Abstract
Background
Aloe is known for its topical use for treating wounds and burns. Many previous studies reported the healing effects of Aloe vera. However, there are few clinical studies on the effect of orally administered A. vera gel on the skin. Aloe sterols are a type of plant sterols that have the capability to regulate the metabolism of glucose and lipids. In a recent study, we confirmed that ingested Aloe sterols reached the peripheral tissues through the bloodstream. However, their influence on dermal fibroblasts has not been investigated.
Methods
First, we investigated the capability of Aloe sterols (cycloartenol and lophenol) to stimulate human dermal fibroblasts in vitro. Then, we investigated the effect of intake of Aloe vera gel powder (AVGP) containing 40 μg Aloe sterols on the skin conditions in Japanese women with dry skin in a randomized, double-blind, placebo-controlled trial.
Results
After cocultivation with Aloe sterols, the production of collagen and hyaluronic acid increased by approximately two-fold and 1.5-fold, and gene expression levels of these enzymes responsible for their synthesis were also observed in human dermal fibroblasts. An increase in arm skin hydration was observed at 8 weeks in the AVGP group, whereas a slight decrease in arm skin hydration was noted in the placebo group. However, there was no statistical difference between AVGP and placebo groups in skin moisture. In subgroup analysis, the change in the mean wrinkle depth was significantly lower in the AVGP group than in the control group. In addition, percent body fat after 8 weeks was significantly lower in the AVGP group. No AVGP intake-dependent harmful phenomenon was observed during the intake period.
Conclusion
The present study confirms that daily oral Aloe sterol-containing AVGP significantly reduced facial wrinkles in women aged ≥40 years, and Aloe sterols stimulate collagen and hyaluronic acid production by human dermal fibroblasts.
Keywords: aloe sterol, collagen, wrinkle
Introduction
Aloe vera (Aloe barbadensis Miller) is a plant species belonging to the family Liliaceae.1 A. vera gel is obtained from the mesophyll and has been used as a herbal medicine.2 The skin was raised and the recovery period was shortened by applying A. vera gel in diabetic rats.3 A clinical trial demonstrated the usefulness of A. vera for the prophylaxis of radiation-induced dermatitis.4
A. vera gel contains polysaccharides, amino acids, lipids, plant sterols, tannins, and enzymes.5,6 Acemannan is the major polysaccharide in A. vera gel and is known to induce immunological responsiveness.7,8 A previous report suggested that acemannan stimulated the synthesis of keratinocyte growth factor-1 and vascular endothelial growth factor by gingival fibroblasts in vitro.9 However, the effects of other ingredients in A. vera on dermal tissues have not been investigated.
Fibroblasts represent the main cellular population of the dermis. Their major function is to maintain extracellular matrix (ECM) homeostasis.10,11 The dermis tissues also contain collagen, elastin, and hyaluronic acid (HA) as other ingredients. Collagen forms the three-dimensional structure, and elastin maintains the elasticity of the skin. HA is responsible for moisture retention in the skin. Fibroblasts play an important role in generating collagen, HA, and elastin in the dermis.
Aloe sterols (lophenol [Lop], 24-methyl-lophenol, 24-ethyl-lophenol, cycloartanol [Cyc], and 24-methylene-cycloartanol) are plant sterols derived from A. vera gel and possess unique efficacy.12 Structurally, Aloe sterols fall into two groups of compounds, the Lop group and the Cyc group. The oral administration of Lop and Cyc reduced visceral fat accumulation and improved hyperglycemia and hyperlipidemia in animal models of diabetes and obesity.13 However, the effect of Aloe sterols on dermal cells and tissues is unknown. In addition, although A. vera is known for its topical effect in promoting wound healing, the active ingredient accounting for the effect on the skin by oral administration has been not considered in detail. Therefore, the aim of the present study was to investigate the effect of Aloe sterols on human dermal fibroblasts in vitro. Next, we examined the influence of the intake of Aloe vera gel powder (AVGP) containing Aloe sterols on the skin conditions in Japanese women with dry skin.
Materials and methods
Cell cultures
Primary adult human dermal fibroblasts (HDFa #2320) were obtained from DS Pharma Biomedical (Tokyo, Japan). The cell cultures were maintained in CSC complete serum-free medium system (Cell Systems Corporation, Kirkland, WA, USA). They were seeded at 5×104 cells/well in 12-well plates and cultured. After 48 hours, cells were incubated for another 48 hours in the absence or presence of Cyc and Lop. RNA was extracted from fibroblasts after 6-hour cultivation, and the culture supernatant was collected after 48 hours. The collagen and HA contents of the supernatant were determined using a soluble collagen assay (Accurate Chemical and Scientific Corporation, Westbury, NY, USA) and HA ELISA assay kit (R&D Systems Inc., Minneapolis, MN, USA).
Cell proliferation assay
Cell viability was assessed by culturing cells in a culture medium containing 10% WST-8 (Dojin Molecular Technologies, Kumanoto, Japan) for 0 hour to 6 hours at 37°C and was obtained by scanning with a microplate reader at 492 nm. This absorbance was expressed as a percentage of that in the control cells, after subtraction of background absorbance.
