Abstract
The development of biological agents with specific immunological targets has revolutionized the treatment of a wide variety of paediatric diseases where traditional immunosuppressive agents have been partly ineffective or intolerable. The increasing requirement for pharmaceutical companies to undertake paediatric studies has provided impetus for studies of biologics in children. The assessment of biological agents in children to date has largely relied upon randomized controlled trials using a withdrawal design, rather than a parallel study design. This approach has been largely used due to ethical concerns, including use of placebo treatments in children with active chronic disease, and justified on the basis that treatments have usually already undergone robust assessment in related adult conditions. However, this study design limits the reliability of the data and can confuse the interpretation of safety results. Careful ongoing monitoring of safety and efficacy in real-world practice through national and international biologics registries and robust reporting systems is crucial. The most commonly used biological agents in children target tumour necrosis factor-α, interleukin-1, interleukin-6 and cytotoxic lymphocyte-associated antigen-4. These agents are most frequently used in paediatric rheumatic diseases. This review discusses the development and assessment of biologics within paediatric rheumatology with reference to the lessons learned from use in other subspecialties.
Keywords: adverse drug reactions, biologics, clinical trials, paediatric rheumatology
Introduction
Biological treatments are defined as ‘a pharmacological group of specific proteins with high molecular weight, specifically targeting pro-inflammatory cytokines or cell surface antigens’ 1. Their mechanism of action contrasts to traditional immunosuppressives and disease-modifying anti-rheumatic drugs (DMARDs) like methotrexate, which inhibit the overall inflammatory process. The identification of the role of the pro-inflammatory cytokines tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) in experimental arthropathy models and human disease has been critical to the development of specific biological treatments 2,3. Evolving knowledge of the nature of such cytokines through preclinical studies of the immunobiology of synovial fluid harvested from patients with active disease and the development of recombinant genetic techniques have facilitated production of humanized monoclonal antibodies and soluble cytokine receptors. These are able to almost completely eliminate or block target pro-inflammatory cytokines. Biologics may also block particular cell-to-cell interactions and, consequently, inhibit cellular activation or deplete specific cell types from the circulation 4,5.
Important changes in the expectations of patients, families and healthcare professionals have contributed towards a significant and continued motivation for developing and improving clinical outcomes using biologics in children. Key drivers for development of biologics include the following: recognition of the need for true disease suppression for the prevention of joint damage in rheumatoid arthritis (RA) and identification of the critical role of TNF-α in juvenile idiopathic arthritis (JIA) 6,7; growing expectations of higher standards of sustained clinical improvement; and the goal of striving for complete disease remission, both on medication and, more importantly, after cessation of treatment, with an ultimate goal of disease cure.
Assessment tools used in clinical trials in juvenile idiopathic arthritis
Table 1 summarizes how clinical response is assessed and defined in JIA clinical trials. The magnitude of the treatment response is generally defined in terms of attainment of the American College of Rheumatology (ACR) Paediatric 30, 50, 70, 90 (PedACR30, 50, 70, 90) response, which assesses the percentage improvement in three of the six JIA core set measures. ‘Flare’ is defined in terms of worsening of these core set measures 8. The proportion of patients achieving ‘inactive disease’ or ‘clinical remission’ is also reported 8,9.
Table 1.
| Criteria | Components | Definitions of end-points used |
|---|---|---|
| PedACR criteria for determining the magnitude of the treatment response in juvenile idiopathic arthritis | Core set measures:
|
|
| Inactive disease |
|
|
| Clinical remission |
|
|
Abbreviation is as follows: PedACR, paediatric American College of Rheumatology criteria.
Design of clinical trials in paediatric rheumatology
To date, many trials of biologics within paediatric rheumatology have used the ‘withdrawal design’ (see Figure 1). All trial participants are initially exposed to the drug. Those who reach a predefined response are randomized to treatment with active drug or placebo, with the trial primary outcome being a predefined disease flare 10 (see Table 1). Efficacy is defined by the difference in the number of patients flaring and the time taken to flare. The rationale for this trial design is that all patients receive the active drug, that a smaller sample size can be used and that the time on placebo is minimized, making it more acceptable to parents and many clinicians. Such biologic treatments have generally already been shown to be efficacious in robust parallel-design randomized control trials (RCTs) in related adult conditions (e.g. RA), providing a priori proof of efficacy in children. However, in some diseases there is arguably inadequate overlap between the pathogenesis of adult-onset diseases for these data to be extrapolated directly to paediatric diseases.
Figure 1.
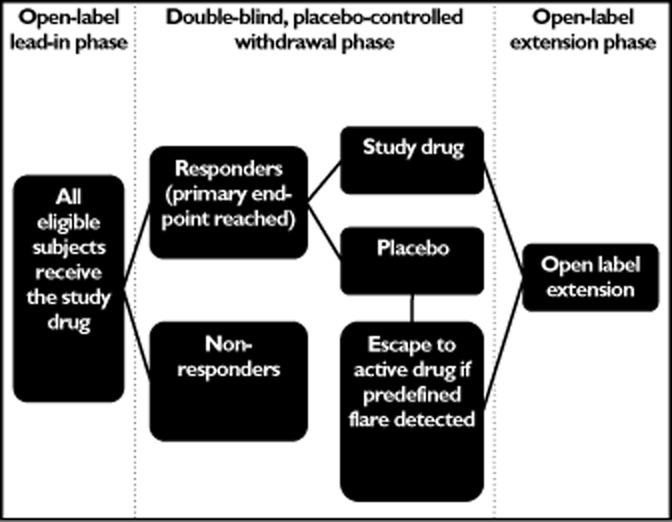
Diagram outlining the withdrawal study design frequently used in clinical trials of biologics in children
The safety profile of the study drug can be difficult to assess when a withdrawal study design is used, owing to potential for a ‘carry-over’ effect during the placebo phase. The relatively short time on placebo treatment also limits the power to compare the safety profile of the active drug with placebo 11. Therefore, although such RCTs have shown important efficacy of biologics in paediatric disease, there is critical need for long-term, open-label studies and registries to determine the long-term safety profile and efficacy of biologic drugs. The withdrawal study design may preselect responders who continue to retain their response throughout all phases of the study, due to the placebo effect 11,12. Inclusion of physicians' and parents' global assessments of disease activity as part of the core set measures detailed in Table 1 is also susceptible to the inadvertent placebo response, whereby the child and parent may report improvement because they are keen to believe that there has been a positive response 13,14.
Overview of biologic treatments and their uses
Anti-tumour necrosis factor-α treatments
Etanercept
Identification and efficacy
Etanercept is a fully human, soluble fusion protein that binds to TNF-α with high affinity, preventing binding to cell surface TNF receptors 15. Etanercept was the first biologic treatment to be used in JIA, following a multicentre, randomized, placebo-controlled trial in 69 methotrexate-resistant JIA patients with a polyarticular disease course 16. At the end of the initial open-label study, 74% responded to etanercept treatment, achieving a PedACR30 response. In the double-blind phase, 81% of patients on placebo flared, compared with 28% of etanercept-treated patients (P = 0.003). The median time to disease flare was 28 days on placebo compared with 116 days with etanercept (P < 0.001) 16. Those flaring on placebo were restarted on etanercept. The response to etanercept improved over 2 years during the open-label extension phase, with 63% of patients achieving clinical remission 17.
Etanercept efficacy noticeably varied according to JIA subtype. Children with systemic-onset JIA (SoJIA) generally responded less favourably than other JIA subtypes 17–19. The ongoing multicentre ‘CLinical Study In Paediatric Patients of Etanercept for Treatment of enthesitis related arthritis (ERA), psoriatic arthritis (PsA) and extended oligoarthritis’ study (CLIPPER) demonstrated etanercept to be effective and well tolerated in patients with these specific subtypes of JIA, with an overall PedACR 50, 70, 90 response and inactive disease being achieved in 81, 62, 30 and 12%, respectively 20.
Longer-term outcomes on etanercept are generally favourable. The German biologics registry has shown that males with a shorter duration of disease, a lower active joint count and lower childhood health assessment questionnaire (CHAQ) disability score at baseline are more likely to achieve inactive disease and remission on etanercept over a mean of 4.6 years 21. Likewise, the Dutch biologics register has shown a lower CHAQ score at baseline, less DMARD failures prior to starting etanercept and a younger age at onset also to be predictive of achieving an excellent response to etanercept, 15 months after initiation of treatment 22. Co-administration of methotrexate raises the chance of remission, especially in patients with rheumatoid factor-negative polyarthritis (odds ratio 2.0, P = 0.03) 21.
Etanercept is currently licensed by the European Medicines Agency (EMA) for the treatment of polyarticular JIA in children >4 years old who have had an inadequate response to methotrexate or who are intolerant 23. In the UK, the National Institute for Clinical Excellence (NICE) guidelines from 2002 recommend use of etanercept in children with an active polyarticular course of JIA, widening its use to patients with extended oligoarthritis, psoriatic arthritis, enthesitis-related and systemic-onset arthritis. These guidelines are due for review in 2015 24.
Long-term efficacy and safety
Long-term open-label follow-up of the original etanercept trial over 8 years showed a good safety profile and a durable response, with sustained reductions in disease activity 17,25,26. To date, some 2896 cumulative patient-years of safety data have been published from 1273 patients, who have individually received a maximum of 6.8 years of etanercept (see Table 2) 18,19,26–29. Both the Dutch and the German national biologics registries have reported a decrease in the rate of adverse events (AEs) over time 19,27, from 0.20 AEs per patient during the first year of treatment, reducing to 0.12 AEs per patient per year thereafter 27. The Italian JIA cohort reported a higher rate of AEs over a mean of 24.1 months of follow-up (0.51 AEs per patient per year) 29. The main AEs associated with etanercept use are shown in Table 3.
Table 2.
