Abstract
Safety and efficacy data on many medicines used in children are surprisingly scarce. As a result children are sometimes given ineffective medicines or medicines with unknown harmful side effects. Better and more relevant clinical trials in children are needed to increase our knowledge of the effects of medicines and to prevent the delayed or non-use of beneficial therapies. Clinical trials provide reliable evidence of treatment effects by rigorous controlled testing of interventions on human subjects. Paediatric trials are more challenging to conduct than trials in adults because of the paucity of funding, uniqueness of children and particular ethical concerns. Although current regulations and initiatives are improving the scope, quantity and quality of trials in children, there are still deficiencies that need to be addressed to accelerate radically equitable access to evidence-based therapies in children.
Keywords: clinical trials, ethics, medicines in children, paediatric drug therapy
Imperative to conduct trials in children
Since the acknowledgement of children as ‘therapeutic or pharmaceutical orphans’ in the 1960s 1–3 there has been a worldwide recognition of the need to conduct trials of medicines used in children as a mechanism to improve the health of children 4–7. Significant advances in child health have resulted from the conduct of paediatric trials. Well-known trials of polio vaccines and the subsequent rapid translation into practice were instrumental in the successful and almost complete eradication of polio 8,9. Recent advances in multicentre cancer trials in children have increased childhood cancer 5 year survival from 28% in the late 1960s to 79% by 2005 10–13. Regrettably, these stories of remarkable benefits cannot be extended to many other childhood conditions 14 because of the dearth of relevant trials.
Prescribing in children is often based on extrapolation from trials in adults due to the lack of paediatric data. Children are not ‘little adults,’ but are a heterogeneous group, ranging from preterm neonates to post-pubertal adolescents 15–17. Their disease presentation may have a different natural history from adults and they may also suffer from diseases which do not occur in adults 18–20. Children have complex physiological, developmental, psychological and pharmacological characteristics that vary from adults and these features are also different across the newborn to adolescent age range 21. They may metabolize certain medicines differently from adults resulting in sub-optimal therapy, unexpected responses, adverse drug reactions and toxicity which may affect development and future reproductive capacity 22–24.
Relying on adult safety and efficacy data when prescribing in children can have unpredictable and tragic effects 4,17,25. For example, faster metabolism of cyclosporin in children could lead to subtherapeutic concentrations because of under-dosing 26. Prescribing tetracycline in children during the period of mineralization of developing teeth results in severe enamel dysplasia 27. Children may experience paradoxical hyperactivity with phenobarbital which is not experienced in adults due to differences in pharmacodynamics 26. In neonates, because of the immaturity of the liver, reduced medicine clearance resulted in the Gray baby syndrome with chloramphenicol 28,29, hepatotoxicity, hypotension, renal failure and death with the solvent propylene glycol which was in E-Ferol® 30 and ‘gasping syndrome’ from metabolic acidosis with formulations containing benzyl alcohol 15. Thalidomide was used for morning sickness in pregnant women with devastating effects on foetal development, resulting in thousands of children being born with phocomelia 31.
More trials are needed, especially in areas of high clinical need. A study in 2007 showed that the number of randomized controlled trials in adults published in five high impact general medical journals has nearly doubled over 20 years, while the number of paediatric trials has not increased 32. Despite about 27% of the world's population being children 33, paediatric trials constitute only 16.7% of the total number of trials registered on the World Health Organization (WHO) portal 34. In a study of trials registered on clinicaltrials.gov on selected medical conditions, only 12% were paediatric trials although children contributed to almost 60% of the total disease burden 35. The WHO Global Burden of Disease study in 2002 estimated 11.4 million deaths in children under 10 years of age with 91% of these in children less than 5 years 36. Fewer trials are conducted in younger children where they are most needed 37. Only 7% (42) of all paediatric trials published in 2007 were in neonates 38,39. There are a disproportionately small number of trials in children in low income countries 40,41. Over 89% of children live in low and lower to middle income countries, but only about a quarter of the 604 trials of medicinal products in children published in 2007 were conducted in these countries 38.
The pharmaceutical industry funds a greater proportion of trials in adults (65%) than children 35,42. Industry may be reluctant to conduct trials in children due to decreased commercial interest, increased cost and greater risk of liability 20,43–45. The more restrictive regulatory oversight for paediatric trials 23,46 which includes the recommendation for provision of medicines post-trial to participants where there is proven therapeutic benefit 47,48, may further discourage industry from conducting trials in children. Funding for paediatric trials therefore often relies on non-profit organizations, which have limited funding. The government favours trials in adults because of political and economic pressures 17,35.
Study design and conduct of paediatric trials
There are special considerations when designing any of the four phases of clinical trials in children 49,50. Phase I trials, which test the safety and pharmacokinetics of a new intervention for the first time, are discouraged in children due to the unknown effects of the intervention 51,52. However, phase I trials are more acceptable in children with severe or life-threatening conditions where there is no proven treatment or when standard therapies have failed 51,52. Phase I trials can only occur when there are appropriate pre-clinical safety and efficacy data available from animal studies, modelling or other predictive studies 53. Phase II trials, which study the safety and efficacy of the intervention 51, are sometimes conducted in children. Generally medicinal product testing in children is deferred until the trials reach phase III which evaluates efficacy, acceptability and adverse effects 54. Although the intent of the deferral is to protect children from exposure to unnecessary harm, it also means a delay to the access of children to potentially useful medications 54. Phase III trials (randomized controlled trials) compare the investigational intervention with standard therapy, another effective therapy or placebo to estimate unbiased treatment effects 54–56. Control groups and placebos are used when there are no established alternative therapies 54,57–59. Phase IV post-marketing trials are infrequently conducted in children. However, the Food and Drug Administration (FDA) Paediatric Research Equity Act (PREA) requires paediatric trials of marketed medicines 60.
Pharmacokinetic studies
Pharmacokinetic studies (which generally occur in phase I) are important in the different paediatric age ranges. Challenges with pharmacokinetic studies include the lack of expertise in paediatric population pharmacokinetic or pharmacodynamic analysis, problems associated with the number, volume and timing of sampling and the absence of sensitive micro-analytical techniques to determine accurately the drug concentration of very small volume specimens 61. If the disease progression is similar between children and adults, an initial dose extrapolated from adult data may be adequate, followed by pharmacokinetic studies to determine the most appropriate paediatric dose 62. Another approach is to conduct single dose paediatric studies in the different age groups if medicines are known to have linear pharmacokinetics in adults 63. In paediatric pharmacokinetic studies, innovative trial design techniques for reducing the number and volume of samples required are sometimes used. These techniques use sparse and scavenged pharmacokinetic samples with population pharmacokinetic methods using non-linear mixed effects 61,63. Opportunistic trials, which collect pharmacokinetic samples from children receiving treatment as part of routine clinical care, is another low risk and high yield design that is efficient and acceptable to parents and ethics committees 61. Pharmacogenomics methods are also being developed for investigating drug disposition, efficacy and safety 46.
Trial registration and publication
Public registration of clinical trials is especially important to protect participants from unnecessary, duplicative studies, to improve transparency and overcome publication and selective outcome reporting bias 37,64–66. Prospective registration of trials is strongly advocated internationally by regulatory authorities, ethics committees and journals as a condition of publication 67. However, a review of published paediatric randomized controlled trials showed that some were poorly reported 42 and had incomplete reporting of adverse drug reactions 68. Disappointingly a considerable number of paediatric trials that were conducted as a result of the paediatric exclusivity legislations were not published 69. For paediatric trial results to be translated into clinical practice, prompt and accessible publication of unbiased results including negative results helps to improve public trust and confidence in paediatric research 70–72.
