical area, thus representing relative values. In previous studies, a cut-off level of 10% of the cervical area covered by columnar epithelium has been used to define a cervix as having ectopy.6 12 The investigators were blinded to the patients’ STI results and questionnaire information.
Laboratory analyses
CVL samples were analysed by strand displacement assay for Neisseria gonorrhoeae and C. trachomatis (ProbeTec CT/GT, Becton, Dickinson and Company (BD), Franklin Lakes, New Jersey, USA). A Gram stain was made from the CVL and scored for bacterial vaginosis using Nugent's scoring methodology. PCR was used to screen for herpes simplex virus types 1 and 2 (HSV 1 and 2) and Trichomonas vaginalis (in-house PCR, Laboratory of Infection, Prevention and Control, UKZN, Durban, South Africa).17 18 Syphilis serology was performed using rapid plasma reagin (Macro Vue test 110/112, BD) and verification was done using Treponema pallidum hemagglutination assay (Omega diagnostics Group PLC, Alva, Scotland, UK). HIV serology was done using rapid test kits (Bioline Rapid Test HIV, New Jersey, USA and Sensa Tri-Line HIV Test Kit, Durban, South Africa). All serology was done on serum samples frozen at −80°C.
Ethics
The study was approved by the Biomedical Research Ethics Administration (BREC), University of KwaZulu-Natal, South Africa 20 February 2009 (Ref BF029/07, renewed yearly) and by the Provincial Department of Health, Pietermaritzburg, South Africa on 3 February 2009 (Reference HRKM010-08). The Regional Committee for Medical and Health Research Ethics (REC) South Eastern Norway gave ethical clearance on 17 September 2007 (Ref 469-07066a1.2007.535). The Departments of Health and Education in Ugu district, KwaZulu-Natal also gave permissions.
Only sexually active women were invited. All participants signed individual, written consent forms. The ethical committees, BREC and REC, were aware that 15-year-olds to 17-year-olds were participating in the study and specifically approved the consent procedure (independent minor consent, no parental consent). According to South African legislation, persons over the age of 12 years could consent independently to participate in research. Those who presented with symptoms, signs or tested positive on subsequent laboratory analyses of STIs were offered treatment in accordance with a South African syndromic protocol. Partner treatment was also offered. HIV testing and follow-up was done in accordance with South African guidelines. HIV positive patients were referred to local clinics for follow-up and antiretroviral treatment. Anti-schistosomal treatment was offered to all.
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics V.20 (SPSS, Armonk, New York, USA). Pearson's χ2 test and Fisher's exact test were used to compare the characteristics of women with and without ectopy. Univariate and multivariate logistic regression models were constructed to analyse the associations between ectopy, age, parity and gynaecological pathology. A significance level of 5% was used throughout.
Results
Out of 781 participants, 694 (88.9%) could be included in the evaluation (figure 1). Mean age was 19.1 years and followed a near normal distribution (SD 2.1, range 15–31). Half of the women had given birth (383/682, 56.2%). Parous women had a mean of 1.2 pregnancies (SD 0.6) and their mean age was 19.8 years (SD 2.0). Injectable contraceptives were used by 30.0% of the women (204/681), while only 1.5% (10/680) used contraceptive pills. At least one STI (not including HIV) was found in 306 women (60.6%). The HIV prevalence was 27.8% (159/572). Only 9.1% (62/681) reported that they knew they were HIV positive, of which 23.0% (14/61) reported to be on antiretroviral treatment (one did not answer).
Cervical ectopy
Ectopy, measured as a fraction of the total ectocervical area, did not follow a normal distribution. The median degree of ectopy was 4.0% (range 0.0%–69.5%). Using a 10% cut-off in defining ectopy, 27.2% (n=189) of the women had ectopy (table 1, Web reference 1). Ectopy was associated with having been pregnant (OR 3.11, 95% CI (2.14 to 4.52), p value <0.001). The level of ectopy was also increasing with age (0.5% per year, p=0.019). However, when adjusted for having given birth, no age-dependent increase was found (p=0.83). The same tendency was found for years since menarche, years since sexual debut and number of lifetime sexual partners, which were significantly associated to degree of ectopy but not when adjusted for having given birth.