Real-time reverse transcription polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from human dermal fibroblasts with the RNeasy Mini Kit (Qiagen NV, Venlo, the Netherlands) according to the manufacturer’s protocol. Its quality was verified by lab-on-a-chip analysis (2100 Bioanalyzer; Agilent Technologies, Santa Clara, CA, USA). Total RNA was used for one-cycle RNA synthesis (Affymetrix, Santa Clara, CA, USA) with the PrimeScript 1st strand cDNA Synthesis Kit (Takara Bio, Otsu, Japan). Then, real-time PCR primer sets were purchased from the Takara Bio Perfect Real Time Support System. Real-time PCR was performed using Fast SYBR® Green Master Mix (product line of Thermo Fisher Scientific, Waltham, MA, USA) or TaqMan® Fast Universal PCR Master Mix (product line of Thermo Fisher Scientific) on a 7500 Fast Real-Time PCR System (product line of Thermo Fisher Scientific). Melting curve analysis showed a single melting curve in each RT-PCR assay using Fast SYBR Green (Figure S1). mRNA expression levels of all genes were normalized to those of β-actin (Cell Signaling Technology, Tokyo, Japan) in the same sample. The differences between the control and Aloe sterol-treated fibroblasts were expressed as relative increases, with the control value set to 100%. The results are expressed as mean ± standard deviation (SD) of three independent experiments.
Preparation and composition of the AVGP tablets
AVGP (Lot 20111027) was prepared by Morinaga Milk Industry Co Ltd (Tokyo, Japan) by drying the mesophyll of A. vera plants cultivated at Thai farms. The compositions of AVGP and the control tablets are presented in Table 1. In the placebo tablets, AVGP was replaced by inert dextrin. The amount of Aloe sterols per daily dose (five tablets; 0.5 g AVGP) was approximately 40 μg (Lop, 24-methyl-lophenol, 24-ethyl-lophenol, cycloartanol, and 24-methylene-cycloartanol were 11, 13, 5, 6, and 8 μg, respectively), as determined by liquid chromatography–tandem mass spectrometry analysis.
Table 1.
Ingredient composition of study tablets
| Placebo (mg/5 tablets) |
AVGP (mg/5 tablets) |
|
|---|---|---|
| AVGP | 0 | 500 |
| Dextrin | 500 | 0 |
| Citric acid | 100 | 100 |
| Maltose | 1,417.5 | 1,417.5 |
| Sour milk | 25 | 25 |
| Cellulose | 250 | 250 |
| Calcium phosphate | 12.5 | 12.5 |
| Flavor | 87.5 | 87.5 |
| Glycerin fatty acid | 50 | 50 |
| Sugar ester | 50 | 50 |
| Food color | 5 | 5 |
Abbreviation: AVGP, Aloe vera gel powder.
Human study design
This double-blinded, placebo-controlled trial was performed from September to December 2012 at the Ceravi Shinbashi Clinic (Tokyo, Japan) by the KSO Corporation Co, Ltd (Tokyo, Japan). The skin parameters were measured by the Skin Research Center of InfoWard Inc. (Tokyo, Japan). A total of 58 Japanese women with dry skin (age: 20–50 years) were randomly assigned to the placebo (n=28) or AVGP (n=28) group. We defined dry skin by an amount value of 60 AU or less for cheek moisture (measured by Corneometer CM 825 [Courage and Khazaka, Cologne, Germany]). Each participant was identified by a code randomly selected by a computer-generated permutation procedure. The codes were allocated sequentially in the order in which participants were enrolled. After all measurements were completed, the randomization code was disclosed to investigators. Study participants, investigators, study staff members, and laboratory technicians were blinded to the group assignment. All participants provided written informed consent to participate before beginning the study and were free to withdraw from the study at any time without obligation. The study protocol was examined and approved by the institutional review board of Ceravi Shinbashi Clinic, and was conducted according to the guidelines of the Declaration of Helsinki.
Dosage regimen
All subjects ingested five tablets per day (AVGP or placebo) for 8 weeks. Assessments of skin parameters and general physical examinations (ie, height, weight, and percent body fat) were conducted at weeks 0, 4, and 8 of the treatment period. Vital signs (heart rate and blood pressure) and laboratory analyses (hematology, blood chemistry, and urinalysis) were performed at each visit.
Measurements of skin parameters
The hydration properties of the skin and crow’s feet wrinkles were measured by noninvasive methods at weeks 0, 4, and 8 of the treatment period. Measurements were performed under standard conditions of room temperature (20°C–22°C) and humidity (45%–55%). Participants were given at least 20 minutes before the examination to adapt to the room conditions. Skin wrinkles were measured using a two-dimensional analysis system of replicas at oblique illumination. Participants were seated and instructed to keep their eyes closed. Skin replicas were obtained according to the guidelines of the Japan Cosmetic Industry Association. Silicone skin replicas were taken from the left crow’s feet region 5 mm from the lateral angle of the eye. Corneometer CM 825 was used to determine the skin hydration level of the inner side of one upper arm and one cheek. Percent body fat was measured by impedance using body weight composition (Omron, Tokyo, Japan).