Small trials sizes for paediatrics
The recruitment of children in trials is more difficult than for adults 45,47 because of the lower burden of disease in children 46,73,74. Most paediatric trials have small sample sizes 35,38,41 with only 38% of 736 paediatric trials published from 1996 to 2002 having a sample of more than 100 40. The small sample size, heterogeneity of response of children to treatments and rarity of certain important outcomes contribute to the problem of inadequate power 75. Underpowered trials may provide inconclusive results and fail to detect modest but clinically relevant outcomes including adverse effects 17,21,55,71. This may waste resources and the efforts of children's participation in trials 76. To address the small sample sizes in children, there have been major achievements in developing statistical methods and collaborative specialist multi-national groups and paediatric clinical trials networks that pool their data and resources 77–82.
Attitudes to participation in trials
There has been a general reluctance about involving children in trials particularly by parents and doctors because of fears of harming children by exposing them to uncertain treatment effects 45,72,83. In particular, parents were anxious about their child being treated as a ‘guinea pig’ and were concerned that investigators may have conflicting interest and do not have their child's health as a priority 45,84. However, in a neonatal study 75% of parents believed that their doctor would not approach them to do research if it might place babies in real danger and 50% reported that they trusted their doctor and would agree to participate in a trial if suggested by their doctor 85. Practitioners interviewed in a study in 2011 were apprehensive and averse to recruiting children for trials due to the trial burdens which included the overwhelming amount of information they had to provide to the families 72. Assisting practitioners to understand families' perceptions of trials and providing ‘moral’ support may improve recruitment of children 72. In contrast, parents who reported a positive recruitment experience, viewed participation in a trial as an ‘exciting’ opportunity, felt a sense of comfort and safety, acknowledged the value of research and desired to be informed about a trial if their child was eligible 72. Other positive aspects parents experience include the altruistic desire to help future children, the opportunity to access new therapies, increased access to health care professionals and medical information, better medical care for their child, meeting other parents in a similar situation and feeling a sense of hope when no other effective therapies are available 72–74,86,87. The high level of threat and need for hope may account for the generally high rates of recruitment to neonatology and childhood cancer trials 83. There are also perceived benefits for children participating in trials. Children often enjoy being part of a trial, interacting with other participants, being able to contribute to helping other children and are empowered about their treatment 84.
There has been some work on developing strategies to aid parents in the decision making process for trial participation 88. Some strategies include improving the readability of the consent 89. Masty et al. conceptualized a ‘goodness-of-fit’ approach to informed consent for paediatric trials that encouraged investigators to create consent procedures that took into account the research context, the child's cognitive and emotional maturity and the family system 90. The James Lind Library has been created to help the public understand that trials are fair tests of treatments in health care 91.
The use of placebo is poorly understood by parents who do not understand the rationale for using placebo to determine whether the intervention is effective or necessary. Parents fear assignment of their child to the placebo arm or to the treatment arm which is later proven to be less effective 45,92,93. This concern may be compensated for by providing the proven effective treatment to all participants at the conclusion of the study. Alternative randomization methods should also be considered which may be more acceptable to parents. In a conventionally randomized trial, parent's views were evenly divided on accepting the controversial Zelen randomization where randomization occurs prior to discussion with the family and only if the child is allocated the experimental treatment arm is consent sought 94. In a survey investigating different types of consent to hypothetical neonatal resuscitation trials, parents wished to be responsible for making the informed decisions and were more comfortable with prospective consent than deferrals, waivers or ‘opt-out’ options 95.
The burdens of trial participation for children are different from adults. For example children's aversion to needles makes obtaining blood samples challenging. To address this burden and protect children from unnecessary testing, the volume of blood sampling generally allowed in paediatric trials is less than 3% of the estimated circulating blood volume over a 2 to 8 week period 20,96,97. Alternative appropriate sampling techniques, for example finger or heel pricks or salivary samples, may be preferred as they minimize discomfort for children 15,20,96,97. There is a strong advocacy that paediatric trials require the same dedicated time and attention to educate families and participants ensuring an appropriate child friendly environment and adapting treatments to their special needs that is generally accepted as routine clinical care 98,99. The use of pragmatic trials where there are no additional burdens of testing and monitoring beyond the requirements of routine clinical care 37–40 may also alleviate some of these concerns, including the use of placebos 55. Participation may also be improved by having trained investigators who understand the complexities of conducting trials in children, appropriate facilities that meet the needs of children and a designated trials co-ordinator to facilitate recruitment and trial conduct 45,93,100. Increasingly the importance of engaging children and families in the recruitment, consent and design of trials has also been recognized 99.
Appropriate medicine formulations in trials
The development of appropriate formulations for children has been slow. This may be due to historical disasters such as the formulation of sulphanilamide as an elixir which used diethylene glycol without animal toxicity testing resulting in poisoning and deaths in the 1930s 101,102. Consequently, risk averse companies may be reluctant to develop paediatric formulations due to the potential harmful effects of excipients used in the products and the low market share of these products. To encourage the pharmaceutical industry to develop paediatric appropriate formulations, the 2003 FDA Best Pharmaceuticals for Children Act (BPCA) 103 and European Union Paediatric regulations were endorsed.
Administration of medicines in children is complex and the trial design needs to consider the child's developmental abilities and the medicine's acceptability and tolerability as this will impact on compliance. Children are fearful of injectable medicines and if an alternative route of administration is not available, the provision of local anaesthetic gels or patches will decrease discomfort. The requirements of child friendly formulations may differ in resource limited settings where there may be a lack of refrigeration facilities which may impact on the stability and efficacy of the medicine 104. When there is a lack of paediatric appropriate formulations, the use of adult dose forms such as tablets and capsules can be problematic for younger children who cannot swallow tablets as crushing tablets or opening capsules and dispersing in liquids may compromise the palatability and bioavailability of the medicine and affect the trial results 96. The inclusion of extemporaneous preparation guidelines in paediatric trials which use solid oral dosage forms, will improve the accuracy and reproducibility of the preparation 73. Skin patches or flexible oral solid dosage forms such as dispersible tablets or powders, melts (wafers, sublingual) and sprinkles may be more suitable across various settings 102.
Outcome measures
As children grow and mature through the developmental stages, it may not be suitable to use the same outcome measures when comparing children of different ages and stages 16. Some outcomes such as pain, nausea, dizziness, level of sedation or visual and auditory responses 42 are difficult to measure and report in young children. When designing how to measure these outcomes, researchers need to consider using age-appropriate tools such as the face pain scale 57,105. Because of differences in body composition in different age groups, pharmacokinetic studies have sometimes resulted in incomplete and incorrect conclusions 96. Trials that include different paediatric ages may need appropriate dose adjustments by weight or body surface area 96,106. Researchers are increasingly recognizing the importance of qualitative outcome measures that are relevant to the child and family, including the impact of the illness and treatment on the quality of life 16. When measuring quality of life outcomes consideration needs to be given about whether to use proxy response by parents or reporting by the child or both 107, as these may differ. Involving parents and children in the selection of outcome measures that are important to them is recommended 16,21.
Ethics of paediatric clinical trials
There is a dilemma in finding a balance between the obligation to conduct trials to protect children from the risk of using untested medicines and to protect children against unknown risks and harms which may occur with trial participation 27,39,108,109. The ethical principles of respect for persons, beneficence, non-maleficence and justice in trials involving children are the same as for adults. There are additional ethical challenges because children lack the capacity to understand the risks involved in trials and depend upon adults to make decisions for them 110. In a review of 739 paediatric trials from 1996 to 2002, 523 (71%) reported adverse drug reactions, but only 13 (2%) of the trials had safety monitoring committees 4. Since then, trial governance is becoming more stringent with a requirement for an independent safety monitoring board with paediatric expertise, who can appreciate the uniqueness and unpredictability of responses in children 76,111. Long term follow up in children is particularly important as many adverse effects may present later in life 17,44.