Table 1.
Characteristics and test results of the study population comparing the group with cervical ectopy (≥10% of the area) to those without ectopy
| Ectopy (%)* | No ectopy (%)* | OR (95% CI) | p Value† | ||
|---|---|---|---|---|---|
| Characteristics | |||||
| Total | 189 (100) | 505 (100) | |||
| Age | <19 | 81/188 (43) | 276/496 (56) | 0.60 (0.43 to 0.84) | 0.003 |
| Never been pregnant | 47/187 (25) | 252/495 (51) | |||
| Pregnant at least once | 141/187 (75) | 243/495 (49) | 3.11 (2.14 to 4.52) | <0.001 | |
| Years since menarche | 0–4 | 66 (35) | 210 (43) | ||
| 5–6 | 65 (35) | 167 (34) | 1.24 (0.83 to 1.84) | 0.293 | |
| ≥7 | 57 (30) | 115 (23) | 1.58 (1.04 to 2.40) | 0.034 | |
| Infections | |||||
| Chlamydia trachomatis | 55/157 (35) | 89/412 (22) | 1.96 (1.31 to 2.93) | 0.001 | |
| HIV | 53/165 (32) | 106/407 (26) | 1.34 (0.91 to 1.99) | 0.142 | |
| Herpes simplex virus | 6/158 (4) | 26/408 (6) | 0.58 (0.23 to 1.44) | 0.239 | |
| Syphilis | 6/147 (4) | 9/344 (3) | 1.58 (0.55 to 4.53) | 0.388 | |
| Trichomonas vaginalis | 40/170 (24) | 87/424 (21) | 1.19 (0.78 to 1.82) | 0.419 | |
| Neisseria gonorrhoeae | 38/156 (24) | 70/412 (17) | 1.57 (1.01 to 2.46) | 0.046 | |
| Bacterial vaginosis | 75/149 (50) | 217/394 (55) | 0.83 (0.57 to 1.21) | 0.323 | |
| Any sexually transmitted infection‡ | 102/150 (68) | 204/355 (58) | 1.57 (1.05 to 2.35) | 0.027 | |
| Grainy sandy patch§ | 28/189 (15) | 70/505 (14) | 1.08 (0.67 to 1.74) | 0.748 | |
| Contraceptives | |||||
| Contraceptive injection | 61/187 (33) | 143/494 (29) | 1.19 (0.83 to 1.71) | 0.350 | |
| Contraceptive pills | 5/187 (3) | 5/493 (1) | 2.68 (0.77 to 9.37) | 0.148¶ | |
| Condom use (last intercourse) | 64/140 (46) | 158/380 (42) | 1.18 (0.80 to 1.75) | 0.398 | |
| Behavioural factors | |||||
| Number of lifetime partners | ≥3 | 41/186 (22) | 147/492 (30) | 0.66 (0.45 to 0.99) | 0.042 |
| Alcohol, age at debut** | ≤15 | 24/187 (13) | 82/491 (17) | 0.66 (0.39 to 1.11) | 0.119 |
| >15 | 83/187 (44) | 229/491 (47) | 0.82 (0.57 to 1.17) | 0.272 | |
| Drugs (ever used) | 11/188 (6) | 51/494 (10) | 0.54 (0.28 to 1.06) | 0.069 | |
| Years since sexual debut | ≥3 | 95/187 (51) | 183/492 (37) | 1.74 (1.24 to 2.45) | 0.001 |
| Age at sexual debut | ≤15 | 52 (28) | 112 (23) | ||
| 16 | 49 (26) | 167 (34) | 0.63 (0.40 to 1.00) | 0.049 | |
| ≥17 | 86 (46) | 215 (44) | 0.86 (0.57 to 1.30) | 0.480 | |
| Days since last intercourse | ≤7 | 53/186 (28) | 135/488 (28) | 0.96 (0.66 to 1.40) | 0.830 |
| Anal sex | 8/187 (4) | 13/494 (3) | 1.65 (0.67 to 4.06) | 0.267 | |
| Forced sex | 20/188 (11) | 45/494 (9) | 1.19 (0.68 to 2.07) | 0.543 | |
*Denominators vary according to specimens available for testing or missing answers in the questionnaire.