Statistical analysis
Typically, dermatological studies assess skin changes over time in comparison with baseline parameters. During this 8-week study, descriptive statistics were calculated at each time point (weeks 0, 4, and 8). For all variables, differences between baseline and weeks 4 and 8, but not between weeks 4 and 8, were calculated. Analysis of covariance was performed for absolute skin hydration levels and the maximal width of the largest wrinkle and mean depth of wrinkles. The percentage of change in skin wrinkle parameters and percent body fat were calculated using the following equation: ([treatment period − baseline value]/baseline values) ×100. Analyses were performed using SPSS Statistics 17.0. A P-value <0.05 was considered significant. The changes in skin wrinkle parameters are expressed as mean ± standard error or the mean (SEM) and the other data as mean ± SD.
Results
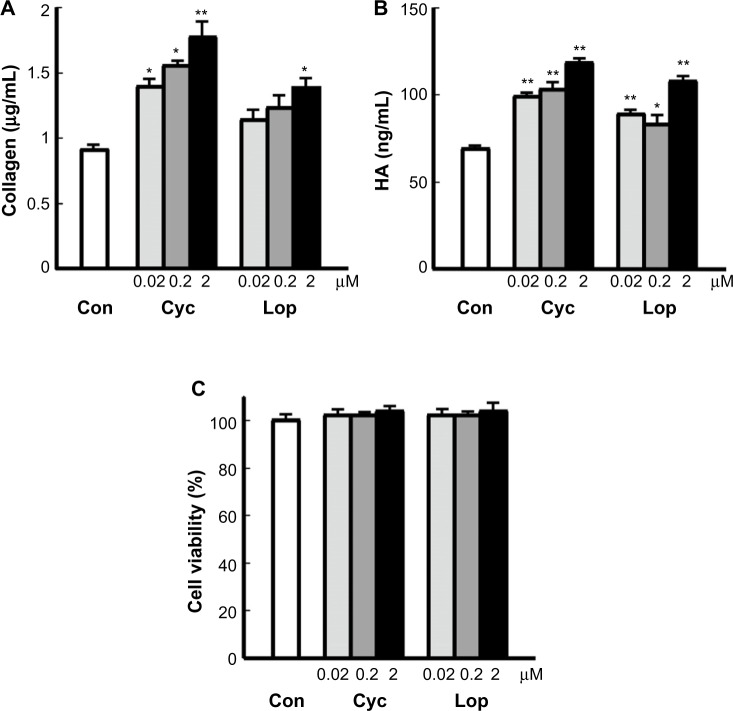
Fibroblasts produce major skin components, including collagen and HA. Therefore, cultured human fibroblasts were used to test the ability of Aloe sterols to stimulate collagen and HA production by human dermal fibroblasts. After a 48-hour coculture, Cyc and Lop increased collagen and HA production by cultured human fibroblasts in a concentration-dependent manner (Figure 1A and B). Treatment with 2 μM Aloe sterols increased collagen production in comparison with control (Cyc: 1.77±0.1, Lop: 1.4±0.06 vs control: 0.9±0.01 μg/mL) (Figure 1A). Similarly, treatment with 2 μM Cyc and Lop increased HA production in comparison with control (Cyc: 118.33±1.53, Lop: 107.69±2.28 vs control: 69.17±0.76 ng/mL) (Figure 1B). We did not observe a change in cell viability after the addition of Cyc and Lop (Figure 1C). These data suggest that the effects of sterols on human fibroblasts are specific for A. vera.
Figure 1.
Effects of Aloe sterols on (A) collagen and (B) HA production in human dermal fibroblasts. Cells were incubated for 48 hours in the absence or presence of 0.02–2 μM Cyc and Lop. The collagen and HA contents of the culture supernatant were determined with a soluble collagen assay and an HA ELISA assay kit. Cell viability was assessed by WST-8 (C).
Notes: The data are expressed as the mean ± SD (n=3). *P<0.05 and **P<0.001 vs control (0 μM).
Abbreviations: HA, hyaluronic acid; Con, control; Cyc, cycloartenol; Lop, lophenol; ELISA, enzyme-linked immunosorbant assay; SD, standard deviation.
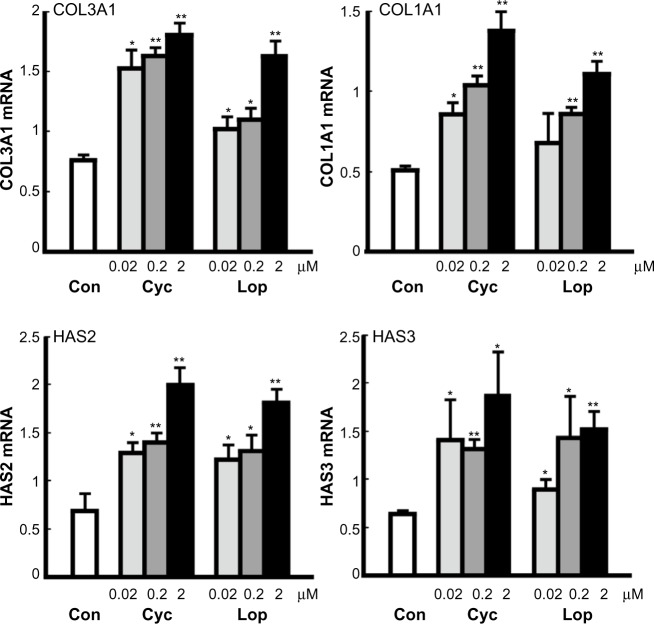
The mechanism of stimulation by Aloe sterols was determined by measuring the expression level of key enzymes responsible for collagen (COL1A1 and COL3A1) and HA (HAS2 and HAS3) production by human fibroblasts. A 6-hour incubation period with 0.02–2.0 μM Cyc and Lop was associated with a dose-dependent increase in the mRNA level of all four enzymes (Figure 2).