Adverse events related to different anti-tumour necrosis factor agents used in juvenile idiopathic arthritis (data mainly from long-term studies and biologics registries)
| References | Etanercept | Adalimumab | Infliximab | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 18 | 26 | 29 | 19 | 28 | 27 | 44* | 29 | 72 | |
| No. of patients | 61 | 58 | 95 | 146 | 397 | 604 | 171 | 68 | 78 |
| Median/mean exposure (years) | 1.1 | NA | 2.0 | 2.5 | NA | NA | NA | 1.8 | 2.2 |
| Maximal exposure (years) | 2.5 | 8 | 5.9 | 7.3 | 3 | NA | 2 | 6.1 | 3.9 |
| Cumulative patient-years of exposure | NA | 318 | 258 | 312 | 859 | 1149 | 319.3 | 140 | NA |
| No. of adverse events | 68 | NA | 133 | 65 | 179 | 190 | 2549 | 71 | 71 |
| No. of serious adverse events | NA | 39 | NA | 9 | NA† | 52 | 17 | NA | 17 |
| Anaphylactoid/serious drug reactions (%) | 0 | 0 | NA | 0 | 0 | 0 | 0 | 1.5 | 3.6 |
| Serious infections (%) | NA | 15.5 | 3.2 | 2.7 | 2.8 | 4.3 | 4.1 | 1.5 | 2.6 |
| Opportunistic infections (%) | NA | 0 | 0 | 0 | NA | 0.2 | 0 | 0 | 0 |
| New auto-antibody development (%) | 20.6 | NA | 2.1 | NA | 3.8 | NA | NA | 10.3 | 32.5 |
| New/worsening uveitis (%) | 2.9 | 0 | 4.2 | 0 | 0.3 | 1.5 | 0 | 4.2 | 5.0 |
| Demyelinating events (%) | 1.5 | 0 | 0 | 0 | 0 | 0.3 | 0 | 0 | 0 |
| Malignancies (%) | 0 | 0 | 1‡ | 0 | 0 | 0.5§ | 0 | 0 | 0 |
| Deaths | 0 | 0 | 0 | 0¶ | 0 | 0 | 0 | 0 | 2.6** |
Abbreviation: NA, not applicable.
Safety data available from the original clinical trial to date.
This study compared patients on methotrexate, etanercept and etanercept plus methotrexate, and found the exposure-adjusted rates of serious adverse events per 100 patient-years to be 4.6, 7.1 and 6.0, respectively, but the study did not provide the absolute number of serious adverse events.
One case of malignancy (thyroid cancer).
Three cases of malignancy (thyroid carcinoma, yolk-sac carcinoma, non-Hodgkin's lymphoma).
Three nonresponders to etanercept subsequently died of tuberculosis, suspected macrophage activation syndrome and sepsis whilst on other immunosuppressives, at least 8 months after etanercept discontinuation.
The first died at week 2 of the trial, 10 days after a placebo infusion, from septic shock and an associated deterioration in cardiac function; the second patient had systemic-onset juvenile idiopathic arthritis, experienced a severe flare of their disease and died of a cardiac arrest 3 months after discontinuation of infliximab (in the 3 mg kg−1 infliximab group), whilst in the open-label extension phase of the study.
Table 3.
Main adverse events reported in association with anti-tumour necrosis factor use in children with rheumatic diseases 17–19, 26, 28–30, 46, 70, 123–127
| AE category | Generic anti-TNF AEs | Etanercept | Adalimumab | Infliximab |
|---|---|---|---|---|
| Musculoskeletal | Arthritis flare, arthralgia | MAS, sarcoidosis, aseptic necrosis (femoral head), vasculitic rash, osteoporosis | Elevated CPK, myositis, back pain, muscle spasms | MAS |
| Haematological | – | Pancytopenia, lymphadenopathy, epistaxis | Neutropenia, leucopenia | – |
| Gastrointestinal and/or renal | Vomiting | CD, UC, aspecific bowel inflammation, biliary calculosis, nausea, abdominal pain, weight loss or gain, anorexia, oral apthosis, proctorrhagia | Increased ALT and AST, appendicitis, abdominal pain, gastroduodenitis, gastrointestinal bleeding | Persistent macroscopic haematuria |
| Cardiorespiratory | Hypertension | Cough, chest pain, asthma, tachycardia, extrasystolia | Chest pain | Cough |
| Infectious | Upper respiratory tract infections, pneumonia, CMV, gastrointestinal infection, soft tissue infections | Urosepsis, pyelonephritis, meningoencephalitis, appendicular abscess, prosthetic hip infection, EBV, recurrent urinary tract infections, dental infections, otitis | Tuberculosis, herpes zoster, herpes simplex, streptococcal infection, urinary tract infection, pharyngitis, Clostridum difficile | Tuberculosis, candidiasis (vaginal and oral), herpes zoster, varicella zoster, viral thyroiditis, histoplasmosis, pharyngitis, bronchiolitis |
| Autoimmune and/or immunological | Injection-site or infusion reactions | New onset of JSLE, Takayasu's arteritis, dermatomyositis, DM, alopecia, new auto-antibodies, autoimmune hepatitis | Lupus-like syndrome, injection-site reaction, new antibodies to ADA, DM | Infusion reactions*, newly induced auto-antibodies, development of antibodies to infliximab |
| Ophthalmological | – | Uveitis, blurred vision | Keratitis | Uveitis |
| Neurological | Headache | Demyelinating retrobulbar neuritis, neuropathy, epileptic insult, hearing loss, vertigo, dysaesthesia, insomnia | ‘Sporadic seizure’, dizziness, syncope | Irritability, paraesthesia |
| Psychiatric | – | Depression, personality disorder, emotional lability, panic attacks, anxiety, agitation, concentration disorder, hallucinations, aggression | – | Psychoses, depression, panic attacks, anxiety, hyperactivity |
| Oncological | – | Lymphomas, thyroid carcinoma, yolk-sac tumour | Lymphomas | – |
| Gynaecological | – | Ovarian cyst, endometriosis, irregular/painful menses | Menorrhagia | – |
| Dermatological | New-onset psoriasis | Skin ulcer, hair loss, pruritic/urticarial rash, pityriasis, onychodystrophy, coccygeal cyst, eczema | Granuloma annulare, excoriation, purpura | Urticaria |
| Deaths | – | – | Four deaths† | Two deaths‡ |
Abbreviations are as follows: ADA, adalimumab; AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CD, Crohn's disease; CMV, cytomegalovirus; CPK, creatinine phosphokinase; DM, diabetes mellitus; EBV, Epstein–Barr virus; JSLE, juvenile-onset systemic lupus erythematosus; MAS, macrophage activation syndrome; TNF, tumour necrosis factor; UC, ulcerative colitis.
Reported infusion reactions to infliximab include anaphylaxis, vomiting, fever, headache, hypotension, abdominal pain, coughing, face oedema, rash, urticaria, chills, fatigue, sleepiness and insomnia.
Described by the FDA US Healthcare AE Reporting System.
Deaths described in Table 2.
Cases of new-onset/worsening uveitis (see Table 3) 18,28–30 have led to avoidance of etanercept if uveitis is present (see adalimumab section below). Notably, no significant adverse events (SAEs) were reported from a French cohort where etanercept was administered weekly (dose 0.8 mg kg−1 week−1) 18, and further studies have demonstrated comparable safety/efficacy of weekly and twice-weekly etanercept administration 30–32. Cases of pulmonary and extrapulmonary Mycobacterium tuberculosis infection have been reported in children with JIA on anti-TNF treatment, and vigilance for the condition must continue to be maintained during treatment, despite negative pretreatment screening 33,34.
Conflicting results exist regarding the relative safety of treating with etanacept and methotrexate in combination. Data from the USA, Canada and Germany have mainly reported a similar rate of AEs in children with JIA receiving etanercept alone or in combination 27,28, although a trend was seen towards a higher number of SAEs in those on combination treatment in the German registry (P = 0.06) 27. Preliminary data from the UK biologics registry have shown nearly twice as many AEs in patients on combination etanercept and methotrexate treatment 35.
Further uses of etanercept
Etanercept use has been reported in children with the following conditions: Behcet's disease 36, Familial Mediterranean Fever (FMF) 37, tumour necrosis factor receptor-associated periodic syndrome (TRAPS) 38, Kawasaki disease 39, cutaneous granulomas, common variable immunodeficiency and idiopathic pneumonia syndrome following allogeneic haematopoietic stem cell transplantation 40. In Crohn's disease (CD), it is avoided due to reports of triggering of inflammatory bowel disease (IBD) 41. Clinical trial data support its use in severe paediatric plaque psoriasis 42; case reports indicate that etanercept may help in other types of psoriasis 43.
Adalimumab
Identification and efficacy
Adalimumab is a humanized monoclonal anti-TNF antibody, administered to JIA patients by subcutaneous injection fortnightly. In RA, concomitant use of methotrexate prolongs its half-life. The clinical trial of adalimumab in JIA compared the efficacy of monotherapy or combination therapy using a withdrawal trial design. In the open-label lead-in phase (first 16 weeks), 171 JIA patients aged between 4 and 17 years with a polyarticular disease course were treated with 24 mg m−2 of adalimumab on alternate weeks; 84 of 171 continued with previous methotrexate treatment. The PedACR 30, 50, 70 and 90% response was achieved in 74, 64, 46 and 26% of children receiving adalimumab monotherapy compared with 94, 91, 71 and 28% receiving combination therapy, respectively. During the double-blind phase of the study, flares were significantly more frequent in those treated with methotrexate and placebo or placebo alone 44. A preliminary open-label study including children <4 years of age or <15 kg has shown a similar improvement in PedACR criteria, and a comparable safety profile to that for older children 45. In Europe, adalimumab is licensed for use in active polyarticular JIA (in combination with methotrexate or alone if methotrexate is inappropriate) and in children with enthesitis-related arthritis who have failed to respond to one or more DMARD 23. NICE guidelines relating to adalimumab use in children are not yet available 24.
Long-term efficacy and safety
Fewer data are available regarding the long-term safety and efficacy of adalimumab (see Tables 2 and 3). During the 2 year RCT open-label extension study, dosing of adalimumab changed, with patients weighing <30 kg receiving 20 mg and patients weighing ≥30 kg receiving 40 mg. Adalimumab showed ongoing efficacy, with sustained PedACR responses and no change in tolerability. Forty per cent of patients were in remission by the end of the open-label extension 44.