†χ2 test.
‡Not including HIV.
§Diagnosed by colposcopy, pathognomonic for female genital schistosomiasis.19
¶Fisher's exact test.
**Those who reported to never have tasted alcohol served as reference group.
Ectopy and C. trachomatis infection
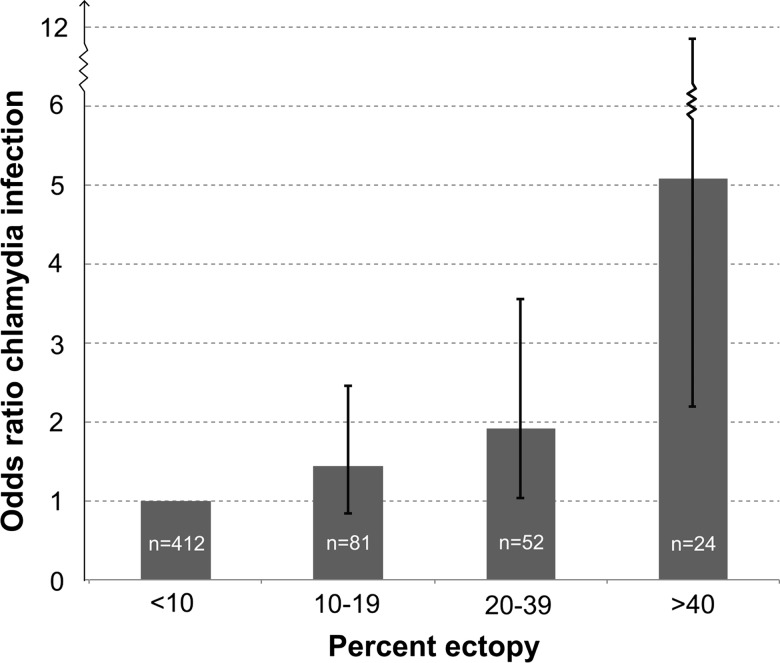
Of the women with ectopy, 35.0% tested positive for genital chlamydia infection as opposed to 21.6% of the women without ectopy (OR 1.96, 95% CI (1.31 to 2.93), p=0.001). Increasing ratio of ectopy was associated with an increasing OR of having chlamydia infection (figure 3). Women with more than 40% ectopy very often had chlamydia infection compared with women without ectopy (OR 5.08, 95% CI (2.18 to 11.83), p<0.001).
Figure 3.
Graph showing the OR, with 95% CIs, of having Chlamydia trachomatis infection (y-axis) with increasing levels of cervical ectopy (x-axis). Participants without ectopy (<10%) serve as the reference group.
We fitted a multivariate logistic regression model with chlamydia as the dependent variable, where we adjusted for other STIs, age, condom use, number of partners and age at sexual debut. We found that women with ectopy had almost twofold increased odds of having chlamydia (adjusted OR (AOR) 1.78, 95% CI (1.05 to 3.01), p=0.033).
Ectopy and N. gonorrhoeae infection
Gonorrhoea was found more frequently in the ectopy group (24.4%) than in the group without ectopy (17.0%, OR 1.57, 95% CI (1.01 to 2.46), p=0.046). None of the other STIs nor FGS was significantly associated with ectopy in univariate analysis (table 1). However, when adjusting for other STIs in a multivariate regression model, gonorrhoea was not significantly associated with ectopy (AOR 1.15, 95% CI (0.70 to 1.88), p=0.578).
Ectopy and HIV infection
For women under 19 years of age (n=282), we found an HIV prevalence of 31.8% in women with ectopy as opposed to 17.6% in women without ectopy (OR 2.19, 95% CI (1.17 to 4.09), p=0.014, Web reference 1). There was no significant association between cervical ectopy and HIV in women over 19 years of age (p=0.452).
Discussion
In this study, we found a twofold increase of the odds of being HIV positive for the youngest women with ectopy. Furthermore, we found that chlamydia infection was independently associated with ectopy.