Figure 2.
Effects of Aloe sterols on the gene expression of enzymes responsible for the synthesis of collagen (COL1A1 and COL3A1) and HA (HAS2 and HAS3) in human dermal fibroblasts.
Notes: Cells were incubated for 6 hours in the absence or presence of 0.02–2 μM and 0.02–2 μM Cyc and lophenol (Lop), and changes in gene expression relative to control (0 μM) were determined by qRT-PCR. The data are expressed as mean ± SD (n=3). *P<0.05 and **P<0.001 vs control.
Abbreviations: Con, control; Cyc, cycloartenol; Lop, lophenol; qRT-PCR, real-time reverse transcription polymerase chain reaction; SD, standard deviation.
A double-blinded, placebo-controlled trial was conducted to determine whether the oral ingestion of AVGP containing Aloe sterols can significantly improve dry skin. A total of 56 women with dry skin were randomly assigned to the placebo group or the AVGP group. One participant in each group withdrew after the baseline tests. Therefore, we analyzed the data from 54 subjects. The two groups presented comparable baseline characteristics (Table 2). They had healthy women in their 40s, with a low incidence of smoking, and about half of them drank alcohol. There was no difference in the rate of tablet intake between placebo (96.1%±18.9%) and AVGP (99.6%±1.6%) groups. No significant treatment-related adverse events were reported during the 8-week study (data not shown).
Table 2.
Demographic and baseline characteristics
| Placebo (n=27) | AVGP (n=27) | P-value | |
|---|---|---|---|
| Number or mean ± SD | Number or mean ± SD | ||
| Agea (range), years | 37.8±6.9 | 39.7±5.0 | 0.254 |
| Heighta (cm) | 158.4±5.8 | 159.3±5.2 | 0.565 |
| Body weighta (kg) | 53.6±11.2 | 53.6±10.7 | 0.999 |
| BMIa (kg/m2) | 21.3±3.9 | 21.1±4.1 | 0.867 |
| Systolic blood pressurea (mmHg) | 108.5±11.7 | 106.3±11.0 | 0.467 |
| Diastolic blood pressurea (mmHg) | 67.3±9.1 | 66.9±8.4 | 0.868 |
| Pulse ratea (bpm) | 71.6±11.0 | 70.9±9.6 | 0.798 |
| Smokerb | 3 | 2 | 1 |
| Drinkerb | 17 | 12 | 0.285 |
Notes:
Data are expressed as mean ± SD;
data are expressed as numbers of persons.
Abbreviations: AVGP, Aloe vera gel powder; BMI, body mass index.
The facial skin hydration levels at 8 weeks were significantly increased in both groups compared with the baseline levels (Table 3). An increase in arm skin hydration was observed at 8 weeks in the AVGP group, whereas a slight decrease in arm skin hydration was observed in the placebo group. No significant differences were observed in skin hydration at both sites between the groups.
Table 3.
Skin hydration of face and arm in women who ingested AVGP or placebo tablets
| Item | Group | n | Baseline value (0 week)
|
4 weeks
|
8 weeks
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ANCOVA | Paired t | Mean ± SD | ANCOVA | Paired t | |||
| Skin hydration | AVGP | 27 | 29.0±10.8 | 30.3±11.6 | 0.055 | 36.8±12.4 | 0.846 | ||
| Face (AU) | Placebo | 27 | 30.4±8.7 | 34.4±7.6 | 37.5±10.6 | ||||
| Δ skin hydration | AVGP | 27 | 1.2±5.2 | 0.223 | 7.7±7.3 | 0.000* | |||
| Face | Placebo | 27 | 4.0±6.5 | 0.004* | 7.1±8.8 | 0.000* | |||
| Skin hydration | AVGP | 27 | 35.4±6.6 | 36.4±6.1 | 0.992 | 37.6±6.8 | 0.671 | ||
| Arm (AU) | Placebo | 27 | 38.7±6.4 | 39.1±9.0 | 38.4±8.7 | ||||
| Δ skin hydration | AVGP | 27 | 1.0±4.0 | 0.230 | 2.2±6.7 | 0.108 | |||
| Arm | Placebo | 27 | 0.4±6.7 | 0.741 | −0.3±8.4 | 0.878 | |||
Notes: Data are expressed as mean ± SD.
Different from week 0.
Abbreviations: AVGP, Aloe vera gel powder; SD, standard deviation; ANCOVA, analysis of covariance; AU, arbitrary unit.
The changes in the maximal width of the largest wrinkle and mean wrinkle depth from the baseline value after 4 weeks and 8 weeks of treatment are shown in Table 4. In the placebo group, the percent change in the maximal width of the largest wrinkle and mean wrinkle depth from baseline increased at 8 weeks (3.96%±4.23% and 0.73%±0.96%, respectively), whereas the values were lower at 8 weeks compared with those at baseline in the AVGP group (−2.18%±4.09% and −0.11%±1.0%). However, the differences in both groups were not significant. Next, we performed a stratified analysis of subjects aged ≥40 years (placebo group, n=12, and AVGP group, n=14). The percent change in the maximal width of the largest wrinkle at 8 weeks in the placebo group was 1.18%±5.85% vs −10.70%±4.65% in the AVGP group (Figure 3A). Furthermore, the percent change in the mean wrinkle depth was significantly lower in the AVGP group than in the placebo group at 8 weeks in this age group (P=0.035) (Figure 3B). In contrast, both parameters were not significantly affected by the placebo.