Cumulative safety data on 398 patient-years of adalimumab exposure have been published from 171 patients, with 103 receiving adalimumab for >3 years 44. The safety profile of adalimumab in children appears similar to that for adults. In 2009, the US Food and Drugs Administration (FDA) collated adalimumab safety data from the US Healthcare AE Reporting System, including 108 SAEs occurring following in utero exposure or in paediatric patients with JIA, CD, ulcerative colitis (UC), uveitis, psoriasis, PsA and vasculitis. Four deaths occurred, three involving in utero exposure; the fourth was of a 16 year old who developed macrophage activation syndrome (MAS), pneumonia and respiratory failure. There were 24 reports of serious infections and two cases of malignancy. Both patients had a ∼10 year history of using multiple immunosuppressants, making it difficult to establish direct causality 46.
Further uses of adalimumab
A recent qualitative study indicated that uveitis is the most important factor impacting on the clinician's choice between adalimumab and etanercept, in light of evidence that etanercept can contribute to worsening or new development of uveitis (see etanercept section above) 19,30,47. Two retrospective observational open-label studies have explored adalimumab use in JIA-associated uveitis. One demonstrated adalimumab to be effective in 16 of 18 patients 48. The other showed seven of 20 (35%), of children to have a reduction in uveitis activity, one of 20 (5%) had worsening activity, and 12 of 20 (60%) had no change 49. Adalimumab and infliximab have been compared in an open-label, prospective, multicentre study including 16 children with JIA-associated uveitis, three with idiopathic uveitis and one with Behcet's disease. No significant difference was noted between the treatments, although a higher probability of uveitis remission was seen with adalimumab 50. A UK-based multicentre RCT of the clinical effectiveness, safety and cost effectiveness of adalimumab in combination with methotrexate vs. methotrexate alone for the treatment of JIA-associated uveitis (SYCAMORE) is underway 51.
In adult CD, adalimumab is effective in the induction and maintenance of remission, reducing hospital admissions and related surgery 52–55. Paediatric studies are limited to open-label prospective studies, retrospective analyses and case series 56–60. The IMAgINE 1 phase III RCT investigated safety and dosing of adalimumab treatment in 191 children with moderate-to-severe CD 61. Following 4 weeks of induction treatment, patients were randomized to receive low- or high-dose adalimumab. Thirty-six per cent were in remission by 22 weeks, with no difference between dosages (P = 0.075). The safety profile was comparable to adult CD studies 61.
Adalimumab is also used in paediatric psoriasis after failure of other systemic and biological agents 62–64, and an ongoing RCT is testing its efficacy and safety in paediatric chronic plaque psoriasis 65. Adalimumab is both effective and well tolerated in juvenile-onset ankylosing spondylitis 66. Patients with primary systemic vasculitis 67 and Behcet's have also received adalimumab 68,69.
Infliximab
Identification and efficacy
Infliximab is a chimeric murine–human monoclonal antibody with high affinity for TNF-α, administered by intravenous infusion at 0, 2 and 4 weeks, with subsequent doses being administered at 4–8 week intervals. A double-blind RCT including 122 children with polyarticular JIA failed to demonstrate superiority of infliximab over placebo. In that study, 3 mg kg−1 of infliximab or placebo was given for 14 weeks in combination with methotrexate. A higher proportion of patients receiving infliximab reached a PedACR30 response, but the difference between the treatment groups was not statistically significant (P = 0.12). Patients on placebo then went on to receive high-dose infliximab (6 mg kg−1). By week 52, a PedACR50 and 70 response was achieved in 70 and 52% of patients overall, with no difference depending on the infliximab dosage 70. Despite these results, infliximab is frequently used in refractory polyarticular JIA (unlicensed indication), with good clinical results reported.
Long-term efficacy and safety
In the RCT discussed above, the 3 mg kg−1 per dose infliximab group had double the number of SAEs and a higher rate of infusion reactions and anaphylactic reactions than the 6 mg kg−1 per dose group, correlating with an increased incidence of anti-infliximab antibodies. New antinuclear and double-stranded DNA antibodies were also seen more frequently in those on low-dose infliximab. The optimal dosage warrants further investigation, because some have reported doses as high as 10–20 mg kg−1 per dose as being rapidly effective and well tolerated 71.
During the open-label extension phase of the study discussed above (weeks 52–204), 42 of 78 patients discontinued infliximab due to consent withdrawal, lack of efficacy or patient/physician/sponsor requirements. By week 204, the proportion of patients with PedACR30, 50, 70, 90 responses or inactive disease was 44, 40, 33, 24 and 13%, respectively. Infusion reactions occurred in 32% of patients, with a higher incidence in patients positive for infliximab antibodies 72. Other studies have also looked at infliximab treatment in paediatric rheumatology practice (see Tables 2 and 3).
Further uses of infliximab
Infliximab is effective as induction 73–75 and maintenance treatment in children with CD 61,75,76, when used regularly 77. US FDA approval for use in paediatric CD was obtained following the randomized, multicentre, open-label REACH study in children with moderate-to-severe CD 75. Of the 112 patients treated, 58% achieved clinical remission following a 5 mg kg−1 induction dose. Longitudinal follow-up of patients on maintenance infliximab showed 56 and 33% still to be in remission at 54 weeks and 3 years, respectively 75,76. This secondary loss of response associated with anti-infliximab antibodies may be reduced by giving infliximab regularly and with a concomitant immunosuppressive 78. Infliximab treatment in CD is also associated with reduced rates of hospitalization and surgery for complications of active disease 79,80 and improvements in growth 81. In 2010, NICE recommended that infliximab is used for the treatment of severe active CD not responding to conventional treatment, or where conventional treatment cannot be used due to intolerance or contraindications 24.
Certolizumab pegol and golimumab
Certolizumab is a PEGylated Fab fragment of a humanized anti-TNF-α antibody, and golimumab is a humanized monoclonal anti-TNF-α antibody. These newer TNF antagonists have been shown to be effective in the treatment of RA, PsA, AS, UC and CD in adults 82–87. Certolizumab does not possess an Fc region and therefore should not lead to cell-mediated cytotoxicity, decreasing the infection risk; however, this does not seem to have translated into a clear reduction in infection risk in clinical practice. It may also be of use in pregnancy, because the lack of an Fc region will prevent transplacental transfer 87. Golimumab is similar to adalimumb with respect to its molecular weight and its affinity for soluble and transmembrane TNF; however, golimumab has a longer half-life and can be administered monthly 4. Currently, there are ongoing multicentre trials looking at the efficacy and safety on golimumab and certolizumab in JIA 88,89. Certolizumab pegol and golimumab are not currently licensed for use in children 23.
Agents targeting interleukin-1
Anakinra
Identification and efficacy
Anakinra is an interleukin-1 (IL-1) receptor antagonist administered by daily subcutaneous injection at a dose of 1–2 mg kg−1 day−1. It has not demonstrated a significant benefit over placebo in polyarticular JIA 90, but in a multicentre RCT of anakinra in SoJIA, eight of 12 patients on anakinra and one of 12 on placebo achieved a PedACR30 response. Nine of 10 of the placebo-treated patients who were switched to anakinra subsequently responded. The tolerability of anakinra was comparable to placebo 91.
Use of anakinra as part of the initial therapeutic strategy in SoJIA has been assessed in a multicentre case series including 46 patients. Anakinra was used as monotherapy in 28%, with 67% and 33% also receiving corticosteroids and additional DMARDs, respectively. Fever and rash resolved within 1 month in >95%, and in 80%, c-reactive protein and ferritin also normalized over this time period. Persistence of arthritis was seen in 39, 27 and 11% after 1, 3 and >6 months of treatment, respectively. Inactive disease was achieved in eight of 10 patients receiving anakinra monotherapy 92. In another study, including patients who had previously received long-term corticosteroids with or without DMARDs, the clinical response to anakinra was more heterogeneous. The majority of patients experienced an initial amelioration of systemic features and acute-phase reactants, but subsequently, one group displayed ongoing disease remission and the other showed a tendency towards recurrence 93.
Long-term efficacy and safety
Studies looking at the efficacy and safety of anakinra beyond 1 year are warranted. The main AEs reported in association with IL-1 blocker use are shown in Table 4. Injection site reactions were the most common AE, decreasing over time 90,94,95.
Table 4.
Adverse events reported in association with interleukin-1 receptor blockers
| Adverse event categories | Examples of adverse events reported during use of interleukin-1 receptor blockers | ||
|---|---|---|---|
| Anakinra 90,94,95 | Rilonacept 97 | Cannakinumab 97,99,101 | |
| Musculoskeletal | Arthritis flare, arthralgia, limb pain, osteonecrosis of the femoral head, vertebral collapse, MAS | Arthritis flare, MAS | Arthritis flare, MAS, leg fracture |
| Haematological | Transient neutropenia, anaemia | Pancytopenia, anaemia | Leucopenia, thrombocytopenia, prolonged activated partial thromboplastin time |
| Gastrointestinal | Diarrhoea, nausea, abdominal pain, vomiting, elevated alanine aminotransferase, new-onset CD | – | Abdominal pain, vomiting, pneumonia, aminotransferase elevations |
| Cardiorespiratory | Cough, sore throat | Pulmonary fibrosis | Cough |
| Infectious | Hepatitis due to CMV, URTI, visceral Leishmania infection, varicella, rhinopharayngitis, labial herpes, uncomplicated hepatitis A | URTI, gastroenteritis, nasopharyngitis, skin infection, ear infection, influenza | Nasopharyngitis, URTI, varicella, otitis media, urosepsis, measles, pneumonia, Epstein–Barr virus |
| Immunological and/or autoimmune | Local injection-site reactions (ecchymosis, erythema, inflammation, pain, pruritis), anti-IL-1 receptor antibody development | Local injection-site reactions (erythema, bruising), development of anti-rilonacept antibodies | Non-neutralizing anti-canakinumab antibody development |
| Ophthalmological | – | – | Uveitis |
| Neurological | Headache | – | Headache, syncope |
| Renal and/or urological | Dysuria, nephrosis | – | – |
| Malignancy | None reported | None reported | None reported |
| Psychiatric | – | Depression | – |
| Dermatological | Rash | – | Rash |
| Other | Fever, whole-body pain | Fever | Fever, arm pain, lymphadenopathy, splenic cyst, haematoma, two deaths* |
Abbreviations are as follows: CD, Crohn's disease; CMV, cytomegalovirus; IL-1, interleukin-1; MAS, macrophage activation syndrome; URTI, upper respiratory tract infection.