A large, longitudinal study conducted in South Africa in 2006 determined that ectopy was a risk factor for HIV.13 They found a weak association, and only for ectopy extending over 20% of the cervical surface. The women were, however, participants of a screening programme for cervical cancer and older than in our study (>35 years). We observed a difference in the association between ectopy and HIV infection between age groups. It has previously been reported that young age is an independent risk factor for acquisition of HIV.1 It is possible that the influence of various HIV risk factors change during adolescence. Stratifying by age might help decipher risk factors in different age groups. A challenge is the timing of HIV acquisition in relation to the ectopy, as there is a natural decrease of ectopy over time.4 The youngest women in this population are likely to have HIV seroconverted more recently and may therefore give a better indication of risk factors present at the time of HIV acquisition. The single-layered columnar epithelium may represent a weaker physical barrier than the stratified squamous epithelium, leaving a woman with ectopy at higher risk of HIV infection.4 Furthermore, HIV target cells have been found in greater abundance in columnar than in squamous epithelium.4 Higher levels of HIV viral shedding in the genital tract have also been associated with ectopy.20
In accordance with other studies, we also found a strong association between ectopy and chlamydia infection.7 9 11 Due to the cross-sectional design of this study, we cannot determine the cause–effect relationship of the association. One longitudinal study reported increased risk of C. trachomatis acquisition when ectopy was present.9 It seems biologically plausible that a larger area of columnar epithelium exposed on the ectocervical surface may increase the risk of infection. Our data indicate a dose–response relationship between ectopy and chlamydia infection (figure 3). In the univariate analysis, both gonorrhoea and chlamydia infection was associated with ectopy. It has been suggested that chlamydia shedding could be increased with concurrent N. gonorrhoeae infection.21 Both these STIs may lead to serious consequences for women and increased risk of HIV acquisition.22 We did not find a significant association between ectopy and syphilis, T. vaginalis, bacterial vaginosis, HSV or FGS in univariate analysis (table 1).
Our finding that parity was associated with ectopy is in accordance with previous studies.10 12 23 24 This can be related to normal changes of the cervix caused by the delivery or hormonal changes during pregnancy. This finding may indicate that parity could influence a woman's susceptibility to STIs. The number of oral contraceptive users was too low to draw any conclusion on the association with ectopy, but contraceptive injections of progestogen were not associated with ectopy.
Limitations
The size of the exposed columnar epithelium might depend on the opening of the speculum. Parous women have a wider and more open cervix and as a result, the ectopy could be overestimated. Even though the columnar epithelium is generally easy to distinguish from the surrounding squamous epithelium, we cannot rule out the possibility that some areas of immature metaplasia, inflammation or otherwise hyperaemic epithelium could mimic ectopy and hence lead to an overestimation of the area. In cases of doubt, the use of acetic acid was helpful.
The planimetric measuring tool provides relative numbers as a fraction of the total ectocervical area. Although this gives an indication of the degree of ectopy, which allows for comparison with other subjects, it may be of more biological importance to measure the absolute area of ectopy. This is only possible if the exact depth of field is known, but this information could not be extracted from our images.
T. vaginalis, N. gonorrhoeae, C. trachomatis and HSV were analysed in vaginal lavage samples. Similar methods have been used in previous studies and have been validated for HSV and T. vaginalis.17 18 25
Our analysis of N. gonorrhoeae and C. trachomatis in lavage showed a similar ratio between the two infections compared to previous reports from South Africa.26 27 Furthermore, the DNA-based detection technique that was used for N. gonorrhoeae and C. trachomatis is reported to have close to 100% specificity.28
We did not collect information about the practice of vaginal douching, which may be negatively associated with cervical ectopy.11
Strengths
The study population was extensively explored for factors that have previously been hypothesised to influence ectopy. As our study participants were recruited from schools regardless of symptoms, the participants were more likely representative of the general population at this age than women attending health facilities.
This study has the advantage that ectopy area calculations were objective. Computer assisted planimetry on digital, colposcopic images offered a precise and reproducible way of measuring the relative area of ectopy on the cervix. The investigation was performed at a point in time where there are almost no adult survivors of mother-to-child HIV transmission, hence almost all HIV infections were likely to have been sexually transmitted. In this young population, it is therefore probable that STIs including HIV infections occurred relatively recent to our measurement of ectopy. As the degree of ectopy changes over time, this temporal proximity of measurement is a clear advantage.