Table 4.
Analysis results of the wrinkle replicas
| Item of wrinkle | Group | n | Baseline value (0 week)
|
4 weeks
|
8 weeks
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ANCOVA | Paired t | Mean ± SD | ANCOVA | Paired t | |||
| Maximal largest width (μm) | AVGP | 27 | 481.0±135.9 | 510.3±174.4 | 0.608 | 469.2±179.3 | 0.817 | ||
| Placebo | 27 | 481.8±214.5 | 493.6±219.4 | 476.7±187.3 | |||||
| Δ maximal largest width | AVGP | 27 | 29.2±126.0 | 0.238 | −12.0±124.5 | 0.622 | |||
| Placebo | 27 | 11.8±123.6 | 0.624 | −5.2±99.7 | 0.789 | ||||
| Mean depth (μm) | AVGP | 27 | 160.2±23.1 | 159.9±24.1 | 0.873 | 159.9±24.1 | 0.564 | ||
| Placebo | 27 | 162.4±26.7 | 161.6±25.4 | 163.4±27.1 | |||||
| Δ mean depth | AVGP | 27 | −0.28±10.2 | 0.888 | −0.29±8.46 | 0.860 | |||
| Placebo | 27 | −0.83±7.7 | 0.579 | 0.98±9.32 | 0.546 | ||||
Note: Data are expressed as mean ± SD.
Abbreviations: SD, standard deviation; ANCOVA, analysis of covariance; AVGP, Aloe vera gel powder.
Figure 3.
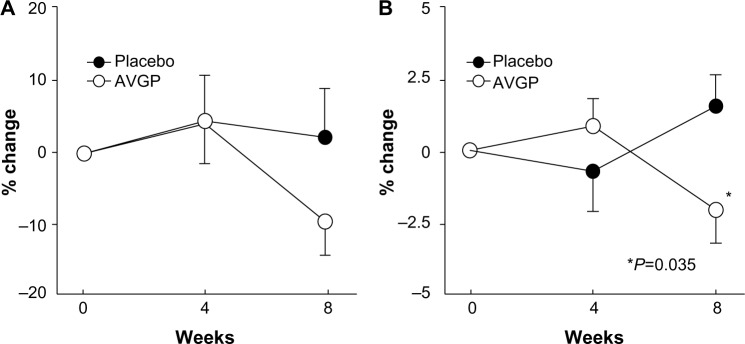
Effects of oral AVGP therapy on the facial wrinkles of participants with dry skin aged ≥40 years.
Notes: Participants ingested placebo or AVGP tablets containing Aloe sterols for 8 weeks, and measurements were taken after 0 week, 4 weeks, and 8 weeks of therapy. (A) Maximal width of the largest wrinkle (mean ± SEM). (B) Mean wrinkle depth (mean ± SD). •, placebo group, n=12; ○, AVGP group, n=14. *P=0.035 vs placebo.
Abbreviations: AVGP, Aloe vera gel powder; SEM, standard error of the mean; SD, standard deviation.
Figure 4 presents typical changes in a replica image in four participants (two from each group) before and after 8 weeks of treatment with the AVGP supplement or placebo.
Figure 4.
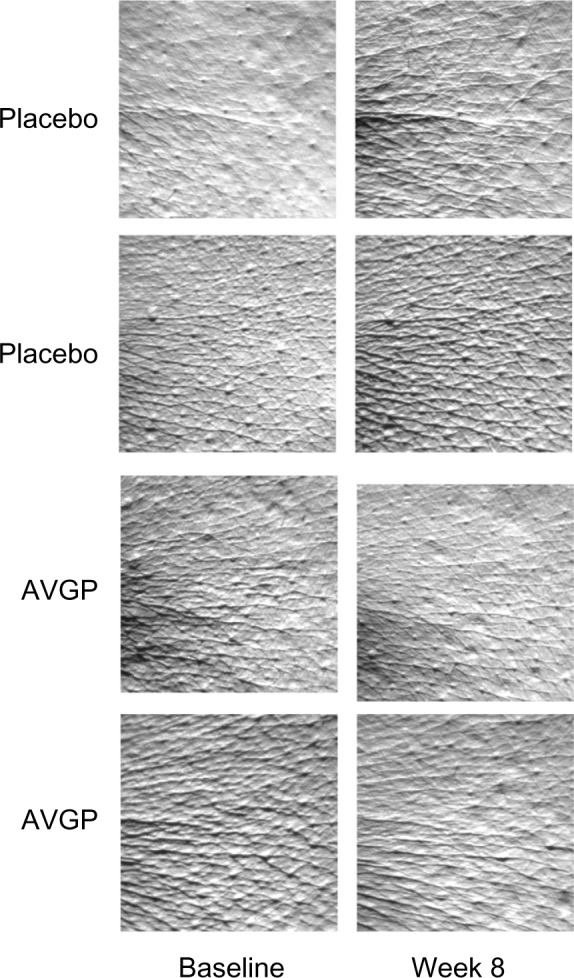
Effects of oral AVGP therapy facial skin hydration.