Informed consent and assent
Informed consent for participation in paediatric trials is more complex than adult studies because consent is by proxy from the parents or guardian, who have a duty to protect the child's welfare 112. Parents are uncomfortable with this responsibility because they are making a decision for their child 87. Mason & Allmark propose that parents' consent in trials is vital to socially recognise parental roles, but does not offer added protection for neonates to that provided by appropriate research ethics, safety monitoring and governance procedures and parent's knowledge of these measures would improve decision making 113. A review by Shilling & Young indicated that parents are keen to take responsibility for the decision to enrol their child in a trial, but are also fearful of making the ‘wrong’ decision' 83. They also reported that individual parent's understanding of the threat of the child's condition and the trial risks depends on their personal values and experiences, their child's medical condition and the type of the trial 83. Suggestions to address some of these parental concerns identified in this review include positive interactions at recruitment with the flexibility to tailor discussions to the needs and circumstances of individual parents 83. The study by Woolfall et al. in 2013 suggest that the investigating team involved in recruitment need to be aware of parents' priorities and the sorts of misunderstandings that can arise with parents 86. A goodness-of-fit approach is described by Masty & Fisher whereby consent procedures are tailored to the research, the cognitive and emotional maturity of the child, the family system, the participants' priorities and well-being and are focussed on the issues that are of concern to potential participants and helping them achieve understanding of a trial 90. To improve recruitment of children the study by Woolfall et al. also recommended providing tailored trial information on aspects that parents considered important in making a decision for their child participating in a trial 86. Also information about the trial should be provided not only to parents but also to children in an age-appropriate method to improve comprehension, show respect, preserve trust and enable co-operation 98,114. The most frequently cited suggestions from interviews and focus groups for improving informed consent related to allowing parents more time to make their decision, the amount and type of information provided, organization of the consent meeting, communication style and providing additional materials 115. Although consent by parents or guardians is a legal requirement for trials, the autonomy of children should be respected and investigators also need to include them in decision making as much as they are capable 27,87,116. Children's dissent should also be respected, particularly if their dissent is different from their usual response to the same procedure in normal clinical care.
Payment for participation
Although it is common practice to compensate trial participants for travel, parking, meal allowance and accommodation 54, payments for participation in trials is more controversial in children 117. The types of payment can be in the form of reimbursement for direct trial-related expenses, compensation for the time and inconvenience of trial participation, appreciation after participation to thank them for their involvement and incentives to reward enrolment above actual out-of-pocket expenses 112,117. While the European Union (EU) advocates banning of all incentive payments to children 118, this is common practice in the United States (US) where almost 25% of paediatric trials offer payment 117. The ethical concern about large incentive payments is that it might entice and distort the judgement and decision of the parent or child about the risks of trial participation 112,118. However, non-payment for direct expenses and inconvenience of trial participation may create unnecessary financial obstacles for participation and risks hindering essential paediatric research 45,117–119. The ethics committees, investigators and sponsors need to ensure that payment for trial participation of children is fair by keeping payments reasonable 98,120.
Advocacy for trials
The 1989 ‘Convention on the Rights of the Child’ ‘recognizes the right of the child to the enjoyment of the highest attainable standard of health’ 121. This includes the right to have research evidence for treatments commonly used in children. It is a reasonable requirement for the developmental pipeline of new interventions to include children in trials when use in children is anticipated 55,122. Several rigorous guidelines including the Belmont Report 123, Declaration of Ottawa on Child Health 124, Declaration of Helsinki and International Conference on Harmonization (ICH) E11 15,125 address the need for paediatric trials and protection of the rights and welfare of children. Many countries have also developed their own regulations, guidelines and standards for the inclusion of children in trials 22,54,57,126–128.
Paediatric legislations
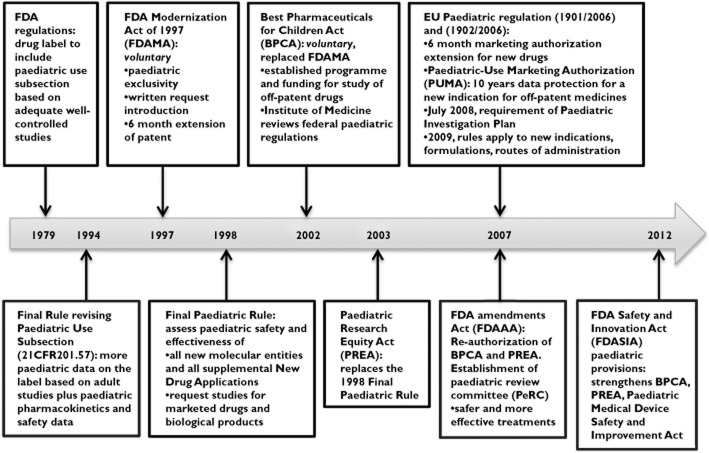
Most medicines used by children internationally are unlicensed or off-label, with no randomized controlled trial data in more than 50% of interventions used in children as compared with adults 44,71,129–131. The US was the first to initiate legislative changes in 1997 to encourage more trials in children to improve the evidence base for medicines in children 28,51, followed by the EU in 2007 7,132–135 (Figure 1). Their expectations were for the pharmaceutical industry to evaluate the safety and efficacy of medicines used by children in all appropriate paediatric age groups, to ensure that product labels contain the known paediatric data, to develop paediatric appropriate formulations and to provide a Paediatric Investigation Plan for testing in children at the time of medicine application submission 122. Both regulations have mandatory requirements with an incentive of a 6 month extension of patent protection to encourage the pharmaceutical industry to conduct paediatric trials. These incentives resulted in an increase in the number of in paediatric trials 136–138. By March 2008, more than 49 000 children were enrolled in trials and the FDA had granted exclusivity to 150 medicines and granted requests for 842 studies which were mostly for new indications of existing medicines 134. The FDA ‘New Paediatric Labelling Information Database’ provides information of new paediatric trials that resulted in new or enhanced safety data in children 137,139. In a retrospective analysis of the applications for Paediatric Investigation Plan and Waivers submitted to the European Medicines Agency (EMA), from 2007 to 2009, there has only been a slight increase in the proportion of paediatric trials from 8.2% of all trials in 2007 to 9.4% in 2009 140. The main therapeutic areas of applications were endocrinology (13.4%), oncology (11%), infectious diseases (10.8%) and cardiovascular diseases (7.1%) 140. This pattern reflected the commercial interest of the pharmaceutical industry to evaluate medicines with a high market value and which are commonly prescribed in adults rather than addressing the priority health needs of children 140,141. Many frequently prescribed, essential medicines that are off-patent and have a small market share in children have yet to be investigated 39,142,143. This is because incentives for the development of off-patent medicines in both the US and EU are small and voluntary and public funding is inadequate 142.
Figure 1.
Timeline of paediatric regulations in the US and EU
The pharmaceutical industry does not appear to be interested in transferring the benefits of approved paediatric appropriate medicines in the US and EU to other countries. This may be due to the lack of economic incentives and the high costs associated with amending the labels of existing medicines with new paediatric data or registering new medicines. Therefore, there needs to be an efficient, harmonized process globally through collaboration of government, pharmaceutical industry and the medical community to ensure that the development of paediatric medicine is optimally aligned with priority health care needs 45,142. Canada and Japan have implemented modest paediatric regulation reforms 142. Other countries need to adopt regulations and incentives for promoting paediatric medicines research with sustained government and societal support.
International paediatric trials initiatives
Global priorities of the WHO Millennium Development Goals include the ‘Make medicines child size’ initiative and the World Health Assembly “Better Medicines for Children” Resolution WHA60.20 in 2007 to improve knowledge, access, research and development of paediatric medicines 143,144. Another WHO initiative is the Paediatric medicines Regulator's Network (PmRN), which involves National Medicines Regulatory Authorities (NMRAs) which aim to harmonize the regulation of manufacture, license and research of medicines for children 144.
International paediatric trial networks have been established in many countries to address some of the challenges by improving the infrastructure and research capacity. The US and EU created networks with specialized expertise in conducting trials in children and have dedicated funding for paediatric research and training 142. The US National Institute of Child Health and Human Development (NICHD) Pediatric Trial Network (PTN) was launched in September 2010 with US $95 million for 7 years to conduct paediatric trials on off-patent medicines 146. This network provides an appropriate environment for performing safe and effective trials in children as recommended by the Best Pharmaceuticals for Children Act (BPCA) drug development programme in a variety of therapeutic areas 146. In 2012 the network, in collaboration with the FDA, has commenced paediatric studies on 30 drugs 147.