One patient had urosepsis and MAS whilst on placebo (following eight doses of canakinumab); the second patient died of MAS and severe pulmonary hypertension whilst on canakinumab.
Further uses of anakinra
Anakinra is licensed for use in cryopyrin-associated periodic syndrome (CAPS), with dramatic amelioration of clinical characteristics. It has also been shown to be of benefit in deficiency of the interleukin-1-receptor antagonist (DIRA), nod-like receptor protein-12 (NLRP-12)-associated periodic fever syndrome, FMF and TRAPS. A variable treatment response has been found in Blau's syndrome, pyogenic sterile arthritis and pyoderma gangrenosum and acne syndrome (PAPA) 96.
Rilonacept
Rilonacept is a fusion protein, which acts as a long-acting soluble IL-1 receptor, with a longer half-life than anakinra. In an RCT, 24 SoJIA patients were treated with weekly rilonacept (2.2–4.4 mg kg−1, maximal dose 360 mg) or placebo for 4 weeks. Twenty-three of 24 patients subsequently entered an open-label trial lasting up to 24 months. There was no significant difference in efficacy between rilonacept and placebo during the initial double-blind phase. Within 3 months, fever and rash resolved in all patients, and a PedACR30, 50, 70 response of 78.3, 60.9 and 34.8%, respectively, was seen and subsequently maintained. All patients developed an AE (see Table 4), with 13% developing an SAE and discontinuing treatment 97. Rilonacept has also been used in CAPS and FMF 96. Rilonacept is not currently licensed for use in children 23.
Canakinumab
Canakinumab is a fully human monoclonal anti-IL-1β antibody with a long half-life, given monthly by subcutaneous injection. In a double-blind study, a PedACR30 response of 84% was seen at 15 days post-treatment in SoJIA patients who received a single dose of canakinumab and 10% who received placebo (P < 0.001) 98. Patients who achieved greater than a PedACR30 response were enrolled into a phase III trial with a two-part withdrawal design. All patients initially received canakinumab, and corticosteroid tapering was attempted on open-label treatment. Forty-five per cent were able to reduce their corticosteroid dosage by at least 50%, and 33% discontinued steroids all together. In the placebo-controlled phase, 75% on placebo flared, in comparison to 26% in the canakinumab group (relative risk reduction of 64%, hazard ratio 0.36; 95% confidence interval 0.17–0.75) 99. Canakinumab was initially licensed for treatment of CAPS, but its license has been extended by the US FDA and EMA to include the treatment of SoJIA patients ≥2 years old 23,100. NICE guidance is not yet available.
Safety
In the placebo-controlled phase of the study detailed above, one patient developed MAS and a serious infection in each treatment group. Seven patients developed serious infections during the open-label treatment phase (two associated with MAS). There were two deaths associated with cannakinumab treatment, but no reports of cancer, tuberculosis or opportunistic infection (see Table 4) 99,101.
Further uses of canakinumab
Canakinumab has been shown to be effective in CAPS. There is an ongoing clinical trial assessing use in TRAPS and anecdotal reports of use in FMF 96.
Tocilizumab
Identification and efficacy
Tocilizumab is a humanized monoclonal antibody that targets the IL-6 receptor, preventing IL-6 from exerting pro-inflammatory effects. Serum and synovial IL-6 levels are elevated in SoJIA and correlate with disease activity, decreasing with effective treatment 102,103. The first phase III RCT of tocilizumab in SoJIA used a withdrawal study design. Fifty-six Japanese SoJIA patients were initially treated with 8 mg kg−1 of tocilizumab, 2 weekly over 6 weeks, with responders subsequently being randomized to tocilizumab or placebo for 12 weeks. A PedACR30, 50, 70 response was achieved in 91, 86 and 68%, respectively. Flares were more common in the placebo group (83 vs. 20%, P < 0.0001). By 48 weeks, in the open-label extension, PedACR30, 50, 70 responses were achieved in 98, 94 and 90%, respectively 104.
In a multinational, phase III, 5 year, double-blind RCT (TENDER trial), significantly more patients on tocilizumab than control subjects achieved a PedACR30, 50, 70 by week 12 of treatment (85, 71 and 37% vs. 24, 8 and 5%, respectively; P < 0.0001). A progressive improvement in treatment response was observed during the 52 week open-label extension, with 59% reaching an ACR90 response and 28% attaining clinically inactive disease 105. Tocilizumab was approved by the US FDA and NICE in 2011, for the treatment of children >2 years old with SoJIA who have not responded adequately to nonsteroidal anti-inflammatory drugs, corticosteroids and methotrexate 24,100.
Safety
The range of AEs associated with tocilizumab is summarized in Table 5. In the Japanese tocilizumab phase III trial mentioned above, 8.9% of patients developed anti-tocilizumab antibodies and mild-to-moderate infusion reactions, leading to discontinuation of tocilizumab in most patients 106. Within the TENDER trial, infusion reactions occurred in 16% on tocilizumab and 5% on placebo 105.
Table 5.
| Adverse event categories | Tocilizumab | Abatacept |
|---|---|---|
| Musculoskeletal | Arthritis flare, fracture, septic arthritis, hip dislocation MAS | Arthritis flare, arthralgia, foot deformity |
| Haematological | Neutropenia, leucopenia | – |
| Gastrointestinal | Gastrointestinal bleeding, increase in transaminases, diarrhoea | Vomiting |
| Cardiorespiratory | Pneumothorax, cardiac failure, pulmonary veno-occlusive disease | – |
| Infectious | Infectious mononucleosis, nasopharyngitis, URTI, gastroenteritis, varicella/herpes zoster infections, pneumonia | Nasopharyngitis, URTI, dengue fever, erysipelas, gastroenteritis, herpes zoster, bacterial meningitis, pyelonephritis, varicella infection |
| Opthalmological | – | Uveitis |
| Immunological and/or autoimmune | Anaphylactic reaction, mild-to-moderate infusion reactions, anti-tocilizumab antibodies, angioedema, urticaria | Anti-abatacept antibodies, multiple sclerosis*, acute infusion reactions† |
| Neurological | Headaches | – |
| Dermatological | Chronic panniculitis | – |
| Oncological | – | Acute lymphoblastic leukaemia‡, benign neoplasms§ |
| Other | Elevated total cholesterol, testicular torsion | Pyrexia, ovarian cyst |
Abbreviation is as follows: MAS, macrophage activation syndrome, URTI, upper respiratory tract infection.
Developed after 19 months of abatacept treatment.
Dizziness, nausea, vomiting, headache, hypersensitivity, rhinitis.
Diagnosed at day 89 of the open-label lead-in phase and thought to have been initially misdiagnosed as juvenile idiopathic arthritis.
Four benign neoplasms were reported but no malignancies.
Further uses of tocilizumab
Preliminary results from the global, phase III, placebo-controlled CHERISH trial of tocilizumab in polyarticular JIA 107 have revealed a significantly higher PedACR30, 50, 70 response with tocilizumab than placebo, with 65% attaining an ACR70 response by week 40. Forty-eight per cent of patients on placebo and 26% on tocilizumab flared within this time period (P = 0.0024) 108. An open-label study is ongoing.
Abatacept
Identification and efficacy
Cytotoxic lymphocyte-associated antigen-4 (CTLA-4) is a potent inhibitor of the co-stimulatory pathway that is necessary to activate T cells. Abatacept is a fully human soluble fusion protein that is composed of a modified Fc portion of IgG1, linked to CTLA-4. It binds to CD80/CD86 on antigen presenting cells, inhibiting its interaction with CD28 on T cells, thereby inhibiting T cell co-stimulation and activation. In a phase III, multinational, double-blind RCT in polyarticular JIA patients using a withdrawal design, 70% of patients responded to abatacept during the open-label lead-in phase. Subsequently, flares occurred in 53% of patients receiving placebo and in 20% of abatacept patients (P = 0.0003), with the median time to flare being shorter in those on placebo. PedACR30, 50, 70 and 90 responses in the abatacept and placebo group were 82, 77, 53 and 40% and 69, 52, 39 and 16%, respectively 109.
The majority of patients (153 of 190) subsequently entered an open-label extension study, for a median of 35 months (range 5.5–47.8 months). By day 589, a PedACR30, 50, 70, 90 and 100% response was achieved in 90, 88, 75 and 57% and 39% of patients who had been treated with abatacept during both the double-blind and the extension phases of the study. The response to abatacept was maintained or progressively improved over the duration of the study, with 73% of children who had not reached a PedACR30 response at the end of the lead-in phase subsequently achieving this during the open-label extension 110. The US FDA approved abatacept for use in children >6 years old with moderate to severe JIA of a polyarticular course in 2009, with the EMA also approving it in 2010.
Safety
In the RCT discussed above, the number of AEs was similar across all treatment groups. The AEs reported in association with abatacept are shown in Table 5. No patients randomized to receive abatacept experienced an SAE, whereas two of the patients receiving the placebo developed SAEs (although placebo patients received 4 months of abatacept prior to randomization). Five patients experienced infusion reactions. Anti-abatacept and anti-CTLA-4 antibodies were present in 11% of the 149 patients with samples available, but did not correlate with occurrence of infusion reactions or loss of treatment efficacy 110.
Further uses of abatacept
Abatacept has been assessed in a small retrospective case series of patients with severe anti-TNF-α refractory JIA-associated uveitis. All patients responded within 6 months of treatment, with the frequency of uveitis flares decreasing from a mean of 3.7 episodes to 0.7 episodes per 6 month period 107. Clinical studies in adults are investigating use of abatacept in RA, UC, CD, diabetes mellitus, systemic lupus erythematosus (SLE), graft vs. host disease, uveitis, Takayasu's arteritis, Wegener's granulomatosis, polymyositis, dermatomyositis and sarcoidosis.
