Abstract
Objective: The objective of this study was to determine the safety of oral Polypodium leucotomos extract administered twice daily to healthy adults for 60 days and assess its ability to provide protection against exposure to ultraviolet radiation. Design: This was a randomized, double-blind, placebo-controlled study. Setting: A single clinical research center. Participants: Healthy adult men and women between 18 and 65 years of age with Fitzpatrick skin types I to IV. Measurements: Safety assessments included a physical examination, vital signs, and clinical laboratory parameters including hematology, comprehensive metabolic panel, and prothrombin time-partial thromboplastin time were obtained at baseline and at the end of the study. Reports of adverse events were recorded. Efficacy assessments were changes in minimal erythema dose testing, ultraviolet-induced erythema intensity response, and sunburn history during the prior 60 days. Results: After two months of treatment, there were no changes in any safety assessments. The subjects in the placebo group showed a greater likelihood of experiencing >1 episodes of sunburn (2 vs. 8 subjects; p=0.04) At Day 28, Polypodium leucotomos extract-treated subjects showed greater likelihood of an increased minimal erythema dose (8 vs. 1 subject; p=0.01) and greater likelihood of decreased ultraviolet-induced erythema intensity (10 subjects vs. 3 subjects; p<0.01). Conclusion: Polypodium leucotomos extract 240mg taken twice daily for 60 days was a safe and effective means for reducing the damaging effects of ultraviolet radiation. Based on the excellent safety profile of Polypodium leucotomos, additional studies using higher doses may be warranted.
Sunburn is an inflammation of the skin caused by overexposure to solar ultraviolet (UV) radiation. Sunburns can affect all people, regardless of age or ethnicity, often resulting in erythema, dryness, and pain. Although the pain and inflammation from sunburn are short-lived, the damage to the skin may be permanent leading to serious, long-term consequences, such as skin cancer.
Non-melanoma skin cancer (NMSC) is the most common form of cancer in the United States. More than 3.5 million skin cancers are diagnosed in more than two million people each year.1 Basal cell carcinoma (BCC) comprises the majority of NMSCs and is more common than all other human malignancies combined.2 More than 2.8 million new cases of BCC are diagnosed in the United States each year and are estimated to result in more than 3,000 deaths.2 Squamous cell carcinoma is the second most common form of NMSC. An estimated 700,000 cases of SCC are also diagnosed in the United States each year.3
The incidence of NMSCs appear to be increasing dramatically. The diagnosis and treatment of NMSCs in the United States alone has increased 77 percent over the past 20 years.2 While there may be numerous reasons for this increase, an aging US population appears to be a significant reason.4 A study from South Florida estimated the annual incidence of NMSC to be 466.5 per 100,000 persons ≥ 65 years of age increasing to 10,689.8 per 100,000 persons 65 years of age.5 The economic burden of NMSCs and other sun-related injury is substantial. According to the National Cancer Institute, the estimated total direct cost associated with the treatment of melanoma in 2010 was $2.36 billion in the United States.6 The annual economic impact for lost work and treatment may exceed $10 million.7
There are a variety of methods available to protect the skin from UV radiation that can prevent sunburns and skin cancer. Behavioral changes, such as the use of hats, protective clothing, and sunscreens are effective and well-known means for preventing skin cancer.8-10 Unfortunately, compliance with sun protection techniques is less than optimal. Frequently cited reasons for not using sun protection include the inconvenience of using sun protection, forgetting to apply sun protection measures, a desire to be tan, and protective clothing being too hot to wear.11 A naturally occurring oral supplement may provide a more acceptable alternative for protecting the skin from the harmful effects of sun exposure.
Polypodium leucotomos is a South American species of fern in the family Polypodiaceae. For many years, extracts of this fern have been used for treating a variety of skin conditions,12 including psoriasis,13 atopic dermatitis,14 vitiligo,15 polymorphic light eruption,16 and melasma.17 Growing evidence indicates the oral administration of oral P. leucotomos extract can provide effective protection against solar UV radiation.18 The objective of this double-blind, placebo-controlled study was to determine the safety of oral P. leucotomos extract (Heliocare®, Ferndale Healthcare®, Ferndale, Michigan) in healthy adults and to assess its ability to provide protection against exposure to UV.