The Network of Paediatric Research at the European Medicines Agency (Enpr-EMA)] was established in March 2011 in collaboration with research networks, investigators and centres with recognized expertise in conducting paediatric trials 148. This network is working towards developing the necessary competences and avoiding unnecessary duplication of paediatric studies, educating parents or carers and children about trials and encouraging their participation, raising awareness among health care professionals of the necessity for trials in children of all ages, supporting their involvement in such studies and engaging in dialogue with ethics committees on paediatric trials issues 148. The National Institute for Health Research (NIHR) Medicines for Children Research Network (MCRN) in the UK was established in 2005. This network has been more successful because of funding from the government 99. The Standards for Research in (StaR) Child Health is an international initiative founded in 2009 to improve the quality of the design, conduct and reporting of paediatric trials by promoting the use of internationally developed research standards to enhance their reliability and relevance 5,149–151.
Professional bodies and research organizations internationally have also recognized the imperative to conduct paediatric trials. These include the International Paediatric Association (IPA 152), the American National Institute of Health (NIH) 153,154 and Academy of Paediatrics (AAP) 54, the UK Medical Research Council (MRC) 155 and Royal College of Paediatrics and Child Health (RCPCH) 156 and the European Agency for the Evaluation of Medicinal Products (EMEA) 125.
International collaborative disease specific groups have been formed to address the logistical and methodological difficulties in conducting trials of diseases with a low prevalence 80. Examples of successful disease specific groups include the Children's Oncology Group (COG 77), Paediatric Rheumatology International Trials Organization (PRINTO) 79 and Paediatric European Network for the Treatment of AIDS (PENTA 81). As a result of collaboration, the Children's Oncology Group has developed a research culture in the participating institutions which accepts protocol-driven trials as part of standard care 13. It has also facilitated rigorous protocol development and review, centralization of pathology review, central database and safety monitoring of toxicity and response, internal auditing to ensure compliance to Good Clinical Practice and involvement of established investigators with oncology paediatric expertise 45,157. PRINTO has a Scientific Advisory Council, International and National Coordinating Centres to facilitate logistics and scientific elements of multicentre, multinational studies 80. PRINTO's achievements include developing standardized treatment outcome measures, training young researchers, access to network facilities for the conduct of trials and establishing a website for families to access health information 80. All these global initiatives can be adopted by other disease specific groups to support the collaborative efforts to increase the number of clinically relevant paediatric trials.
The way forward
Although progress has been slow, paediatric clinical trials have undergone a renaissance with international recognition of the importance of trials in children. However, there continue to be deficiencies including inadequate funding and conflicts of interest with trials still being driven by financial and political incentives. Health policy makers need to consider the needs of children by setting priorities, developing infrastructure and providing sufficient funding 158 that is sustainable to accelerate the progress of equitable health care. The future health of children hinges on the success of paediatric trials. Greater advocacy and collaboration between all major stakeholders including regulatory authorities, pharmaceutical industries, scientific community, clinicians and the public at the national and international level is crucial to this success. Investment into better evidence-based treatments for our children is an investment into a better future for all.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare PDJ had support from National Health and Medical Research Council for the submitted work. There are no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- Wilson JT. An update on the therapeutic orphan. Pediatrics. 1999;104(Suppl. 3):585–590. [PubMed] [Google Scholar]
- Shirkey H. Therapeutic orphans. J Pediatr. 1968;72:119–120. doi: 10.1016/s0022-3476(68)80414-7. [DOI] [PubMed] [Google Scholar]
- Shirkey H. Editorial comment: therapeutic orphans. J Pediatr. 1999;104:583–584. [PubMed] [Google Scholar]
- Sammons H, Gray C, Hudson H, Cherrill J, Choonara I. Safety in paediatric clinical trials – a 7-year review. Acta Paediatr. 2008;97:474–477. doi: 10.1111/j.1651-2227.2008.00676.x. [DOI] [PubMed] [Google Scholar]
- Klassen TP, Hartling L, Hamm M, van der Lee JH, Ursum J, Offringa M. StaR Child Health: an initiative for RCTs in children. Lancet. 2009;374:1310–1312. doi: 10.1016/S0140-6736(09)61803-1. [DOI] [PubMed] [Google Scholar]
- Smyth RL. Making a difference: the clinical research programme for children. Arch Dis Child. 2007;92:835–837. doi: 10.1136/adc.2006.113357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner MS. Paediatric clinical trials: redressing the imbalance. Nat Rev Drug Discov. 2003;2:949–961. doi: 10.1038/nrd1253. [DOI] [PubMed] [Google Scholar]
- Dawson L. The Salk Polio Vaccine Trial of 1954: risks, randomization and public involvement in research. Clin Trials. 2004;1:122–130. doi: 10.1191/1740774504cn010xx. [DOI] [PubMed] [Google Scholar]
- Juskewitch JE, Tapia CJ, Windebank AJ. Lessons from the Salk Polio Vaccine: methods for and risks of rapid translation. Clin Transl Sci. 2010;3:182–185. doi: 10.1111/j.1752-8062.2010.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy ME, Sawczyn KK, Garrington TP, Graham DK, Gore L. Pediatric developmental therapies: interesting new drugs now in early-stage clinical trials. Curr Oncol Rep. 2008;10:477–490. doi: 10.1007/s11912-008-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard-Jones K, Europe S. Clinical trials for children with cancer in Europe - still a long way from harmonisation: a report from SIOP Europe. Eur J Cancer. 2008;44:2106–2111. doi: 10.1016/j.ejca.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Stiller CA, Kroll ME, Pritchard-Jones K. Population survival from childhood cancer in Britain during 1978–2005 by eras of entry to clinical trials. Ann Oncol. 2012;23:1–6. doi: 10.1093/annonc/mds183. [DOI] [PubMed] [Google Scholar]
- UK Cancer Research. Childhood cancer survival statistics. Section updated 14/11/11. Available at http://www.cancerresearchuk.org/cancer-info/cancerstats/childhoodcancer/survival/ (last accessed 5 August 2013)
- Martinez-Castaldi C, Silverstein M, Bauchner H. Child versus adult research: the gap in high-quality study design. Pediatrics. 2008;122:52–57. doi: 10.1542/peds.2007-2849. [DOI] [PubMed] [Google Scholar]
- Foex BA. The ethics of clinical trials. Anaesth Intensive Care Med. 2009;10:98–101. [Google Scholar]
- Sinha I, Jones L, Smyth RL, Williamson PR. A systematic review of studies that aim to determine which outcomes to measure in clinical trials in children. PLoS Med. 2008;5:e96. doi: 10.1371/journal.pmed.0050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen TP, Hartling L, Craig JC, Offringa M. Children are not just small adults: the urgent need for high-quality trial evidence in children. PLoS Med. 2008;5:e172. doi: 10.1371/journal.pmed.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint Raymond A, Brasseur D. Development of medicines for children in Europe: ethical implications. Paediatr Respir Rev. 2005;6:45–51. doi: 10.1016/j.prrv.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Amann JP, Dulac O. Trials in children. Epilepsy Res. 2001;45:133–136. doi: 10.1016/s0920-1211(01)00237-6. discussion 7–40. [DOI] [PubMed] [Google Scholar]
- Conroy S, McIntyre J, Choonara I, Stephenson T. Drug trials in children: problems and the way forward. Br J Clin Pharmacol. 2000;49:93–97. doi: 10.1046/j.1365-2125.2000.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi F, Tomasi P. The development of medicines for children. Part of a series on Pediatric Pharmacology, guest edited by Gianvincenzo Zuccotti, Emilio Clementi, and Massimo Molteni. Pharmacol Res. 2011;64:169–175. doi: 10.1016/j.phrs.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Canadian Paediatric Society. 2003. Drug investigation for Canadian children: the role of the Canadian Paediatric Society. Review. Apr. Report No.: 1205-7088 Contract No.: 4.