Notes: Participants ingested placebo or AVGP tablets containing Aloe sterols for 8 weeks, and measurements were taken after 0 week and 8 weeks. Silicone skin replicas of the left crow’s feet were analyzed with a corneometer to determine the skin hydration level. The images present typical replicas for two subjects of each group.
Abbreviation: AVGP, Aloe vera gel powder.
The oral AVGP and placebo regimens did not affect body weight (Table 5). In contrast, the mean percent body fat in the AVGP group was significantly lower compared with the placebo group after 8 weeks (P=0.036). When the data are expressed relative to baseline values for each subject, AVGP did not affect percent body fat, whereas the placebo induced a significant increase in percent body fat (P=0.037).
Table 5.
Values and changes of body weight and body fat percentage 4 weeks and 8 weeks after treatment
| Item | Group | n | Baseline value (0 week)
|
4 weeks
|
8 weeks
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ANCOVA | Paired t | Mean ± SD | ANCOVA | Paired t | |||
| Body weight (kg) | AVGP | 27 | 53.8±10.7 | 53.9±10.8 | 0.511 | 53.7±10.9 | 0.474 | ||
| Placebo | 27 | 53.7±11.9 | 54.0±11.5 | 53.5±10.7 | |||||
| Δ body weight | AVGP | 27 | 0.1±1.1 | 0.539 | −0.2±1.2 | 0.523 | |||
| Placebo | 27 | 0.3±1.2 | 0.153 | 0.2±2.1 | 0.640 | ||||
| Body fat percentage (%) | AVGP | 27 | 27.7±6.1 | 27.3±6.0 | 0.051 | 27.3±6.3 | 0.036a | ||
| Placebo | 27 | 27.9±4.9 | 28.1±4.9 | 28.3±4.6 | |||||
| Δ body fat percentage | AVGP | 27 | −0.4±1.2 | 0.141 | −0.4±1.7 | 0.279 | |||
| Placebo | 27 | 0.2±1.0 | 0.207 | 0.5±1.1 | 0.037b | ||||
Notes: Data are expressed as mean ± SD.
P=0.036 vs placebo;
P=0.037 vs week 0.
Abbreviations: SD, standard deviation; ANCOVA, analysis of covariance; AVGP, Aloe vera gel powder.
Discussion
Skin aging is caused by repeated exposure to UV radiation (photoaging) or by naturally occurring biological processes (intrinsic aging). The skin collagen content declines significantly with age in people aged ≥40 years and after menopause.14 Aging causes a reduction in skin collagen synthesis by at least two mechanisms: cellular fibroblast aging and a lower level of mechanical stimulation.15 In the dermis, heterotypic collagen fibrils contain mainly type I and type III collagens. The major function of type III collagen is associated with the fibrogenesis of type I collagen.16 Type III collagen is abundant at sites of healing and repair in the skin and other tissues. COL1A1 produces a component of type I collagen, whereas COL3A1 produces a component of type III collagen. In this study, we confirmed that Aloe sterols promote the production of collagen and increase the gene expression level of type I and type III collagen synthesis in human dermal fibroblasts. To observe the effects of Cyc and Lop to collagen and HA synthesizing enzyme at the protein level, Western blot analysis was performed. As shown in Figure S2, COL3A1 expression in human dermal fibroblasts was upregulated by Aloe sterols at the protein level. However, further examination is necessary to elucidate effect of Aloe sterols to protein level of COL1A1. This result suggests that Aloe sterols affect the ECM structure of the dermis layer.
Dermal fibroblasts also synthesize HA, which plays an important role in skin hydration. Age-related declines in total HA production have been documented in human skin.1 The synthesis of HA is accomplished by three isoforms of hyaluronan synthase: HAS1, HAS2, and HAS3. Almost all known regulatory systems induce HA synthesis, including growth factors and cytokines. In the present study, Aloe sterols stimulated HA production and gene expression of HA synthesis in human dermal fibroblasts. The protein levels of HAS2 and HAS3 of dermal fibroblasts were also increased by Aloe sterols (Figure S2). These data suggest that Aloe sterols have the capability to improve skin moisture by increasing the HA content in the dermal ECM.
Adiponectin is a novel adipocyte-specific protein with important insulin-sensitizing, anti-atherogenic,17 and anti-inflammatory18 properties. A recent study indicated that skin adiponectin was found in plasma, subcutaneous adipose tissue, and dermal sebaceous tissue.19 Adiponectin promoted HA synthesis and increased HAS2 mRNA level through an AMPK/PPARα-dependent signaling pathway in human dermal fibroblasts.20 We previously showed that the oral administration of Aloe sterols caused an increase in serum adiponectin level.13 In Apc-deficient Min/+ mice, the ingestion of A. vera gel supercritical CO2 extract induced an increase in the serum level of high-molecular-weight adiponectin.21 We recently initiated a new study to determine the effect of Aloe sterols on adiponectin in the epidermis, dermis, and fat tissues. In our latest study, using hairless mice, which we irradiated with UVB light, we confirm that adiponectin level in serum and gene expression level of HAS2 of mouse skin were increased by AVGP intake (Saitou M, unpublished data, 2015).