Clinical relevance and conclusion
STIs and especially HIV have serious consequences for women. The high prevalence of STIs including HIV in this group of young women is worrying and warrants further studies. Thorough knowledge about possible risk factors is needed. Ectopy may be a biological risk factor for chlamydia infection and for HIV in the young women. Treatment of ectopy by methods like cryocauterisation and electrocoagulation has been discussed, but the effect is still uncertain.29 Counselling on barrier contraceptives might be of special importance when cervical ectopy is found in young women at risk.
Key messages.
Columnar epithelium found on the ectocervical surface is called ectopy and it can be a normal finding in young women.
Young women with ectopy were found to have more Chlamydia trachomatis, Neisseria gonorrhoeae and HIV infections.
Ectopy may render the woman more susceptible to sexually transmitted infections.
Supplementary Material
Acknowledgments
Thanks to the local staff, professors Bjørn Myrvang and Leiv Sandvik.
Footnotes
Handling editor: Jackie A Cassell
Contributors: EFK, SGG, MO, MT, EK and SDH contributed to the conception and design of the work. Acquisition, analysis or interpretation of the data was done by EK, SDH, KL, PM and MO. EK and SDH drafted the first version of the paper, and KL, EFK, SGG, MT, PM and MO revised the draft. All the co-authors approved the final version of the manuscript.
Funding: The research project was funded by grants from the South-Eastern Norway Regional Health Authority and European Research Council under the European Union’s Seventh Framework Programme.
Competing interests: None.
Ethics approval: Biomedical Research Ethics Administration, University of KwaZulu-Natal, South Africa and by the Provincial Department of Health, Pietermaritzburg, South Africa and The Regional Committee for Medical and Health Research Ethics (REC) South Eastern Norway.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sarkar K, Bal B, Mukherjee R, et al. Young age is a risk factor for HIV among female sex workers—an experience from India. J Infect 2006;53:255–9. [DOI] [PubMed] [Google Scholar]
- 2.Ramjee G, Wand H. Geographical clustering of high risk sexual behaviors in “hot-spots” for HIV and sexually transmitted infections in Kwazulu-Natal, South Africa. AIDS Behav 2014;18:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi TJ, Shannon B, Prodger J, et al. Genital immunology and HIV susceptibility in young women. Am J Reprod Immunol 2013;69(Suppl 1):74–9. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesh KK, Cu-Uvin S. Assessing the relationship between cervical ectopy and HIV susceptibility: implications for HIV prevention in women. Am J Reprod Immunol 2013;69(Suppl 1):68–73. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson DL, Peralta L, Graham NM, et al. Histologic development of cervical ectopy: relationship to reproductive hormones. Sex Transm Dis 2000;27:252–8. [DOI] [PubMed] [Google Scholar]
- 6.Moscicki a B, Ma Y, Holland C, et al. Cervical ectopy in adolescent girls with and without human immunodeficiency virus infection. J Infect Dis 2001;183:865–70. [DOI] [PubMed] [Google Scholar]
- 7.Lee V, Tobin JM, Foley E. Relationship of cervical ectopy to chlamydia infection in young women. J Fam Plann Reprod Health Care 2006;32:104–6. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson DL, Peralta L, Farmer M, et al. Relationship of hormonal contraception and cervical ectopy as measured by computerized planimetry to chlamydial infection in adolescents. Sex Transm Dis 2000;27:313–19. [DOI] [PubMed] [Google Scholar]
- 9.Morrison CS, Bright P, Wong EL, et al. Hormonal contraceptive use, cervical ectopy, and the acquisition of cervical infections. Sex Transm Dis 2004;31:561–7. [DOI] [PubMed] [Google Scholar]