- Contopoulos-Ioannidis DG, Baltogianni MS, Loannidis JPA. Comparative effectiveness of medical interventions in adults versus children. J Pediatr. 2010;157:322–330. doi: 10.1016/j.jpeds.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Noah B. Just a spoonful of sugar: drug safety for pediatric populations. J Law Med Ethics. 2009;37:280–291. doi: 10.1111/j.1748-720X.2009.00372.x. [DOI] [PubMed] [Google Scholar]
- Pandolfini C, Bonati M, Sammons HM. Registration of trials in children: update of current international initiatives. Arch Dis Child. 2009;94:717–719. doi: 10.1136/adc.2008.148155. [DOI] [PubMed] [Google Scholar]
- Institute of Medicines of the National Academies: Committee on Clinical Research Involving Children. The Ethical Conduct of Clinical Research Involving Children. Washington, DC: The National Academies Press; 2004. [PubMed] [Google Scholar]
- Ackerman TF. The ethics of drug research in children. Paediatr Drugs. 2001;3:29–41. doi: 10.2165/00128072-200103010-00003. [DOI] [PubMed] [Google Scholar]
- Christensen ML, Helms RA, Chesney RW. Is pediatric labeling really necessary? Pediatrics. 1999;104(Suppl. 3):593–597. [PubMed] [Google Scholar]
- Burns LE, Hodgman JE, Cass AB. Fatal circulatory collapse in premature infants receiving chloramphenicol. N Engl J Med. 1959;261:1318–1321. doi: 10.1056/NEJM195912242612604. [DOI] [PubMed] [Google Scholar]
- Balistreri WF, Farrell MK, Lessons BKE. From the E-ferol tragedy. Pediatrics. 1986;78:503–506. [PubMed] [Google Scholar]
- Curran WJ. The thalidomide tragedy in Germany: the end of a historic medicolegal trial. N Engl J Med. 1971;284:481–482. doi: 10.1056/NEJM197103042840906. [DOI] [PubMed] [Google Scholar]
- Cohen E, Uleryk E, Jasuja M, Parkin PC. An absence of pediatric randomized controlled trials in general medical journals, 1985–2004. J Clin Epidemiol. 2007;60:118–123. doi: 10.1016/j.jclinepi.2006.03.015. [DOI] [PubMed] [Google Scholar]
- The World Bank. Population ages 0–14 (% of total) 2011. The United Nations population division's world population prospects. Available at http://data.worldbank.org/indicator/SP.POP.0014.TO.ZS/countries/1W?display=default (last accessed 30 May 2013)
- Joseph DP, Caldwell PHY, Craig JC. Registered paediatric clinical trials: a global context. 2013. Poster presentation International Congress of Pediatrics 2013 (ICP), The 27th Congress of International Pediatric Association 24–29 August 2013 Melbourne, Australia.
- Bourgeois FT, Murthy S, Pinto C, Olson KL, Ioannidis JP, Mandl KD. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics. 2012;130:285–292. doi: 10.1542/peds.2012-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers C. Global burden of disease among women, children, and adolescents. In: Ehiri JE, editor. Maternal and Child Health. New York, NY: Springer Science, Business Media, LLC; 2009. pp. 19–42. [Google Scholar]
- Hoppu K. Can we get the necessary clinical trials in children and avoid the unnecessary ones? Eur J Clin Pharmacol. 2009;65:747–748. doi: 10.1007/s00228-009-0675-y. [DOI] [PubMed] [Google Scholar]
- Aripin KNBN, Choonara I, Sammons HM. A systematic review of paediatric randomised controlled drug trials published in 2007. Arch Dis Child. 2010;95:469–473. doi: 10.1136/adc.2009.173591. [DOI] [PubMed] [Google Scholar]
- Salazar JC. Pediatric clinical trial experience: government, child, parent and physician's perspective. Pediatr Infect Dis J. 2003;22:1124–1127. doi: 10.1097/01.inf.0000101847.22501.e7. [DOI] [PubMed] [Google Scholar]
- Sammons HM, Choonara I. Clinical trials of medication in children 1996–2002. Eur J Clin Pharmacol. 2005;61:165–167. doi: 10.1007/s00228-005-0894-9. [DOI] [PubMed] [Google Scholar]
- Aripin KNBN, Choonara I, Sammons HM. Published paediatric randomized drug trials in developing countries, 1996–2002. Paediatr Drugs. 2010;12:99–103. doi: 10.2165/11316260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Thomson D, Hartling L, Cohen E, Vandermeer B, Tjosvold L, Klassen TP. Controlled trials in children: quantity, methodological quality and descriptive characteristics of pediatric controlled trials published 1948–2006. PLoS ONE. 2010;5:e13106. doi: 10.1371/journal.pone.0013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlberg JPE. Clinical trials in paediatrics. In: Machin D, Day S, Green S, editors. Textbook in Clinical Trials. Chichester: John Wiley and Sons, Ltd; 2006. pp. 701–710. [Google Scholar]
- Hill P. Off licence and off label prescribing in children: litigation fears for physicians. Arch Dis Child. 2005;90(Suppl. I):i17–18. doi: 10.1136/adc.2004.058867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell PHY, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet. 2004;364:803–811. doi: 10.1016/S0140-6736(04)16942-0. [DOI] [PubMed] [Google Scholar]
- Krekels EHJ, van den Anker JN, Baiardi P, Cella M, Cheng KY, Gibb DM, Green H, Iolascon A, Jacqz-Aigrain EM, Knibbe CAJ, Santen GWE, van Schaik RHN, Tibboel D, Della Pasqua OE. Pharmacogenetics and paediatric drug development: issues and consequences to labelling and dosing recommendations. Expert Opin Pharmacother. 2007;8:1787–1799. doi: 10.1517/14656566.8.12.1787. [DOI] [PubMed] [Google Scholar]
- Lim A, Cranswick N. Clinical trials, ethical issues and patient recruitment: an australian perspective. Paediatr Perinat Drug Ther. 2003;5:183–187. [Google Scholar]
- Cohen ERM, O'Neill JM, Joffres M, Upshur REG, Mills E. Reporting of informed consent, standard of care and post-trial obligations in global randomized intervention trials: a systematic survey of registered trials. Developing World Bioeth. 2009;9:74–80. doi: 10.1111/j.1471-8847.2008.00233.x. [DOI] [PubMed] [Google Scholar]
- National Institute of Health. 2001. Glossary of terms for human subjects protection and inclusion issues. Available at http://grants.nih.gov/grants/peer/tree_glossary.pdf (last accessed 4 August 2013)
- Australian Government National Health and Medical Research Council and Department of Industry, innovation, science, research and tertiary education. Australian Clinical Trials.Phases of clinical trials. Available at http://www.australianclinicaltrials.gov.au/node/5 (last accessed 5 August 2013)
- US Food and Drug Administration (FDA) 2011. Title 21 – food and drugs. Chapter 1 – Food and Drug administration, Department of Health and Human Services. Subchapter C- drugs: general, code of federal regulations, volume 4, Revised as of 1 April. Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=201.57 (last accessed 10 May 2013)
- Aleksa K, Koren G. Ethical issues in including pediatric cancer patients in drug development trials. Paediatr Drugs. 2002;4:257–265. doi: 10.2165/00128072-200204040-00005. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (EMA) 2008. Ethical considerations for clinical trials on medicinal products conducted with the paediatric population. Recommendations of the ad hoc group for the development of implementing Guidelines for Directive 2001/20/EC relating to good clinical practice in the conduct of clinical trials on medicinal products for human use. Available at ftp://ftp.cordis.europa.eu/pub/fp7/docs/ethical-considerations-paediatrics_en.pdf (last accessed 5 August 2013)
- Bellanti F, Della Pasqua O. Modelling and simulation as research tools in paediatric drug development. Eur J Clin Pharmacol. 2011;67(Suppl. 1):75–86. doi: 10.1007/s00228-010-0974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaddy RE, Denne SC The Committee on Drugs and Committee on Pediatric Research. Guidelines for the ethical conduct of studies to evaluate drugs in pediatric populations. Pediatrics. 2010;125:850. doi: 10.1542/peds.2010-0082. [DOI] [PubMed] [Google Scholar]
- Henschel AD, Rothenberger LG, Boos J. Randomized clinical trials in children – ethical and methodological issues. Curr Pharm Des. 2010;16:2407–2415. doi: 10.2174/138161210791959854. [DOI] [PubMed] [Google Scholar]
- Zhang B, Schmidt B. Do we measure the right end points? A systematic review of primary outcomes in recent neonatal randomized clinical trials. J Pediatr. 2001;138:76–80. doi: 10.1067/mpd.2001.110299. [DOI] [PubMed] [Google Scholar]
- Flynn JT. Ethics of placebo use in pediatric clinical trials: the case of antihypertensive drug studies. Hypertension. 2003;42:865–869. doi: 10.1161/01.HYP.0000095616.91352.2E. [DOI] [PubMed] [Google Scholar]
- Fost N. Ethical issues in research and innovative therapy in children with mood disorders. Biol Psychiatry. 2001;49:1015–1022. doi: 10.1016/s0006-3223(01)01181-7. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration. Postmarketing requirements and commitments: Frequently Asked Questions (FAQ). Updated: 02/08/2012. Available at http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Post-marketingPhaseIVCommitments/ucm070766.htm#q15 (last accessed 18 August 2013)
- Laughon MM, Benjamin DK, Jr, Capparelli EV, Kearns GL, Berezny K, Paul IM, Wade K, Barrett J, Smith PB, Cohen-Wolkowiez M. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4:643–652. doi: 10.1586/ecp.11.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TN. Modelling approaches to dose estimation in children. Br J Clin Pharmacol. 2005;59:663–669. doi: 10.1111/j.1365-2125.2005.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP) Guideline on the role of pharmacokinetics in the development of medicinal products in the paediatric population. 2006. Doc. Ref. EMEA/CHMP/EWP/147013/2004 Corrigendum London, 28 June.