METHODS
Study subjects. Healthy adult men and women between 18 and 65 years of age with Fitzpatrick skin types I to IV were enrolled in the study. Women of childbearing potential agreed to use an effective method of birth control during the study. All subjects expressed their willingness to maintain their outdoor lifestyle and forgo any skin procedures, such as microdermabrasion, intense pulsed light, light-emitting diode, or radiofrequency therapy. Subjects were excluded from participation if they had a known medical condition or were using a medication or other treatment that might interfere with the objective of the study or place them at risk, were known to be hypersensitive to P. leucotomos extract, were pregnant or lactating, or had participated in a clinical trial using investigational drugs or devices within the previous 30 days. Using the stratified block technique, subjects were randomized to receive P. leucotomos extract (N=20) or placebo (N=20).
Study drug. Each capsule contains 240mg of P. leucotomos extract (Heliocare® Capsules; Ferndale Healthcare®, Inc.). The placebo was an inert capsule of similar appearance.
Study procedures. Twenty subjects were randomized to receive 240mg of P. leucotomos extract or placebo twice daily at approximately 8AM and 2PM for two months. In addition, 10 subjects from each treatment group were randomized to undergo minimal erythema dose (MED) testing,19 consisting of three sessions of two-minute exposures to UV-B on an area of the buttocks that normally does not receive sun exposure (Dermalight® 80; National Biological Corporation, Beachwood, Ohio).
Assessments. A urine pregnancy test was performed for women of childbearing potential prior to treatment. Safety assessments included a physical examination, vital signs, and clinical laboratory parameters including hematology, comprehensive metabolic panel, and prothrombin time-partial thromboplastin time (PT-PTT) were obtained at baseline and at the end of the study. Volunteered, observed, and elicited reports of adverse events (AEs) were recorded. Safety assessments were done on Day 0 (baseline) and Days 14, 28, and 56. In addition, the subjects randomized to undergo MED assessments were evaluated for UV-associated erythema and skin damage on Days 0, 14, and 28. Efficacy assessments included the response to treatment with P. leucotomos extract by changes in MED testing, UV-induced erythema intensity response, and sunburn history during the prior 60 days.
Statistical analysis. A sample size of 40 subjects randomly assigned to two treatment groups was determined to be sufficient to achieve the safety endpoints. These endpoints were evaluated using a 2-sided Chi square test at the 0.05 significance level, assuming 80-percent power. Continuous data were summarized by treatment group using descriptive statistics. Categorical data were summarized by treatment group using frequency tables. Ninety-five percent confidence intervals were constructed for proportions of successes. Statistical tests comparing placebo to the active treatment group were 2-sided and conducted at the 0.05 significance level.
Ethics. The protocol used in this study adhered to the Good Clinical Practice guidelines of the International Conference on Harmonization.20 All subjects provided informed consent prior to participating in any study-related procedures. The protocols used in this study were approved by an independent institutional review board (US Investigational Review Board, Miami, FL, IRB # USIRB2013CCCR/03).
RESULTS
All subjects completed the study. After two months of treatment, there were no changes in any safety assessments. Four P. leucotomos extract-treated subjects reported mild episodic fatigue, bloating, and headaches and one subject in the placebo group reported fatigue; however, these were not believed to be treatment-related.