Rituximab
Rituximab is a chimeric anti-CD20 monoclonal antibody that binds and causes apoptosis of CD20-positive B cells, leading to their prolonged depletion. It is licensed for use in RA and has shown promising results in children for a variety of off-label indications, including juvenile-onset systemic lupus erythematosus (JSLE), JIA, primary systemic vasculitis, relapsed non-Hodgkin's lymphoma and leukaemia, chronic immune thrombocytopenic purpura, autoimmune haemolytic anaemia, nephrotic syndrome, acute and chronic solid organ transplant rejection and post-transplantation lymphoproliferative disease 111. There are, however, no RCTs of rituximab use in children.
In paediatric rheumatology practice, rituximab is most frequently used in JSLE, despite robust evidence for its efficacy being limited. A retrospective case series of rituximab treatment in 19 JSLE patients with severe general symptoms or acute life/organ-threatening manifestations, unresponsive to standard treatment, demonstrated a rapid reduction in disease activity after two infusions in the majority of patients, with improvements in renal, immunological and haematological parameters and no serious side-effects 112. In a French cohort, 11 children with severe JSLE received two to 12 infusions of rituximab in addition to standard immunosuppressive agents in six of 11 patients. Remission was achieved in eight of 11 patients and maintained over a mean of 13.2 months, but SAEs occurred in 45% 113.
The largest rituximab study in JIA included 55 children with refractory disease (polyarticular and SoJIA patients) who received 4 weekly infusions as necessary. Within 6–8 weeks, there was a decrease in systemic, articular and laboratory disease manifestations, and by 24 weeks, 98, 50 and 40% achieved an ACR 30, 50, 70 response respectively 114. Due to the uncontrolled nature of the study and the range of concomitant medications, the results should be interpreted with caution. In contrast to adults treated with rituximab, children may develop long-standing B cell depletion and hypogammaglobuminaemia requiring intravenous immunoglobulin 115.
Belimumab
Belimumab is a fully human monoclonal antibody, which blocks soluable BLyS, a B cell survival factor, and prevents it from binding to B cell receptors. The US FDA and the EMA approved belimumab use in adults with serologically positive, SLE in 2011 following the outcome of the BLISS trials, which showed that belimumab was associated with a reduction in disease activity, prevented worsening of internal organ involvement and reduced the rate of severe flares over 52 weeks in 1684 patients 116,117. Belimumab is currently being evaluated alongside standard JSLE therapy.
Specific considerations
Biologics and risk of malignancy
In 2009, the US FDA reported an increased risk of lymphoma and other cancers associated with anti-TNF treatment in children and adolescents in light of postmarketing surveillance data. Interpretation of these data is complex due to the potential confounding effects of concomitant immunosuppressives (used in 88% reported upon) and uncertainty regarding the incidence of malignancy in uncontrolled inflammatory diseases 118. A Swedish study looking at cancer risk in biologic-naïve JIA patients (using linkage through national databases and matching general population comparators) found an elevated risk of malignancy in biologic-naïve JIA patients in whom the diagnosis was made during the past 20 years 119. In the US FDA report, 10 cases of hepatosplenic T cell lymphoma were reported in IBD patients; however, the concomitant medications used (6-mercaptopurine and azathioprine) are also independently associated with hepatosplenic T cell lymphoma 120. Likewise, there are case reports of non-Hodgkin's lymphoma in JIA patients treated with methotrexate 121.
Biologics registries
In order to understand the long-term safety profile of biological therapies, it is important to collect data through Registries and to continue data collection into adult years. Clearly, there are significant challenges involved, especially as some SAEs (such as malignancy) are likely to be rare. A summary of the current biologics Registries for children is given in Table 6, and it is hoped that pooling of data will help to address long-term safety issues. There are challenges with the governance and structure of data pooling, but to this end, the international pharmacovigilance databank (Pharmachild) has recently started 122. Whilst uncertainties exist, it is important that experienced specialist teams use biological agents, that patients and parents are aware of the rationale, and that discussions regarding risk vs. benefit are carefully discussed and documented.
Table 6.
Registries for monitoring biological treatments in juvenile idiopathic arthritis
| Registry name | Country | Web link |
|---|---|---|
| Pharmachild | Participating centres of >50 countries belonging to PRINTO or PRES | http://www.pharmachild.org/ |
| Childhood Arthritis and Rheumatology Research Alliance (CARRA) | North America | https://www.carranetwork.org |
| Biologics for children with rheumatic disease (BCRD) – the extended biologics study | UK | http://www.bcrdstudy.org |
| British Society of Paediatric and Adolescent rheumatology (BSPAR) etanercept cohort | UK | https://www.aruk.manchester.ac.uk/bspar |
| Dutch national arthritis and biological in children (ABC) register | The Netherlands | https://www.abc-register.nl/ |
| Biologika in der Kinderrheumatologie (BIKER) register (paediatric) | Germany | http://biker-register.de |
| Juvenile arthritis methotrexate/biologics long-term observation (JuMBO study including adults) | Germany | http://dgrh.de/jumbo-forschung.html |
Abbreviations are as follows: PRES, Paediatric Rheumatology European Society; PRINTO, Paediatric Rheumatology International Trials Organization.
Conclusions
The development of biologics for use in children has significantly changed the treatment pathways of a wide range of autoimmune diseases, enabling clinicians to aim for complete disease remission in complex conditions that were previously associated with long-term damage and disability. Reliance upon the withdrawal study design during the assessment of biologics in children has complicated the interpretation of efficacy and safety data, leading to a need for national and international collaboration for delivery of biologics registries and long-term, open-label studies. Development of new biologics and personalized treatment strategies based on biology, genetics and pharmacogenetics will be crucial for further improvements in treatment options and patient outcomes.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- Horneff G. Update on biologicals for treatment of juvenile idiopathic arthritis. Expert Opin Biol Ther. 2013;13:361–376. doi: 10.1517/14712598.2013.735657. [DOI] [PubMed] [Google Scholar]
- Munro JE, Murray KJ. Advances in paediatric rheumatology: beyond NSAIDs and joint replacement. J Paed Child Health. 2004;40:161–169. doi: 10.1111/j.1440-1754.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- Brennan FM, Feldmann R. Cytokines in autoimmunity. Curr Opin Autoimmune. 1992;4:1520–1530. doi: 10.1016/0952-7915(92)90057-l. [DOI] [PubMed] [Google Scholar]
- Taylor PC. Pharmacology of TNF blocade in rheumatoid arthrtis and other chronic inflammatory disease. Curr Opin Pharmacol. 2010;10:308–315. doi: 10.1016/j.coph.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Kok MR, Tak PP. Taking advances from bench to bedside during the last decade. Best Pract Res Clin Rheumatol. 2012;26:225–236. doi: 10.1016/j.berh.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Mangge H, Kenzian H, Gallistl S, Neuwirth G, Liebmann P, Kaulfersch W, Beaufort F, Muntean W, Schauenstein K. Serum cytokines in juvenile rheumatoid arthritis. Correlation with conventional inflammation parameters and clinical subtypes. Arthritis Rheum. 1995;38:211–220. doi: 10.1002/art.1780380209. [DOI] [PubMed] [Google Scholar]
- Eberhard BA, Laxer RM, Andersson U, Silverman ED. Local synthesis of both macrophage and T cell cytokines by synovial fluid cells from children with juvenile rheumatoid arthritis. Clin Exp Immunol. 1994;96:260–266. doi: 10.1111/j.1365-2249.1994.tb06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40:1202–1209. doi: 10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Wallace CA, Ruperto N, Giannini E. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31:2290–2294. [PubMed] [Google Scholar]
- Brunner HI, Lovell DJ, Finck BK, Giannini EH. Preliminary definition of disease flare in juvenile rheumatoid arthritis. J Rheumatol. 2002;29:1058–1064. [PubMed] [Google Scholar]
- Lehman TJ. Are withdrawal trials in paediatric rheumatic disease helpful? Lancet. 2008;372:348–350. doi: 10.1016/S0140-6736(08)60999-X. [DOI] [PubMed] [Google Scholar]
- Irvine EJ, Whitehead WE, Chey WD, Matsueda K, Shaw M, Talley NJ, Veldhuyzen van Zanten SJ. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2006;130:1538–1551. doi: 10.1053/j.gastro.2005.11.058. [DOI] [PubMed] [Google Scholar]