We present results from the first randomized, double-blind, placebo-controlled trial on the effects of oral A. vera gel supplementation for dry skin in Japanese women. The placebo and AVGP groups responded to treatment by an increase in facial moisture. This study was conducted during the peak season for dry skin: from September to December. The continual use of facial cosmetics during the testing period could explain these findings. We recognized that cosmetics affect mainly the epidermis. On the other hand, oral ingestion of AVGP was expected to act on the dermis through the blood. Therefore, we did not stop the use of cosmetics on the subject. Therefore, in order to examine the effect of oral ingestion of AVGP to face moisture content, additional study of subjects that do not use the cosmetics is required. On the other hand, arm skin moisture decreased in the placebo group and increased in the AVGP group, but these responses were not statistically significant. Thus, the possible moisturizing benefits of AVGP would not supplement the regular use of facial and body creams. On the other hand, we demonstrate that daily oral AVGP (40 μg of Aloe sterols) significantly reduced facial wrinkles in women aged ≥40 years in terms of the mean wrinkle depth. The depth of wrinkles rapidly increased in women aged ≥40 years.22 Thus, the daily oral intake of AVGP could reduce skin aging by targeting wrinkles rather than by acting as a systemic moisturizing agent.
An animal study showed that AVGP ingestion significantly reduced subcutaneous and visceral fat weight, and percent body fat in diet-induced obese rats.23 The present study showed, for the first time, that oral AVGP caused a significant reduction in percent body fat in human subjects. However, additional testing using suitable candidates (overweight person, etc) for examining the effect of AVGP on body fat is required. We recently demonstrated the contribution of Aloe sterols in the gel powder in an animal model of type 2 diabetes.24 The Aloe sterols Cyc and Lop improved fatty acid metabolism in the liver by upregulation of many genes targeted by PPARs. To confirm the anti-obesity effect of AVGP containing Aloe sterols, it is necessary to conduct clinical tests on overweight or obese subjects.
A. vera whole leaf may contain anthraquinones, which have been shown to generate reactive oxygen species in the presence of UVA light. Exposure to UVA light can also generate reactive oxygen species and is associated with photo-damaged and photo-aged skin in humans.25 A. vera gel, which is taken from the leaf, does not include anthraquinones. Furthermore, we confirmed that the AVGP tablet did not include anthraquinones before carrying out this examination. In this study, there were less signs indicating dermal irritancy due to AVGP intake, as per the skin diagnosis performed by a dermatologist on each subject during the testing period. We previously tested the safety of AVGP in vitro and in vivo. In a 90-day toxicity test in which rats were continuously administered AVGP at 1,328 mg/kg, euthanized, and subjected to pathological examinations, no abnormalities attributable to the AVGP were found. AVGP was non-mutagenic in either the Ames test or in a chromosomal aberration test at concentrations of up to 5,000 μg/plate, or in an in vivo bone marrow micronucleus test at up to 2,000 mg/kg/d. Therefore, AVGP can be safely used as a functional food material.
In conclusion, the present study showed that Aloe sterols stimulated collagen and HA production by human dermal fibroblasts, and that AVGP containing Aloe sterols reduced facial wrinkles in women. More recently, we confirmed that ingested Aloe sterols reached the peripheral tissues through the bloodstream (Ikeda I, unpublished data, 2010). Together, these data suggest that AVGP alters skin metabolism in vivo. We are currently testing the protective efficacy of Aloe sterols against skin damage by UV radiation. Future studies are also required to elucidate the protective mechanism of Aloe sterols in the skin.
Supplementary materials
Melting curve analysis of qRT-PCR assay.
Effect by Cyc and Lop in COL3A1, HAS2, and HAS3 expression at the protein level (Western blot analysis).
Notes: Cells were incubated for 6 hours in the absence or presence of 2 μM Cyc and Lop. Western blot analysis was performed using monoclonal antibodies: COL3A1 (abcam®), HAS2 (abcam®) and HAS3 (abcam®), and β-actin abcam® (Cambridge, England, United Kingdom).