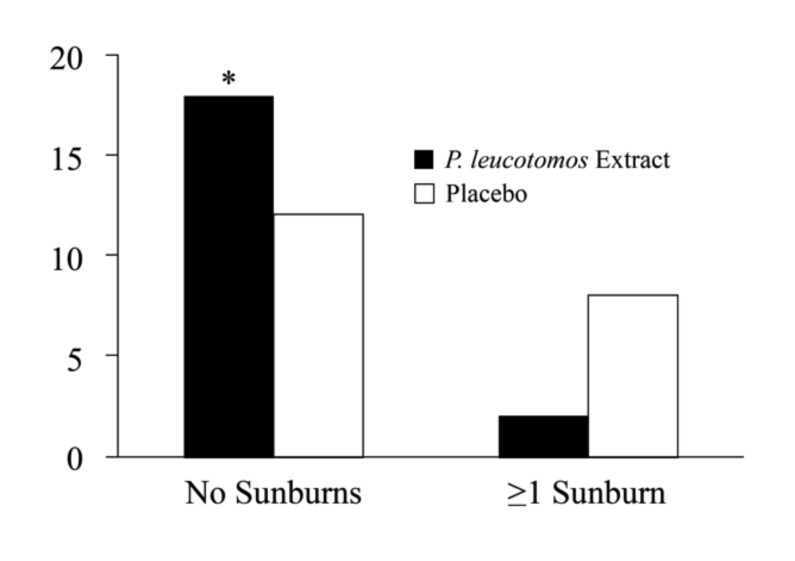
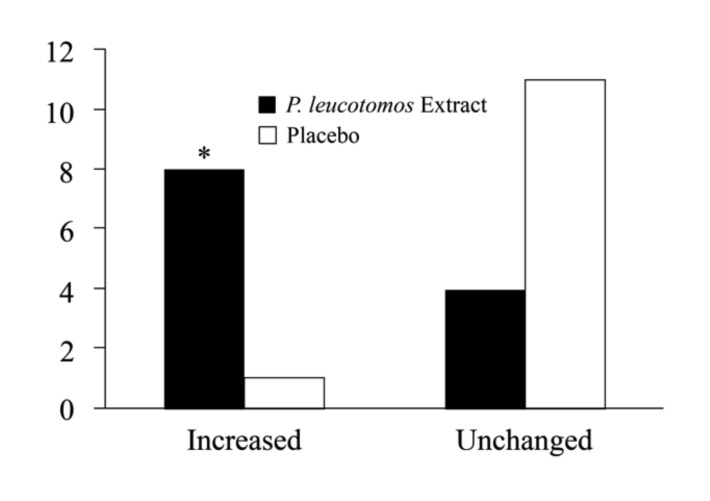
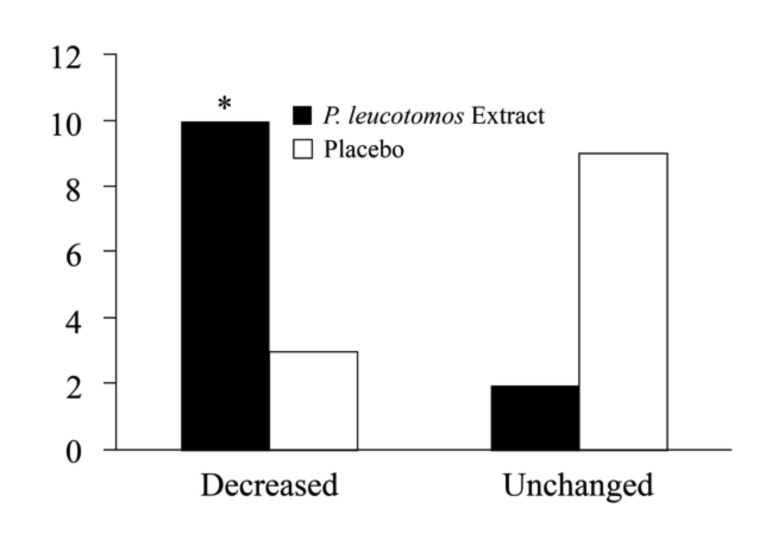
Although there was no significant intergroup difference in the number of hours of sun exposure before or during the study, subjects in the placebo group showed a greater likelihood of experiencing one or more episodes of sunburn during the study (2 subjects vs. 8 subjects; p=0.04) (Figure 1). At Day 28, P. leucotomos extract-treated subjects showed greater likelihood of an increased MED compared to subjects treated with placebo (8 subjects vs. 1 subject; p=0.01) (Figure 2). Subjects in the P. leucotomos extract group also showed a greater chance of demonstrating decreased UV-induced erythema intensity compared to those assigned to the placebo (10 subjects vs. 3 subjects; p<0.01) (Figure 3).