- Fernandes R, Ferreira JJ, Sampaio C. The placebo response in studies of acute migraine. J Pediatr. 2008;152:527–533. doi: 10.1016/j.jpeds.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Rothner AD, Wasiewski W, Winner P, Lewis D, Stankowski J. Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents. Headache. 2006;46:101–109. doi: 10.1111/j.1526-4610.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- Mohler KM, Torrance DS, Smith CA, Goodwin RG, Stremler KE, Fung VP, Madani H, Widmer MB. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993;151:1548–1561. [PubMed] [Google Scholar]
- Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, Stein LD, Gedalia A, Ilowite NT, Wallace CA, Whitmore J, Finck BK. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000;342:763–769. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- Lovell DJ, Giannini EH, Reiff A, Jones OY, Schneider R, Olson JC, Stein LD, Gedalia A, Ilowite NT, Wallace CA, Lange M, Finck BK, Burge DJ. Long-term efficacy and safety of etanercept in children with polyarticular-course juvenile rheumatoid arthritis: interim results from an ongoing multicenter, open-label, extended-treatment trial. Arthritis Rheum. 2003;48:218–226. doi: 10.1002/art.10710. [DOI] [PubMed] [Google Scholar]
- Quartier P, Taupin P, Bourdeaut F, Lemelle I, Pillet P, Bost M, Sibilia J, Koné-Paut I, Gandon-Laloum S, LeBideau M, Bader-Meunier B, Mouy R, Debré M, Landais P, Prieur AM. Efficacy of etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthritis Rheum. 2003;48:1093–1101. doi: 10.1002/art.10885. [DOI] [PubMed] [Google Scholar]
- Prince FH, Twilt M, ten Cate R, van Rossum MA, Armbrust W, Hoppenreijs EP, van Santen-Hoeufft M, Koopman-Keemink Y, Wulffraat NM, van Suijlekom-Smit LW. Long-term follow-up on effectiveness and safety of etanercept in juvenile idiopathic arthritis: the Dutch national register. Ann Rheum Dis. 2009;68:635–641. doi: 10.1136/ard.2007.087411. [DOI] [PubMed] [Google Scholar]
- Horneff G, Burgos-Vargas R, Constantin T, Foeldvari I, Vojinovic J, Chasnyk VG, Dehoorne J, Panaviene V, Susic G, Stanevica V, Kobusinska K, Zuber Z, Mouy R, Rumba-Rozenfelde I, Breda L, Dolezalova P, Job-Deslandre C, Wulffraat N, Alvarez D, Zang C, Wajdula J, Woodworth D, Vlahos B, Martini A, Ruperto N. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Ann Rheum Dis. 73:1114–1122. doi: 10.1136/annrheumdis-2012-203046. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papsdorf V, Horneff G. Complete control of disease activity and remission induced by treatment with etanercept in juvenile idiopathic arthritis. Rheumatol (Oxford) 2011;50:214–221. doi: 10.1093/rheumatology/keq292. [DOI] [PubMed] [Google Scholar]
- Otten MH, Prince FH, Armbrust W, ten Cate R, Hoppenreijs EP, Twilt M, Koopman-Keemink Y, Gorter SL, Dolman KM, Swart JF, van den Berg JM, Wulffraat NM, van Rossum MA, van Suijlekom-Smit LW. Factors associated with treatment response to etanercept in juvenile idiopathic arthritis. JAMA. 2011;306:2340–2347. doi: 10.1001/jama.2011.1671. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. 2014. Opinion and decisions on paediatric investigation plans. Available at http://www.ema.europa.eu (last accessed 26 March 2014)
- National Institute for Health and Care Excellence. 2014. NICE Guidance. Available at http://www.nice.org.uk (last accessed 26 March 2014)
- Lovell DJ, Reiff A, Jones OY, Schneider R, Nocton J, Stein LD, Gedalia A, Ilowite NT, Wallace CA, Whitmore JB, White B, Giannini EH. Long-term safety and efficacy of etanercept in children with polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2006;54:1987–1994. doi: 10.1002/art.21885. [DOI] [PubMed] [Google Scholar]
- Lovell DJ, Reiff A, Ilowite NT, Wallace CA, Chon Y, Lin SL, Baumgartner SW, Giannini EH. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 2008;58:1496–1504. doi: 10.1002/art.23427. [DOI] [PubMed] [Google Scholar]
- Horneff G, De Bock F, Foeldvari I, Girschick HJ, Michels H, Moebius D, Schmeling H. Safety and efficacy of combination of etanercept and methotrexate compared to treatment with etanercept only in patients with juvenile idiopathic arthritis (JIA): preliminary data from the German JIA Registry. Ann Rheum Dis. 2009;68:519–525. doi: 10.1136/ard.2007.087593. [DOI] [PubMed] [Google Scholar]
- Giannini EH, Ilowite NT, Lovell DJ, Wallace CA, Rabinovich CE, Reiff A, Higgins G, Gottlieb B, Singer NG, Chon Y, Lin SL, Baumgartner SW. Long-term safety and effectiveness of etanercept in children with selected categories of juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:2794–2804. doi: 10.1002/art.24777. [DOI] [PubMed] [Google Scholar]
- Gerloni V, Pontikaki I, Gattinara M, Fantini F. Focus on adverse events of tumour necrosis factor alpha blockade in juvenile idiopathic arthritis in an open monocentric long-term prospective study of 163 patients. Ann Rheum Dis. 2008;67:1145–1152. doi: 10.1136/ard.2007.069484. [DOI] [PubMed] [Google Scholar]
- Horneff G, Ebert A, Fitter S, Minden K, Foeldvari I, Kummerle-Deschner J, Thon A, Girschick HJ, Weller F, Huppertz HI. Safety and efficacy of once weekly etanercept 0.8 mg/kg in a multicentre 12 week trial in active polyarticular course juvenile idiopathic arthritis. Rheumatol (Oxford) 2009;48:916–919. doi: 10.1093/rheumatology/kep122. [DOI] [PubMed] [Google Scholar]
- Kuemmerle-Deschner JB, Horneff G. Safety and efficacy of once-weekly application of Etanercept in children with juvenile idiopathic arthritis. Rheumatol Int. 2007;28:153–156. doi: 10.1007/s00296-007-0399-1. [DOI] [PubMed] [Google Scholar]
- Prince FH, Twilt M, Jansen-Wijngaarden NC, van Suijlekom-Smit LW. Effectiveness of a once weekly double dose of etanercept in patients with juvenile idiopathic arthritis: a clinical study. Ann Rheum Dis. 2007;66:704–705. doi: 10.1136/ard.2006.067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussi SS, Pan N, Walters HM, Walsh TJ. Infections in children and adolescents with juvenile idiopathic arthritis and inflammatory bowel disease treated with tumor necrosis factor-α inhibitors: a systematic review of the literature. Clin Infect Dis. 2013;57:1318–1330. doi: 10.1093/cid/cit489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A, Clark J, Foster H. Tuberculosis and treatment with infliximab. N Engl J Med. 2002;346:623–626. [PubMed] [Google Scholar]
- Southwood TR. Adverse events in juvenile idiopathic arthritis patients treated with etanercept or the combination of etanercept and methotrexate. Arthritis Rheum. 2005;55(Suppl):S85. [Google Scholar]
- Kaneko U, Kishi T, Kikuchi M, Hara R, Shinoki T, Miyamae T, Imagawa T, Mori M, Yokota S. Two patients with childhood-onset Behcet's disease successfully treated by anti-tumor necrosis factor therapy. Jpn J Clin Immunol. 2010;33:157–161. doi: 10.2177/jsci.33.157. [DOI] [PubMed] [Google Scholar]
- Sakallioglu O, Duzova A, Ozen S. Etanercept in the treatment of arthritis in a patient with familial Mediterranean fever. Clin Exp Rheumatol. 2006;24:435–437. [PubMed] [Google Scholar]
- Kusuhara K, Hoshina T, Saito M, Ishimura M, Inoue H, Horiuchi T, Sato T, Hara T. Successful treatment of a patient with tumor necrosis factor receptor-associated periodic syndrome using a half-dose of etanercept. Pediatr Int. 2012;54:552–555. doi: 10.1111/j.1442-200X.2011.03525.x. [DOI] [PubMed] [Google Scholar]
- Choueiter NF, Olson AK, Shen DD, Portman MA. Prospective open-label trial of etanercept as adjunctive therapy for kawasaki disease. J Pediatr. 2010;157:960–966. doi: 10.1016/j.jpeds.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Liebhaber M, Roberts RL, Dyer Z, Stiehm ER. Etanercept treatment of cutaneous granulomas in common variable immunodeficiency. J Aller Clin Immunol. 2006;117:878–882. doi: 10.1016/j.jaci.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Akobeng AK, Zachos M. Tumor necrosis factor-alpha antibody for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2004;(1) doi: 10.1002/14651858.CD003574.pub2. CD003574. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller AS, Siegfried EC, Langley RG, Gottlieb AB, Pariser D, Landells I, Hebert AA, Eichenfield LF, Patel V, Creamer K, Jahreis A. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241–251. doi: 10.1056/NEJMoa066886. [DOI] [PubMed] [Google Scholar]
- Lofgren S, Krol A. New therapies in pediatric dermatology. Curr Opin Pediat. 2011;23:399–402. doi: 10.1097/MOP.0b013e3283483ecf. [DOI] [PubMed] [Google Scholar]
- Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, Nemcova D, Mouy R, Sandborg C, Bohnsack J, Elewaut D, Foeldvari I, Gerloni V, Rovensky J, Minden K, Vehe RK, Weiner LW, Horneff G, Huppertz HI, Olson NY, Medich JR, Carcereri-De-Prati R, McIlraith MJ, Giannini EH, Martini A. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359:810–820. doi: 10.1056/NEJMoa0706290. [DOI] [PubMed] [Google Scholar]
- Kingsbury D, Quartier P, Santra S. Safety and efficacy of adalimumab in children with active polyarticular juvenile idiopathic arthritis aged 2 to < 4 years or > 4 years weighing < 15 kg. Ann Rheum Dis. 2012;71(Suppl 3):428. [Google Scholar]
- FDA Pediatric Advisory Committee. 2009. Pediatric focused safety review: adalimumab (humira). Available at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM193197.pdf (last accessed 13 June 2013)