Abbreviations: Con, control; Cyc, cycloartenol; Lop, lophenol.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Grindlay D, Reynolds T. The Aloe vera phenomenon: a review of the properties and modern uses of the leaf parenchyma gel. J Ethnopharmacol. 1986;16:117–151. doi: 10.1016/0378-8741(86)90085-1. [DOI] [PubMed] [Google Scholar]
- 2.Little JW. Complementary and alternative medicine: impact on dentistry. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:137–145. doi: 10.1016/j.tripleo.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol. 1998;59:195–201. doi: 10.1016/s0378-8741(97)00124-4. [DOI] [PubMed] [Google Scholar]
- 4.Heggie S, Bryant GP, Tripcony L, et al. A Phase III study on the efficacy of topical Aloe vera gel on irradiated breast tissue. Cancer Nurs. 2002;25:442–451. doi: 10.1097/00002820-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Shelton RM. Aloe vera. Its chemical and therapeutic properties. Int J Dermatol. 1991;30:679–683. doi: 10.1111/j.1365-4362.1991.tb02607.x. [DOI] [PubMed] [Google Scholar]
- 6.Vogler BK, Ernst E. Aloe vera: a systematic review of its clinical effectiveness. Br J Gen Pract. 1999;49:823–828. [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Tizard IR. Activation of a mouse macrophage cell line by acemannan: the major carbohydrate fraction from Aloe vera gel. Immunopharmacology. 1996;35:119–128. doi: 10.1016/s0162-3109(96)00135-x. [DOI] [PubMed] [Google Scholar]
- 8.Djeraba A, Quere P. In vivo macrophage activation in chickens with Acemannan, a complex carbohydrate extracted from Aloe vera. Int J Immunopharmacol. 2000;22:365–372. doi: 10.1016/s0192-0561(99)00091-0. [DOI] [PubMed] [Google Scholar]
- 9.Jettanacheawchankit S, Sasithanasate S, Sangvanich P, Banlunara W, Thunyakitpisal P. Acemannan stimulates gingival fibroblast proliferation; expressions of keratinocyte growth factor-1, vascular endothelial growth factor, and type I collagen; and wound healing. J Pharmacol Sci. 2009;109:525–531. doi: 10.1254/jphs.08204fp. [DOI] [PubMed] [Google Scholar]
- 10.Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci. 2004;117:667–675. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- 11.Sorrell JM, Caplan AI. Fibroblast – a diverse population at the center of it all. Int Rev Cell Mol Biol. 2009;276:161–214. doi: 10.1016/S1937-6448(09)76004-6. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Misawa E, Ito Y, et al. Identification of five phytosterols from Aloe vera gel as anti-diabetic compounds. Biol Pharm Bull. 2006;29:1418–1422. doi: 10.1248/bpb.29.1418. [DOI] [PubMed] [Google Scholar]
- 13.Misawa E, Tanaka M, Nomaguchi K, et al. Administration of phytosterols isolated from Aloe vera gel reduce visceral fat mass and improve hyperglycemia in Zucker diabetic fatty (ZDF) rats. Obes Res Clin Pract. 2008;2:239–245. doi: 10.1016/j.orcp.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Castelo-Branco C, Pons F, Gratacós E, Fortuny A, Vanrell JA, González-Merlo J. Relationship between skin collagen and bone changes during aging. Maturitas. 1994;18:199–206. doi: 10.1016/0378-5122(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 15.Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin. Am J Pathol. 2006;168:1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Wu H, Byrne M, Krane S, Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A. 1997;94:1852–1856. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokota T, Oritani K, Takahashi I, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 18.Ouchi N, Kihara S, Arita Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 19.Akazawa Y, Sayo T, Sugiyama Y, et al. Adiponectin resides in mouse skin and upregulates hyaluronan synthesis in dermal fibroblasts. Connect Tissue Res. 2011;52:322–328. doi: 10.3109/03008207.2010.528566. [DOI] [PubMed] [Google Scholar]
- 20.Yamane T, Kobayashi-Hattori K, Oishi Y. Adiponectin promotes hyaluronan synthesis along with increases in hyaluronan synthase 2 transcripts through an AMP-activated protein kinase/peroxisome proliferator-activated receptor-α-dependent pathway in human dermal fibroblasts. Biochem Biophys Res Commun. 2011;18(415):235–238. doi: 10.1016/j.bbrc.2011.09.151. [DOI] [PubMed] [Google Scholar]
- 21.Chihara T, Shimpo K, Beppu H, et al. Reduction of intestinal polyp formation in min mice fed a high-fat diet with Aloe vera gel extract. Asian Pac J Cancer Prev. 2013;14:4435–4440. doi: 10.7314/apjcp.2013.14.7.4435. [DOI] [PubMed] [Google Scholar]
- 22.Akazaki S, Nakagawa H, Kazama H, et al. Age-related changes in skin wrinkles assessed by a novel three-dimensional morphometric analysis. Br J Dermatol. 2002;147:689–695. doi: 10.1046/j.1365-2133.2002.04874.x. [DOI] [PubMed] [Google Scholar]
- 23.Misawa E, Tanaka M, Nabeshima K, et al. Administration of dried Aloe vera gel powder reduced body fat mass in diet-induced obesity (DIO) rats. J Nutr Sci Vitaminol. 2012;58:195–201. doi: 10.3177/jnsv.58.195. [DOI] [PubMed] [Google Scholar]
- 24.Nomaguchi K, Tanaka M, Misawa E, et al. Aloe vera phytosterols act as ligands for PPAR and improve the expression levels of PPAR target genes in the livers of mice with diet-induced obesity. Obes Res Clin Pract. 2011;5:e169–e266. doi: 10.1016/j.orcp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Xia Q, Yin JJ, Fu PP, Boudreau MD. Photo-irradiation of Aloe vera by UVA – formation of free radicals, singlet oxygen, superoxide, and induction of lipid peroxidation. Toxicol Lett. 2007;168(2):165–175. doi: 10.1016/j.toxlet.2006.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Melting curve analysis of qRT-PCR assay.
Effect by Cyc and Lop in COL3A1, HAS2, and HAS3 expression at the protein level (Western blot analysis).
Notes: Cells were incubated for 6 hours in the absence or presence of 2 μM Cyc and Lop. Western blot analysis was performed using monoclonal antibodies: COL3A1 (abcam®), HAS2 (abcam®) and HAS3 (abcam®), and β-actin abcam® (Cambridge, England, United Kingdom).
Abbreviations: Con, control; Cyc, cycloartenol; Lop, lophenol.