Figure 1.
Change in sunburn frequency. Odds ratio calculations showed subjects in the placebo group had a 6-fold greater likelihood of experiencing at least one sunburn during the study. * Denotes p=0.04.
Figure 2.
Change in minimal erythema dose (MED). Odds ratio calculations showed subjects in the Polypodium leucotomos extract group had a 22-fold greater likelihood of experiencing an increased MED. * Denotes p=0.01.
Figure 3.
Change in UV-induced erythema intensity. Odds ratio calculations showed subjects in the Polypodium leucotomos extract group had a 15-fold greater likelihood of experiencing decreased UV-induced erythema intensity. *Denotes p<0.01.
Female subjects treated with P. leucotomos extract showed a greater likelihood of experiencing decreased erythema intensity compared to the male subjects after 28 days of treatment (9 subjects vs. 1 subject) and a similar trend was observed for increased MED (7 subjects vs. 1 subject); however, statistical relevance could not be established as there were only two male subjects enrolled in the P. leucotomos extract group.
DISCUSSION
The primary harmful effects of solar UV radiation exposure are sunburn and the development of NMSC.8 UV radiation induces cancer by damaging deoxyribonucleic acid (DNA) and reducing the ability of skin cells to control cell proliferation. This damage occurs when DNA absorbs UV energy—primarily UV-B—which causes adjacent thymine base pairs to bond together into pyrimidine dimers. As a result of this disruption, the DNA strand cannot be copied. Both UV-A and UV-B can also promote the indirect formation of oxidized DNA bases.11 Although skin cells possess mechanisms to repair minor DNA damage, excessive damage may lead to the development of skin cancer.
Orally administered P. leucotomos extract decreases UV-mediated oxidative damage to DNA by enhancing the activity of endogenous antioxidant systems responsible for blocking the formation of reactive oxygen species.18 Several preclinical studies have demonstrated the antioxidant and photoprotective properties of P. leucotomos extract in mice exposed to UV radiation.21,22 Other animal studies have specifically assessed the ability of P. leucotomos to mitigate UV radiation-associated skin cancers.23,24 These beneficial effects have been attributed to the phenolic components of P. leucotomos extract including chlorogenic acid, coumaric acid, vanillic acid, and especially the potent oxidation inhibitors caffeic and ferulic.25,26
The safety and beneficial effects of P. leucotomos extract have been evaluated in relatively few clinical studies. In two reports, the photoprotective effects were demonstrated in healthy volunteers following acute exposure to P. leucotomos using daily doses similar to those used in the present study. Orally administered P. leucotomos at a daily dose of 7.5mg/kg (about 480mg/kg in a 65kg person) for two days provided significant photoprotection to subsequent exposure to artificial UV radiation by reducing the presence of several markers of UV injury.27 In another study, oral P. leucotomos 240mg administered eight and two hours before exposure also provided significant protection to UV-A radiation by minimizing DNA damage.28
Two clinical studies assessed the beneficial effects of P. leucotomos in patients with polymorphic light eruption (PLE). In the first study, patients with PLE or solar urticaria were treated with oral P. leucotomos 480mg daily beginning 15 days prior to sun exposure.29 The majority of treated subjects achieved a significant reduction of skin reactions and subjective symptoms. In the second study, subjects were treated with daily doses of P. leucotomos ranging from 720 to 1200mg daily for two weeks based on body weight with subjects weighing >70kg receiving the highest dose.16 After two weeks, significantly more repeated exposures to UV-A and UV-B were required to elicit PLE reactions.
Previous studies assessed the photoprotective effects of P. leucotomos 480mg for 15 days29 and as much as 1200mg daily for two weeks.16 In the present study, subjects received 480mg of P. leucotomos for 60 days, representing a greater total exposure to the product. Similar to previous clinical studies,16,27-29 there were no reports of adverse events.