- Anink J, Otten MH, Gorter SL, Prince FH, van Rossum MA, van den Berg JM, van Pelt PA, Kamphuis S, Brinkman DM, Swen WA, Swart JF, Wulffraat NM, Dolman KM, Koopman-Keemink Y, Hoppenreijs EP, Armbrust W, Ten Cate R, van Suijlekom-Smit LW. Treatment choices of paediatric rheumatologists for juvenile idiopathic arthritis: etanercept or adalimumab? Rheumatol (Oxford) 2013;52:1674–1679. doi: 10.1093/rheumatology/ket170. [DOI] [PubMed] [Google Scholar]
- Biester S, Deuter C, Michels H, Haefner R, Kuemmerle-Deschner J, Doycheva D, Zierhut M. Adalimumab in the therapy of uveitis in childhood. Br J Ophthalmol. 2007;91:319–324. doi: 10.1136/bjo.2006.103721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynjala P, Kotaniemi K, Lindahl P, Latva K, Aalto K, Honkanen V, Lahdenne P. Adalimumab in juvenile idiopathic arthritis-associated chronic anterior uveitis. Rheumatol (Oxford) 2008;47:339–344. doi: 10.1093/rheumatology/kem356. [DOI] [PubMed] [Google Scholar]
- Simonini G, Taddio A, Cattalini M, Caputo R, De Libero C, Naviglio S, Bresci C, Lorusso M, Lepore L, Cimaz R. Prevention of flare recurrences in childhood-refractory chronic uveitis: an open-label comparative study of adalimumab versus infliximab. Arthritis Care Res. 2011;63:612–618. doi: 10.1002/acr.20404. [DOI] [PubMed] [Google Scholar]
- 2013. Randomised controlled trial of the clinical effectiveness, safety and cost effectiveness of Adalimumab for the treatment of juvenile idiopathic arthritis associated uveitis (SYCAMORE). Available at http://www.sycamoretrial.org.uk (last accessed 13 June 2013)
- Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, Panaccione R, Wolf D, Kent JD, Bittle B, Li J, Pollack PF. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Feagan BG, Panaccione R, Sandborn WJ, D'Haens GR, Schreiber S, Rutgeerts PJ, Loftus EV, Jr, Lomax KG, Yu AP, Wu EQ, Chao J, Mulani P. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn's disease: results from the CHARM study. Gastroenterology. 2008;135:1493–1499. doi: 10.1053/j.gastro.2008.07.069. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, Pollack PF. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- Rosenbach Y, Hartman C, Shapiro R, Hirsch A, Avitzur Y, Shamir R. Adalimumab treatment in children with refractory Crohn's disease. Dig Dis Sci. 2010;55:747–753. doi: 10.1007/s10620-009-0791-7. [DOI] [PubMed] [Google Scholar]
- Wyneski MJ, Green A, Kay M, Wyllie R, Mahajan L. Safety and efficacy of adalimumab in pediatric patients with Crohn disease. J Pediatr Gastroenterol Nutr. 2008;47:19–25. doi: 10.1097/MPG.0b013e318174e886. [DOI] [PubMed] [Google Scholar]
- Viola F, Civitelli F, Di Nardo G, Barbato MB, Borrelli O, Oliva S, Conte F, Cucchiara S. Efficacy of adalimumab in moderate-to-severe pediatric Crohn's disease. Am J Gastroenterol. 2009;104:2566–2571. doi: 10.1038/ajg.2009.372. [DOI] [PubMed] [Google Scholar]
- Russell RK, Wilson ML, Loganathan S, Bourke B, Kiparissi F, Mahdi G, Torrente F, Rodrigues A, Davies I, Thomas A, Akobeng AK, Fagbemi A, Hyer W, Spray C, Vaish S, Rogers P, McGrogan P, Heuschkel RB, Ayub N, Fell JM, Afzal NA, Green M, Murphy MS, Rao P, Shah N, Ho GT, Naik S, Wilson DC. A British Society of Paediatric Gastroenterology, Hepatology and Nutrition survey of the effectiveness and safety of adalimumab in children with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:946–953. doi: 10.1111/j.1365-2036.2011.04603.x. [DOI] [PubMed] [Google Scholar]
- Rosh JR, Lerer T, Markowitz J, Goli SR, Mamula P, Noe JD, Pfefferkorn MD, Kelleher KT, Griffiths AM, Kugathasan S, Keljo D, Oliva-Hemker M, Crandall W, Carvalho RS, Mack DR, Hyams JS. Retrospective Evaluation of the Safety and Effect of Adalimumab Therapy (RESEAT) in pediatric Crohn's disease. Am J Gastroenterol. 2009;104:3042–3049. doi: 10.1038/ajg.2009.493. [DOI] [PubMed] [Google Scholar]
- Hyams JS, Griffiths A, Markowitz J, Baldassano RN, Faubion WA, Jr, Colletti RB, Dubinsky M, Kierkus J, Rosh J, Wang Y, Huang B, Bittle B, Marshall M, Lazar A. Safety and efficacy of adalimumab for moderate to severe Crohn's disease in children. Gastroenterology. 2012;143:365–374. doi: 10.1053/j.gastro.2012.04.046. [DOI] [PubMed] [Google Scholar]
- Callen JP, Jackson JH. Adalimumab effectively controlled recalcitrant generalized pustular psoriasis in an adolescent. J Dermatol Treat. 2005;16:350–352. doi: 10.1080/09546630500430604. [DOI] [PubMed] [Google Scholar]
- Alvarez AC, Rodriguez-Nevado I, De Argila D, Rubio FP, Rovira I, Torrelo A, Zambrano A. Recalcitrant pustular psoriasis successfully treated with adalimumab. Pediatr Dermatol. 2011;28:195–197. doi: 10.1111/j.1525-1470.2010.01219.x. [DOI] [PubMed] [Google Scholar]
- Luu M, Cordoro KM. The evolving role of biologics in the treatment of pediatric psoriasis. Skin Ther Lett. 2013;18:1–4. [PubMed] [Google Scholar]
- 2013. A multicenter, randomized, double-dummy, double-blind study evaluating two doses of adalimumab versus methotrexate (MTX) in pediatric subjects with chronic plaque psoriasis.: ClinicalTrial.gov. Available at http://www.clinicaltrials.gov/ct2/show/NCT01251614 (last accessed 13 June 2013)
- Horneff G, Fitter S, Foeldvari I, Minden K, Kuemmerle-Deschner J, Tzaribacev N, Thon A, Borte M, Ganser G, Trauzeddel R, Huppertz HI. Double-blind, placebo-controlled randomized trial with adalimumab for treatment of juvenile onset ankylosing spondylitis (JoAS): significant short term improvement. Arthritis Res Ther. 2012;14:R230. doi: 10.1186/ar4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftheriou D, Melo M, Marks SD, Tullus K, Sills J, Cleary G, Dolezalova P, Ozen S, Pilkington C, Woo P, Klein N, Dillon MJ, Brogan PA. Biologic therapy in primary systemic vasculitis of the young. Rheumatol (Oxford) 2009;48:978–986. doi: 10.1093/rheumatology/kep148. [DOI] [PubMed] [Google Scholar]
- De Cassan C, De Vroey B, Dussault C, Hachulla E, Buche S, Colombel JF. Successful treatment with adalimumab in a familial case of gastrointestinal Behcet's disease. J Crohn's Colitis. 2011;5:364–368. doi: 10.1016/j.crohns.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Robinson AB, Gallentine WB, Rabinovich CE. Pediatric neuro-Behcet's disease responsive to adalimumab. Pediatr Neurol. 2010;43:291–293. doi: 10.1016/j.pediatrneurol.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G, Wouters C, Silverman ED, Balogh Z, Henrickson M, Apaz MT, Baildam E, Fasth A, Gerloni V, Lahdenne P, Prieur AM, Ravelli A, Saurenmann RK, Gamir ML, Wulffraat N, Marodi L, Petty RE, Joos R, Zulian F, McCurdy D, Myones BL, Nagy K, Reuman P, Szer I, Travers S, Beutler A, Keenan G, Clark J, Visvanathan S, Fasanmade A, Raychaudhuri A, Mendelsohn A, Martini A, Giannini EH Paediatric Rheumatology International Trials Organisation; Pediatric Rheumatology Collaborative Study Group. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56:3096–3106. doi: 10.1002/art.22838. [DOI] [PubMed] [Google Scholar]
- Kahn P, Weiss M, Imundo LF, Levy DM. Favorable response to high-dose infliximab for refractory childhood uveitis. Ophthalmol. 2006;113:860–864. doi: 10.1016/j.ophtha.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Uperto N, Lovell DJ, Cuttica R, Woo P, Meiorin S, Wouters C, Silverman ED, Balogh Z, Henrickson M, Davidson J, Foeldvari I, Imundo L, Simonini G, Oppermann J, Xu S, Shen YK, Visvanathan S, Fasanmade A, Mendelsohn A, Martini A, Giannini EH. Long-term efficacy and safety of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis: findings from an open-label treatment extension. Ann Rheum Dis. 2010;69:718–722. doi: 10.1136/ard.2009.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassano R, Braegger CP, Escher JC, DeWoody K, Hendricks DF, Keenan GF, Winter HS. Infliximab (REMICADE) therapy in the treatment of pediatric Crohn's disease. Am J Gastroenterol. 2003;98:833–838. doi: 10.1111/j.1572-0241.2003.07343.x. [DOI] [PubMed] [Google Scholar]
- Cezard JP, Nouaili N, Talbotec C, Hugot JP, Gobert JG, Schmitz J, Mougenot JF, Alberti C, Goulet O. A prospective study of the efficacy and tolerance of a chimeric antibody to tumor necrosis factors (remicade) in severe pediatric crohn disease. J Pediatr Gastroenterol Nutr. 2003;36:632–636. doi: 10.1097/00005176-200305000-00007. [DOI] [PubMed] [Google Scholar]
- Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, Liu G, Travers S, Heuschkel R, Markowitz J, Cohen S, Winter H, Veereman-Wauters G, Ferry G, Baldassano R, Group RS. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007;132:863–873. doi: 10.1053/j.gastro.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Hyams JS, Lerer T, Griffiths A, Pfefferkorn M, Kugathasan S, Evans J, Otley A, Carvalho R, Mack D, Bousvaros A, Rosh J, Mamula P, Kay M, Crandall W, Oliva-Hemker M, Keljo D, LeLeiko N, Markowitz J Pediatric Inflammatory Bowel Disease Collaborative Research Group. Long-term outcome of maintenance infliximab therapy in children with Crohn's disease. Inflamm Bowel Dis. 2009;15:816–822. doi: 10.1002/ibd.20845. [DOI] [PubMed] [Google Scholar]