- Viergever RF, Ghersi D. The quality of registration of clinical trials. PLoS ONE. 2011;6:1–8. doi: 10.1371/journal.pone.0014701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South African Cochrane Centre. Pan African clinical trials registry (PACTR). Available at http://www.pactr.org (last accessed 9 April 2013)
- Thomas-Urban KB, Joergensen M, Lynch G, Rubison M, Porter BD, Sayers J, Tesch C. Clinical trial disclosure: global overview and implications of new laws and guidelines. Drug Inf J. 2010;44:213–225. [Google Scholar]
- International Committee of Medical Journal. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med. 2004;351:1250–1251. doi: 10.1056/NEJMe048225. [DOI] [PubMed] [Google Scholar]
- de Vries TW, van Roon EN. Low quality of reporting adverse drug reactions in paediatric randomised controlled trials. Arch Dis Child. 2010;95:1023–1026. doi: 10.1136/adc.2009.175562. [DOI] [PubMed] [Google Scholar]
- Benjamin DK, Smith P, Murphy M, Roberts R, Mathis L, Avant D, Califf RM, Li JS. Peer-reviewed publication of clinical trials completed for pediatric exclusivity. JAMA. 2006;296:1266–1273. doi: 10.1001/jama.296.10.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet Oncol. 2009;374:86–89. doi: 10.1016/S0140-6736(09)60329-9. [DOI] [PubMed] [Google Scholar]
- Cramer K, Wiebe N, Moyer V, Hartling L, Williams K, Swingler G, Klassen TP. Children in reviews: methodological issues in child-relevant evidence syntheses. BMC Pediatr. 2005;5:38. doi: 10.1186/1471-2431-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B, Shilling V, Hickey H, Sowden E, Smyth R, Williamson P. What parents think about being approached about children's trials, how this differs from what practitioners expect, and what this tells us about enhancing recruitment. Oral presentation: clinical trials methododlogy conference 2011, Bristol UK 4–5 October 2011. Trials. 2011;12(Suppl. 1):A116. [Google Scholar]
- Morales-Olivas FJ, Morales-Carpi C. Clinical trials in children. Rev Recent Clin Trials. 2006;1:251–258. doi: 10.2174/157488706778250087. [DOI] [PubMed] [Google Scholar]
- Deal G, Nikitin N. European and non-European paediatric clinical trials. Regul Rapportteur. 2009;6:4–7. [Google Scholar]
- Foster BJ, Warady BA. Clinical research in pediatric nephrology: challenges, and strategies to address them. J Nephrol. 2009;22:685–693. [PubMed] [Google Scholar]
- Bavdekar SB. Pediatric clinical trials. Perspect Clin Res. 2013;4:89–99. doi: 10.4103/2229-3485.106403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Children's Oncology Group (COG) Available at http://www.childrensoncologygroup.org/ (last accessed 2 August 2013)
- Children's Cancer and Leukaemia Group (CCLG) Available at http://www.cclg.org.uk/ (last accessed 2 August 2013) [DOI] [PubMed]
- Paediatric Rheumatology International Trials Organisation (PRINTO) Available at http://www.printo.it/ (last accessed 3 August 2013) [DOI] [PubMed]
- Ruperto N, Martini A. Networking in paediatrics: the example of the Paediatric Rheumatology International Trials Organisation (PRINTO) Arch Dis Child. 2011;96:596–601. doi: 10.1136/adc.2010.188946. [DOI] [PubMed] [Google Scholar]
- Paediatric European Network for the Treatment of AIDS (PENTA) Available at http://www.pentatrials.org/network.htm (last accessed 5 August 2013)
- Paediatric Emergency Care Applied Research Network (PECARN) Available at http://www.pecarn.org/ (last accessed 1 August 2013)
- Shilling V, Young B. How do parents experience being asked to enter a child in a randomised controlled trial? BMC Med Ethics. 2009;10:1. doi: 10.1186/1472-6939-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K. Current issues in paediatric clinical trials. Meeting report. Available at http://www.abpi.org.uk (last accessed 3 October 20136) [Google Scholar]
- Singhal N, Oberle K, Burgess E, Huber-Okrainec J. Parents' perceptions of research with newborns. J Perinatol. 2002;22:57–63. doi: 10.1038/sj.jp.7210608. [DOI] [PubMed] [Google Scholar]
- Woolfall K, Shilling V, Hickey H, Smyth RL, Sowden E, Williamson PR, Young B. Parents' agendas in paediatric clinical trial recruitment are different from researchers' and often remain unvoiced: a qualitative study. PLoS ONE. 2013;8:e67352. doi: 10.1371/journal.pone.0067352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell PHY, Butow PN, Craig JC. Parents' attitudes to randomised controlled trials involving children. J Pediatr. 2003;145:555–560. [Google Scholar]
- Caldwell PHY, Hamilton S, Tan A, Craig JC. Strategies for increasing recruitment to randomised controlled trials: systematic review. PLoS Med. 2010;7:1–16. doi: 10.1371/journal.pmed.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnowski KJ, Allen DM, Mayhall C, Kelly PA. Readability of pediatric biomedical research informed consent forms. Pediatrics. 2007;119:849–859. [PubMed] [Google Scholar]
- Masty J, Fisher C. A goodness-of-fit approach to informed consent for pediatric intervention research. Ethics Behav. 2008;18:139–160. [Google Scholar]
- Why fair tests are needed. Available at http://www.jameslindlibrary.org/essays/fair_tests/why-fair-tests-are-needed.html (last accessed 2 November 2013)
- Allmark P, Spedding M. Clinical trials in neonates: ethical issues. Semin Fetal Neonatal Med. 2007;12:318–323. doi: 10.1016/j.siny.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Caldwell P, Butow P, Craig J. Parents' attitudes to children's participation in randomized controlled trials. J Pediatr. 2003;142:554–559. doi: 10.1067/mpd.2003.192. [DOI] [PubMed] [Google Scholar]
- Snowdon C, Elbourne D, Garcia J. Zelen randomization: attitudes of parents participating in a neonatal clinical trial. Control Clin Trials. 1999;20:149–171. doi: 10.1016/s0197-2456(98)00049-x. [DOI] [PubMed] [Google Scholar]
- Culbert A, Davis DJ. Parental preferences for neonatal resuscitation research consent: a pilot study. J Med Ethics. 2005;31:721–726. doi: 10.1136/jme.2004.011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Rahman SM, Reed MD, Wells TG, Kearns GL. Considerations in the rational design and conduct of phase I/II pediatric clinical trials: avoiding the problems and pitfalls. Clin Pharmacol Ther. 2007;81:483–494. doi: 10.1038/sj.clpt.6100134. [DOI] [PubMed] [Google Scholar]