These results indicate that twice-daily use of oral P. leucotomos extract provides photoprotection from the harmful effects of UV radiation. P. leucotomos continues to maintain an excellent safety profile making it suitable for long-term use. Further studies using higher doses of P. leucotomos to achieve even greater photoprotection may be warranted.
CONCLUSION
P. leucotomos extract 240mg taken twice daily for 60 days was a safe and effective means for reducing the damaging effects of UV radiation. Based on the excellent safety profile of P. leucotomos, additional studies using higher doses may be warranted.
ACKNOWLEDGMENT
This study was sponsored by Ferndale Healthcare®, Ferndale, Michigan. The authors gratefully acknowledge the assistance of Dr. Carl Hornfeldt during the preparation of this manuscript.
Footnotes
DISCLOSURE:Financial support for this study was provided by Ferndale Healthcare®. Drs. Nestor and Berman are consultants for and receive research grants from Ferndale Healthcare. Dr. Swenson reports no relevant conflicts of interest.
REFERENCES
- 1.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 2.Mohan SV, Chang AL. Advanced basal cell carcinoma: epidemiology and therapeutic innovations. Curr Dermatol Rep. 2014;3:40–45. doi: 10.1007/s13671-014-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957–966. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Dacosta Byfield S, Chen D, Yim YM, et al. Age distribution of patients with advanced non-melanoma skin cancer in the United States. Arch Dermatol Res. 2013;305:845–850. doi: 10.1007/s00403-013-1357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nestor MS, Zarraga MB. The incidence of nonmelanoma skin cancers and actinic keratoses in South Florida. J ClinAesthet Dermatol. 2012;5:20–24. [PMC free article] [PubMed] [Google Scholar]
- 6.National Cancer Institute, National Institutes of Health. The Cost of Cancer, 2011. [August 2014]. http://www.cancer.gov/aboutnci/servingpeople/cancer-statistics/costofcancer
- 7.Warthan MM, Sewell DS, Marlow RA, et al. The economic impact of acute sunburn. Arch Dermatol. 2003;139:1003–1006. doi: 10.1001/archderm.139.8.1003. [DOI] [PubMed] [Google Scholar]
- 8.Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet. 2007;370:528–537. doi: 10.1016/S0140-6736(07)60638-2. [DOI] [PubMed] [Google Scholar]
- 9.Almutawa F, Buabbas H. Photoprotection: clothing and glass. Dermatol Clin. 2014;32:439–448. doi: 10.1016/j.det.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 10.DeHaven C, Hayden PJ, Armento A, et al. DNA photoprotection conveyed by sunscreen. J Cosmet Dermatol. 2014;13:99–102. doi: 10.1111/jocd.12087. [DOI] [PubMed] [Google Scholar]
- 11.Boggild AK, From L. Barriers to sun safety in a Canadian outpatient population. J Cutan Med Surg. 2003;7:292–299. doi: 10.1007/s10227-003-0126-9. [DOI] [PubMed] [Google Scholar]
- 12.Choudhry SZ, Bhatia N, Ceilley R, et al. Role of oral Polypodium leucotomos extract in dermatologic diseases: a review of the literature. J Drugs Dermatol. 2014;13:148–153. [PubMed] [Google Scholar]
- 13.Padilla HC, Laínez H, Pacheco JA. A new agent (hydrophilic fraction of Polypodium leucotomos) for management of psoriasis. Int J Dermatol. 1974;13:276–282. doi: 10.1111/j.1365-4362.1974.tb05081.x. [DOI] [PubMed] [Google Scholar]
- 14.Ramírez-Bosca A, Zapater P, Betlloch I, et al. Polypodium leucotomos extract in atopic dermatitis: a randomized, double-blind, placebo-controlled, multicenter trial. Adas Dermosifiliogr. 2012;103:599–607. doi: 10.1016/j.ad.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Middelkamp-Hup MA, Bos JD, Rius-Diaz F, et al. Treatment of vitiligo vulgaris with narrow-band UVB and oral Polypodium leucotomos extract: a randomized double-blind placebo-controlled study. J Eur Acad Dermatol Venereol. 2007;21:942–950. doi: 10.1111/j.1468-3083.2006.02132.x. [DOI] [PubMed] [Google Scholar]