- 10.Bright PL, Norris Turner A, Morrison CS, et al. Hormonal contraception and area of cervical ectopy: a longitudinal assessment. Contraception 2011;84:512–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Critchlow CW, Wölner-Hanssen P, Eschenbach DA, et al. Determinants of cervical ectopia and of cervicitis: age, oral contraception, specific cervical infection, smoking, and douching. Am J Obstet Gynecol 1995;173:534–43. [DOI] [PubMed] [Google Scholar]
- 12.Moss GBG, Clemetson D, D'Costa L, et al. Association of cervical ectopy with heterosexual transmission of human immunodeficiency virus: results of a study of couples in Nairobi, Kenya. J Infect Dis 1991;164:588–91. [DOI] [PubMed] [Google Scholar]
- 13.Myer L, Wright TC, Denny L, et al. Nested case-control study of cervical mucosal lesions, ectopy, and incident HIV infection among women in Cape Town, South Africa. Sex Transm Dis 2006;33:683–7. [DOI] [PubMed] [Google Scholar]
- 14.Kjetland EF, Leutscher PD, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol 2012;28:58–65. [DOI] [PubMed] [Google Scholar]
- 15.Gilmour E, Ellerbrock TV, Koulos JP, et al. Measuring cervical ectopy: direct visual assessment versus computerized planimetry. Am J Obstet Gynecol 1997;176(1 Pt 1):108–11. [DOI] [PubMed] [Google Scholar]
- 16.Morrison CS, Bright P, Blumenthal PD, et al. Computerized planimetry versus clinical assessment for the measurement of cervical ectopia. Am J Obstet Gynecol 2001;184:1170–6. [DOI] [PubMed] [Google Scholar]
- 17.Pillay A, Radebe F, Fehler G, et al. Comparison of a TaqMan-based real-time polymerase chain reaction with conventional tests for the detection of Trichomonas vaginalis. Sex Transm Infect 2007;83:126–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aumakhan B, Hardick A, Quinn TC, et al. Genital herpes evaluation by quantitative TaqMan PCR: correlating single detection and quantity of HSV-2 DNA in cervicovaginal lavage fluids with cross-sectional and longitudinal clinical data. Virol J 2010;7:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjetland EF, Ndhlovu PD, Gomo E, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS 2006;20:593–600. [DOI] [PubMed] [Google Scholar]
- 20.Homans J, Christensen S, Stiller T, et al. Permissive and protective factors associated with presence, level, and longitudinal pattern of cervicovaginal HIV shedding. J Acquir Immune Defic Syndr 2012;60:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes RC, Katz BP, Rolfs RT, et al. Quantitative culture of endocervical Chlamydia trachomatis. J Clin Microbiol 1990;28:774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS 2010;5:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson DL, Peralta L, Farmer M, et al. Cervical ectopy and the transformation zone measured by computerized planimetry in adolescents. Int J Gynaecol Obstet 1999;66:7–17. [DOI] [PubMed] [Google Scholar]
- 24.Louv WC, Austin H, Perlman J, et al. Oral contraceptive use and the risk of chlamydial and gonococcal infections. Am J Obstet Gynecol 1989;160:396–402. [DOI] [PubMed] [Google Scholar]
- 25.Kjetland EF, Gwanzura L, Ndhlovu PD, et al. Herpes simplex virus type 2 prevalence of epidemic proportions in rural Zimbabwean women: association with other sexually transmitted infections. Arch Gynecol Obstet 2005;272:67–73. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson D, Abdool Karim SS, Harrison A, et al. Unrecognized sexually transmitted infections in rural South African women: a hidden epidemic. Bull World Heal Organ 1999;77:22–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Pettifor AE, Kleinschmidt I, Levin J, et al. A community-based study to examine the effect of a youth HIV prevention intervention on young people aged 15–24 in South Africa: results of the baseline survey. Trop Med Int Health 2005;10:971–80. [DOI] [PubMed] [Google Scholar]
- 28.Fontana C, Favaro M, Cicchetti O, et al. Performance of strand displacement amplification assay in the detection of Chlamydia trachomatis and Neisseria gonorrhoeae. Jpn J Infect Dis 2005;58:283–8. [PubMed] [Google Scholar]
- 29.Machado Junior LC, Dalmaso ASW, Carvalho HB. Evidence for benefits from treating cervical ectopy: literature review. Sao Paulo Med J 2008;126:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.