- de Wildt SN, Ito S, Koren G. Challenges for drug studies in children: CYP3A phenotyping as example. Drug Discov Today. 2009;14:6–15. doi: 10.1016/j.drudis.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Caldwell PHY, Dans L, de Vries MC, Newman J, Sammons H, Spriggs M, Tambe P, Van't Hoff W, Woolfall K, Young B, Offringa M. Standard 1: consent and recruitment. Pediatrics. 2012;129(Suppl. 3):S118–123. doi: 10.1542/peds.2012-0055D. [DOI] [PubMed] [Google Scholar]
- NHS National Institute for Health Research. Medicines for children research network. Available at http://www.mcrn.org.uk/ (last accessed 12 April 2012)
- Caldwell PHY, Butow PN, Craig JC. Paediatricians' attitudes to randomised controlled trials involving children. J Pediatr. 2002;141:798–803. doi: 10.1067/mpd.2002.129173. [DOI] [PubMed] [Google Scholar]
- Wax PM. Elixirs, diluents, and the passage of the 1938 Federal Food, Drug and Cosmetic Act. Ann Intern Med. 1995;122:456–461. doi: 10.7326/0003-4819-122-6-199503150-00009. [DOI] [PubMed] [Google Scholar]
- Ernest TB, Elder DP, Martini LG, Roberts M, Ford JL. Developing paediatric medicines: identifying the needs and recognizing the challenges. J Pharm Pharmacol. 2007;59:1043–1055. doi: 10.1211/jpp.59.8.0001. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration (FDA) 2007. FDA Amendments Act of 2007 (FDAAA),Title IV: Pediatric Research Equity Act of 2007 (PREA) and Title V: Best Pharmaceuticals for Children Act of 2007 (BPCA). September 27. (Public Law No. 110-85). Available at http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm049867.htm (last accessed 25 January 2013)
- World Health Organization. Report of the Informal Expert Meeting on Dosage Forms of Medicines for Children. Geneva: WHO; 2008. Available at http://www.who.int/selection_medicines/committees/expert/17/application/paediatric/Dosage_form_reportDEC2008.pdf (last accessed 12 August 2013) [Google Scholar]
- von Baeyer CL. Children's self-reports of pain intensity: scale selection, limitations and interpretation. Pain Res Manag. 2006;11:157–162. doi: 10.1155/2006/197616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligi I, Boubred F, Grandvuillemin I, Simeoni U. Clinical research in newborn infants: difficulties and specificity. Eur J Clin Pharmacol. 2011;67(Suppl. 1):29–32. doi: 10.1007/s00228-010-0921-3. [DOI] [PubMed] [Google Scholar]
- Abbott J, Gee L. Quality of life in children and adolescents with cystic fibrosis: implications for optimizing treatments and clinical trial design. Paediatr Drugs. 2003;5:41–56. doi: 10.2165/00128072-200305010-00004. [DOI] [PubMed] [Google Scholar]
- O'Lonergan TA, Milgrom H. Ethical considerations in research involving children. Curr Allergy Asthma Rep. 2005;5:451–458. doi: 10.1007/s11882-005-0025-9. [DOI] [PubMed] [Google Scholar]
- Ross LF, Newburger JW, Sanders SP. Ethical issues in pediatric trials. Am Heart J. 2001;142:233–236. doi: 10.1067/mhj.2001.117065. [DOI] [PubMed] [Google Scholar]
- Dixon-Woods M, Ashcroft RE, Jackson CJ, Tobin MD, Kivits J, Burton PR, Samani NJ. Beyond ‘misunderstanding’: written information and decisions about taking part in a genetic epidemiology study. Soc Sci Med. 2007;65:2212–2222. doi: 10.1016/j.socscimed.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Hirtz DG, Gilbert PR, Terrill CM, Buckman SY. Clinical trials in children - How are they implemented? Pediatr Neurol. 2006;34:436–438. doi: 10.1016/j.pediatrneurol.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Spriggs M, Caldwell P. The ethics of paediatric research. J Paediatr Child Health. 2011;47:664–667. doi: 10.1111/j.1440-1754.2011.02166.x. [DOI] [PubMed] [Google Scholar]
- Mason SA, Allmark PJ. Obtaining informed consent to neonatal randomised controlled trials: interviews with parents and clinicians in the Euricon study. Lancet. 2000;356:2045–2051. doi: 10.1016/s0140-6736(00)03401-2. [DOI] [PubMed] [Google Scholar]
- World Medical Association. 2008. Declaration of Helsinki. Available at http://www.wma.net/en/30publications/10policies/b3/index.html (last accessed 9 February 2013)
- Eder M, Yamokoski A, Wittmann P, Kodish E. Improving informed consent: suggestions from parents of children with leukemia. Pediatrics. 2007;119:e849–859. doi: 10.1542/peds.2006-2208. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, O'Brien M. Ethics and medical research in children. Paediatr Anaesth. 2009;19:994–1004. doi: 10.1111/j.1460-9592.2009.03117.x. [DOI] [PubMed] [Google Scholar]
- Wendler D, Rackoff JE, Emanuel EJ, Grady C. The ethics of paying for children's participation in research. J Pediatr. 2002;141:166–171. doi: 10.1067/mpd.2002.124381. [DOI] [PubMed] [Google Scholar]
- Dobson R. Lump sums for children taking part in research may distort parents' judgment. BMJ. 2002;325:796. [Google Scholar]
- Tishler CL, Reiss NS. Pediatric drug-trial recruitment: enticement without coercion. Pediatrics. 2011;127:949–954. doi: 10.1542/peds.2010-2585. [DOI] [PubMed] [Google Scholar]
- Glasser S, Howard G. Clinical trial design issues: at least 10 things you should look for in clinical trials. J Clin Pharmacol. 2006;46:1106–1115. doi: 10.1177/0091270006290336. [DOI] [PubMed] [Google Scholar]
- United Nations General Assembly. 1989. ‘Convention on the rights of the child’ Part 1, Article1, A/RES/44/25, 61st plenary meeting. 20 November. Available at http://www.un.org/documents/ga/res/44/a44r025.htm (last accessed 4 April 2013)
- Tassinari MS, editor. Pediatric Regulations in the US and Europe. Silver Spring, MD: FDA Office of New Drugs, ODAC Pediatric Subcommittee; 2010. November. Available at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM236390.pdf (last accessed 1 July 2013) [Google Scholar]
- Office of the Secretary: The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. 1979. The Belmont Report: ethical principles and guidelines for the protection of human subjects of research. 18 April. Available at http://science.education.nih.gov/supplements/nih9/bioethics/guide/teacher/Mod5_Belmont.pdf (last accessed 9 July 2013)
- World Medical Association I. WMA Declaration of Ottawa on child health. Available at http://www.wma.net/en/30publications/10policies/c4/ (last accessed 3 August 2013)
- The European Agency for the Evaluation of Medicinal Products (EMA) Evaluation of medicines for human use. 2000. pp. 1–12. ICH Topic E11 Clinical Investigation of Medicinal Products in the Paediatric Population. CPMP/ICH/2711/99.