- 16.Tanew A, Radakovic S, Gonzalez S, et al. Oral administration of a hydrophilic extract of Polypodium leucotomos for the prevention of polymorphic light eruption. J Am Acad Dermatol. 2012;66:58–62. doi: 10.1016/j.jaad.2010.09.773. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed AM, Lopez I, Perese F, et al. A randomized, double-blinded, placebo-controlled trial of oral Polypodium leucotomos extract as an adjunct to sunscreen in the treatment of melasma. JAMA Dermatol. 2013;149:981–983. doi: 10.1001/jamadermatol.2013.4294. [DOI] [PubMed] [Google Scholar]
- 18.El-Haj N, Goldstein N. Sun protection in a pill: the photoprotective properties of Polypodium leucotomos extract. Int J Dermatol. 2014 doi: 10.1111/ijd.12611. Jul 11. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Heckman CJ, Chandler R, Kloss JD, et al. Minimal erythema dose (MED) testing. J Vis Exp. 2013 doi: 10.3791/50175. e50175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.http://www.fda.gov/downloads/Drugs/Guidances/ucm073122.pdf ICH. Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance. April, 1996.
- 21.Alcaraz MV, Pathak MA, Rius F, et al. An extract of Polypodium leucotomos appears to minimize certain photoaging changes in a hairless albino mouse animal model. A pilot study. Photodermatol Photoimmunol Photomed. 1999;15:120–126. doi: 10.1111/j.1600-0781.1999.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 22.Zattra E, Coleman C, Arad S, et al. Polypodium leucotomos extract decreases UV-induced Cox-2 expression and inflammation, enhances DNA repair, and decreases mutagenesis in hairless mice. Am J Pathol. 2009;175:1952–1961. doi: 10.2353/ajpath.2009.090351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siscovick JR, Zapolanski T, Magro C, et al. Polypodium leucotomos inhibits ultraviolet B radiation-induced immuno-suppression. Photodermatol Photoimmunol Photomed. 2008;24:134–141. doi: 10.1111/j.1600-0781.2008.00352.x. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-Yanes E, Juarranz Á, Cuevas J, et al. Polypodium leucotomos decreases UV-induced epidermal cell proliferation and enhances p53 expression and plasma antioxidant capacity in hairless mice. Exp Dermatol. 2012;21:638–640. doi: 10.1111/j.1600-0625.2012.01544.x. [DOI] [PubMed] [Google Scholar]
- 25.Garcia F, Pivel JP, Guerrero A, et al. Phenolic components and antioxidant activity of Fernblock, an aqueous extract of the aerial parts of the fern. Polypodium leucotomos. Methods Find Exp Clin Pharmacol. 2006;28:157–160. doi: 10.1358/mf.2006.28.3.985227. [DOI] [PubMed] [Google Scholar]
- 26.Gombau L, García F, Lahoz A, et al. Polypodium leucotomos extract: antioxidant activity and disposition. Toxicol In Vitro. 2006;20:464–171. doi: 10.1016/j.tiv.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Middelkamp-Hup MA, Pathak MA, Parrado C, et al. Oral Polypodium leucotomos extract decreases ultraviolet-induced damage of human skin. J Am Acad Dermatol. 2004;51:910–918. doi: 10.1016/j.jaad.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 28.Koch H, Wittern KP, Bergemann J. In human keratinocytes the common deletion reflects donor variabilities rather than chronologic aging and can be induced by ultraviolet A irradiation. J Invest Dermatol. 2001;117:892–897. doi: 10.1046/j.0022-202x.2001.01513.x. [DOI] [PubMed] [Google Scholar]
- 29.Caccialanza M, Percivalle S, Piccinno R, et al. Photoprotective activity of oral Polypodium leucotomos extract in 25 patients with idiopathic photodermatoses. Photodermatol Photoimmunol Photomed. 2007;23:46–47. doi: 10.1111/j.1600-0781.2007.00274.x. [DOI] [PubMed] [Google Scholar]