- US Food and Drug Administration (FDA) Fact sheet: pediatric provisions in the Food and Drug Administration Safety and Innovation Act (FDASIA). 07092012. Available at http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/ucm311038.htm (last accessed 6 July 2013)
- US Food and Drug Administration. Running clinical trials. Additional protections for children. Last update 08/05/2010. Available at http://www.fda.gov/ScienceResearch/SpecialTopics/RunningClinicalTrials/ucm119111.htm (last accessed 31 July 2013)
- National Health and Medical Research Council. Clinical trials. Available at http://www.nhmrc.gov.au/health-ethics/human-research-ethics/clinical-trials (last accessed 10 August 2013)
- Smit-Marshall P. Pediatric clinical trials: a worldview. Appl Clin Trials. 2010;19:32–37. [Google Scholar]
- Smyth AR, Barbato A, Beydon N, Bisgaard H, de Boeck K, Brand P, Bush A, Fauroux B, de Jongste J, Korppi M, O'Callaghan C, Pijnenburg M, Ratjen F, Southern K, Spencer D, Thomson A, Vyas H, Warris A, Merkus PJ. Respiratory medicines for children: current evidence, unlicensed use and research priorities. Eur Respir J. 2010;35:247–265. doi: 10.1183/09031936.00139508. [DOI] [PubMed] [Google Scholar]
- Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A, Knoeppel C, Seyberth H, Pandolfini C, Raffaelli MP, Rocchi F, Bonati M, Jong G, de Hoog M, van den Anker J. Survey of unlicenced and off label drug use in paediatric wards in European countries. BMJ. 2000;320:79–82. doi: 10.1136/bmj.320.7227.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permanand G, Mossialos E, McKee M. The EU's new paediatric medicines legislation: serving children's needs? Arch Dis Child. 2007;92:808–811. doi: 10.1136/adc.2006.105692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammons H. Ethical issues of clinical trials in children: a European perspective. Arch Dis Child. 2009;94:474–477. doi: 10.1136/adc.2008.149898. [DOI] [PubMed] [Google Scholar]
- Greener M. Bitter medicine. New regulations aim to address the dearth of clinical safety trials for drugs used in children. EMBO Rep. 2008;9:505–508. doi: 10.1038/embor.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency (EMA) Paediatric regulation. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000068.jsp (last accessed 5 August 2013)
- Li J, Eisenstein E, Grabowski H, Reid E, Mangum B, Schulman K, Goldsmith J, Murphy MD, Califf R, Benjamin D. Economic return of clinical trials performed under the pediatric exclusivity program. JAMA. 2007;297:480–488. doi: 10.1001/jama.297.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration (FDA) New pediatric labeling information database. Available at http://www.accessdata.fda.gov/scripts/sda/sdNavigation.cfm?sd=labelingdatabase (last accessed 22 May and 19 July 2013)
- WHO and StaR Child Health. Report of the WHO and StaR Child Health Paediatric Trials in Developing Countries Meeting: Use of Standards in Paediatric Clinical Trials in Developing Countries Meeting. Amsterdam, The Netherlands: WHO and StaR Child Health; 2009. 28 October. Available at http://www.who.int/childmedicines/progress/Amsterdam_Meeting.pdf (last accessed 5 May 2013) [Google Scholar]
- US Food and Drug Administration. Database is one-stop resource on kids' medications. Available at http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm305040.htm?source=govdelivery (last accessed 30 May 2012)
- Boots I, Sukhai RN, Klein RH, Holl RA, Wit JM, Cohen AF, Burggraaf J. Stimulation programs for pediatric drug research – do children really benefit? Eur J Pediatr. 2007;166:849–855. doi: 10.1007/s00431-006-0381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppu K, Anabwani G, Garcia-Bournissen F, Gazarian M, Kearns G, Nakamura H, Peterson R, Sri Ranganathan S, Wildt S. The status of paediatric medicines initiatives around the world – what has happened and what has not? Eur J Clin Pharmacol. 2012;68:1–10. doi: 10.1007/s00228-011-1089-1. [DOI] [PubMed] [Google Scholar]
- Flynn JT. Successes and shortcomings of the Food and Drug Modernization Act. Am J Hypertens. 2003;16:889–891. doi: 10.1016/s0895-7061(03)01007-0. [DOI] [PubMed] [Google Scholar]
- World Health Organisation (WHO) Essential medicines for children. Available at http://www.who.int/childmedicines/ (last accessed 12 April 2013)
- World Health Organisation. 2010. Medicines for children:Resources, progress reports and scientific publications: medicines: medicines for children.Fact sheet N°34, 1 June. Available at http://www.who.int/mediacentre/factsheets/fs341/en/index.html (last accessed 9 April 2013)
- Institute DtM. Pediatric Trials Network funded with $95M NIH grant, has FDA approval to study 4 drugs, now enrolling. Available at https://www.dtmi.duke.edu/news-publications/news/dtmi-news-archives/putting-an-end-to-2018ballpark-dosing2019-in-children (last accessed 20 October 2013)
- Benjamin D. Clinical Trials Approach and the Pediatric Trials Network. American Society for Clinical Pharmacology and Therapeutics (ASCPT) conference, 12–17 March 2012. Available at https://pediatrictrials.org/background/ptn-news-papers/ptn-presentations (last accessed 20 October 2013)
- European Medicines Agency (EMA) Network of paediatric research at the European Medicines Agency (Enpr-EMA). Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/partners_and_networks/general/general_content_000303.jsp&mid=WC0b01ac05801df74a (last accessed 12 April 2012)
- The Global Research in Paediatrics (GRIP) network for excellence. Available at http://www.grip-network.org/index.php/cms/en/paediatric_clinical_pharmacology (last accessed 15 August 2011)
- Star Child Health. Standards for research in child health. Available at http://ifsrc.org/ (last accessed 30 July 2013)
- Frakking FNJ, Van der Lee JH, Klassen JH, Offringa M the StaR-Child Health Group. 2009. for Report: survey of current guidance for child health clinical trials. The star child health project: standards for research with children. Available at http://www.who.int/childmedicines/publications/GUIDANCECHILDHEALTH.pdf (last accessed 5 August 2013)
- Hartling L, Wittmeier KDM, Caldwell P, van der Lee H, Klassen TP, Craig JC, Offringa M the StaR Child Health Group. StaR child health: developing evidence-based guidance for the design, conduct, and reporting of pediatric trials. Pediatrics. 2012;129(Suppl. 3):S112–117. doi: 10.1542/peds.2012-0055C. [DOI] [PubMed] [Google Scholar]
- International Pediatric Association. IPA Statement on equity and quality healthcare for children of the world. 2006. Fifty-seventh Session of the WHO Regional Committee for the Western Pacific Auckland, New Zealand, 18–22nd September. Agenda Item 7: Report of the Regional Director. Available at http://www.ipa-world.org/uploadedbyfck/WSO_0001.pdf (last accessed 3 August 2013)
- National Institutes of Health. NIH policy and guidelines on the inclusion of children as participants in Research involving human subjects. 1998. 6 March. Available at http://grants.nih.gov/grants/guide/notice-files/not98-024.html (last accessed 10 August 2013)
- National Institute of Health (NIH) and Child Development (NICHD) Clinical trials & clinical research. Available at http://www.nichd.nih.gov/health/clinicalresearch/ (last accessed 5 July 2013)
- UK Medical Research Council. MRC ethics guide. 2004. Medical research involving children. Available at http://www.mrc.ac.uk/consumption/idcplg?IdcService=GET_FILE&dID=11512&dDocName=MRC002430&allowInterrupt=1 (last accessed 10 August 2013)
- Brooke A. Paediatric clinical research manual. Arch Dis Child. 2006;91:951–952. doi: 10.1136/adc.2005.076141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine S, Dagher RN, Weiss KD, Santana VM. Good clinical practice and the conduct of clinical studies in pediatric oncology. Pediatr Clin North Am. 2008;55:187–209. doi: 10.1016/j.pcl.2007.10.008. xi–xii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]