- Ruemmele FM, Lachaux A, Cezard JP, Morali A, Maurage C, Ginies JL, Viola S, Goulet O, Lamireau T, Scaillon M, Breton A, Sarles J. Efficacy of infliximab in pediatric Crohn's disease: a randomized multicenter open-label trial comparing scheduled to on demand maintenance therapy. Inflamm Bowel Dis. 2009;15:388–394. doi: 10.1002/ibd.20788. [DOI] [PubMed] [Google Scholar]
- Vermeire S, Noman M, Van Assche G, Baert F, D'Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut. 2007;56:1226–1231. doi: 10.1136/gut.2006.099978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall W, Hyams J, Kugathasan S, Griffiths A, Zrubek J, Olson A, Liu G, Heuschkel R, Markowitz J, Cohen S, Winter H, Veereman-Wauters G, Ferry G, Baldassano RN. Infliximab therapy in children with concurrent perianal Crohn disease: observations from REACH. J Pediatr Gastroenterol Nutr. 2009;49:183–190. doi: 10.1097/MPG.0b013e3181a70f21. [DOI] [PubMed] [Google Scholar]
- Gasparetto M, Corradin S, Vallortigara F, Cananzi M, Guariso G. Infliximab and pediatric stricturing Crohn's disease: a possible alternative to surgery? Experience of seven cases. Acta Gastroenterol Belg. 2012;75:58–60. [PubMed] [Google Scholar]
- Walters TD, Gilman AR, Griffiths AM. Linear growth improves during infliximab therapy in children with chronically active severe Crohn's disease. Inflamm Bowel Dis. 2007;13:424–430. doi: 10.1002/ibd.20069. [DOI] [PubMed] [Google Scholar]
- Braun J, Deodhar A, Inman RD, van der Heijde D, Mack M, Xu S, Hsu B. Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 104-week results of the GO-RAISE study. Ann Rheum Dis. 2012;71:661–667. doi: 10.1136/ard.2011.154799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy EH, Hazleman B, Smith M, Moss K, Lisi L, Scott DG, Patel J, Sopwith M, Isenberg DA. Efficacy of a novel PEGylated humanized anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomized, dose-escalating trial. Rheumatol (Oxford) 2002;41:1133–1137. doi: 10.1093/rheumatology/41.10.1133. [DOI] [PubMed] [Google Scholar]
- Kavanaugh A, McInnes IB, Mease PJ, Krueger GG, Gladman DD, van der Heijde D, Mudivarthy S, Xu W, Mack M, Xu Z, Beutler A. Clinical efficacy, radiographic and safety findings through 2 years of golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of the randomised, placebo-controlled GO-REVEAL study. Ann Rheum Dis. 2013;72:1777–1785. doi: 10.1136/annrheumdis-2012-202035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, Adedokun OJ, Guzzo C, Colombel JF, Reinisch W, Gibson PR, Collins J, Jarnerot G, Rutgeerts P. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2013;146:96–109. doi: 10.1053/j.gastro.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, Bloomfield R, Schreiber S. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med. 2007;357:228–238. doi: 10.1056/NEJMoa067594. [DOI] [PubMed] [Google Scholar]
- Fleischmann R, Vencovsky J, van Vollenhoven RF, Borenstein D, Box J, Coteur G, Goel N, Brezinschek HP, Innes A, Strand V. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis. 2009;68:805–811. doi: 10.1136/ard.2008.099291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2013. A study of the safety and efficacy of CNTO 148 (Golimumab) in children with Juvenile Idiopathic Arthritis (JIA) and multiple joint involvement who have poor response to methotrexate (GO KIDS). Available at http://clinicaltrials.gov/show/NCT01230827 (last accessed 20 June 2013)
- 2012. Pediatric Arthritis Study of Certolizumab Pegol (PASCAL) U.S.: U.S. National Institutes of Health. Available at http://clinicaltrials.gov/ct2/show/NCT01550003?term=certolizumab+AND+JIA&rank=1 (last accessed 20 June 2013)
- Lowite N, Porras O, Reiff A, Rudge S, Punaro M, Martin A, Allen R, Harville T, Sun YN, Bevirt T, Aras G, Appleton B. Anakinra in the treatment of polyarticular-course juvenile rheumatoid arthritis: safety and preliminary efficacy results of a randomized multicenter study. Clin Rheumatol. 2009;28:129–137. doi: 10.1007/s10067-008-0995-9. [DOI] [PubMed] [Google Scholar]
- Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, Bardin C, Bossuyt X, Boutten A, Bienvenu J, Duquesne A, Richer O, Chaussabel D, Mogenet A, Banchereau J, Treluyer JM, Landais P, Pascual V. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial) Ann Rheum Dis. 2011;70:747–754. doi: 10.1136/ard.2010.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigrovic PA, Mannion M, Prince FH, Zeft A, Rabinovich CE, van Rossum MA, Cortis E, Pardeo M, Miettunen PM, Janow G, Birmingham J, Eggebeen A, Janssen E, Shulman AI, Son MB, Hong S, Jones K, Ilowite NT, Cron RQ, Higgins GC. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis Rheum. 2011;63:545–555. doi: 10.1002/art.30128. [DOI] [PubMed] [Google Scholar]
- Gattorno M, Piccini A, Lasiglie D, Tassi S, Brisca G, Carta S, Delfino L, Ferlito F, Pelagatti MA, Caroli F, Buoncompagni A, Viola S, Loy A, Sironi M, Vecchi A, Ravelli A, Martini A, Rubartelli A. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2008;58:1505–1515. doi: 10.1002/art.23437. [DOI] [PubMed] [Google Scholar]
- Zeft A, Hollister R, LaFleur B, Sampath P, Soep J, McNally B, Kunkel G, Schlesinger M, Bohnsack J. Anakinra for systemic juvenile arthritis: the Rocky Mountain experience. J Clin Rheumatol. 2009;15:161–164. doi: 10.1097/RHU.0b013e3181a4f459. [DOI] [PubMed] [Google Scholar]
- Lequerre T, Quartier P, Rosellini D, Alaoui F, De Bandt M, Mejjad O, Kone-Paut I, Michel M, Dernis E, Khellaf M, Limal N, Job-Deslandre C, Fautrel B, Le Loet X, Sibilia J. Interleukin-1 receptor antagonist (anakinra) treatment in patients with systemic-onset juvenile idiopathic arthritis or adult onset Still disease: preliminary experience in France. Ann Rheum Dis. 2008;67:302–308. doi: 10.1136/ard.2007.076034. [DOI] [PubMed] [Google Scholar]
- Caorsi R, Federici S, Gattorno M. Biologic drugs in autoinflammatory syndromes. Autoimmun Rev. 2012;12:81–86. doi: 10.1016/j.autrev.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Lovell DJ, Giannini EH, Reiff AO, Kimura Y, Li S, Hashkes PJ, Wallace CA, Onel KB, Foell D, Wu R, Biedermann S, Hamilton JD, Radin AR. Long-term safety and efficacy of rilonacept in patients with Systemic Juvenile Idiopathic Arthritis (sJIA) Arthritis Rheum. 2013;65:2486–2496. doi: 10.1002/art.38042. [DOI] [PubMed] [Google Scholar]
- Quatrier P, Erguven M, Horneff G. IL-1b Inhibition with Canakinumab in patients with systemic juvenile idiopathic arthritis: efficacy and safety outcomes from a single dose, placebo-controlled study. Ann Rheum Dis. 2012;71(Suppl. 3):705. [Google Scholar]
- Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, Brik R, McCann L, Kasapcopur O, Rutkowska-Sak L, Schneider R, Berkun Y, Calvo I, Erguven M, Goffin L, Hofer M, Kallinich T, Oliveira SK, Uziel Y, Viola S, Nistala K, Wouters C, Cimaz R, Ferrandiz MA, Flato B, Gamir ML, Kone-Paut I, Grom A, Magnusson B, Ozen S, Sztajnbok F, Lheritier K, Abrams K, Kim D, Martini A, Lovell DJ. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367:2396–2406. doi: 10.1056/NEJMoa1205099. [DOI] [PubMed] [Google Scholar]
- Food and Drugs Administration. 2014. Drug approvals and databases. Available at http://www.fda.gov (last accessed 26 March 2014)
- Ruperto N, Quartier P, Wulffraat N, Woo P, Ravelli A, Mouy R, Bader-Meunier B, Vastert SJ, Noseda E, D'Ambrosio D, Lecot J, Chakraborty A, Martini A, Chioato A Paediatric Rheumatology International Clinical Trials Organisation. A phase II, multicenter, open-label study evaluating dosing and preliminary safety and efficacy of canakinumab in systemic juvenile idiopathic arthritis with active systemic features. Arthritis Rheum. 2012;64:557–567. doi: 10.1002/art.33342. [DOI] [PubMed] [Google Scholar]
- Kutukculer N, Caglayan S, Aydogdu F. Study of pro-inflammatory (TNF-alpha, IL-1alpha, IL-6) and T-cell-derived (IL-2, IL-4) cytokines in plasma and synovial fluid of patients with juvenile chronic arthritis: correlations with clinical and laboratory parameters. Clin Rheumatol. 1998;17:288–292. doi: 10.1007/BF01451007. [DOI] [PubMed] [Google Scholar]
- De Benedetti F, Massa M, Pignatti P, Albani S, Novick D, Martini A. Serum soluble interleukin 6 (IL-6) receptor and IL-6/soluble IL-6 receptor complex in systemic juvenile rheumatoid arthritis. J Clin Invest. 1994;93:2114–2119. doi: 10.1172/JCI117206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Miyamae T, Imagawa T, Iwata N, Katakura S, Mori M, Woo P, Nishimoto N, Yoshizaki K, Kishimoto T. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2005;52:818–825. doi: 10.1002/art.20944. [DOI] [PubMed] [Google Scholar]
- De Benedetti F, Brunner HI, Ruperto N, Kenwright A, Wright S, Calvo I, Cuttica R, Ravelli A, Schneider R, Woo P, Wouters C, Xavier R, Zemel L, Baildam E, Burgos-Vargas R, Dolezalova P, Garay SM, Merino R, Joos R, Grom A, Wulffraat N, Zuber Z, Zulian F, Lovell D, Martini A. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367:2385–2395. doi: 10.1056/NEJMoa1112802. [DOI] [PubMed] [Google Scholar]
- Yokota S, Imagawa T, Mori M, Miyamae T, Takei S, Iwata N, Umebayashi H, Murata T, Miyoshi M, Tomiita M, Nishimoto N, Kishimoto T. Long-term treatment of systemic juvenile idiopathic arthritis with tocilizumab: results of an open-label extension study in Japan. Ann Rheum Dis. 2013;72:627–628. doi: 10.1136/annrheumdis-2012-202310. [DOI] [PubMed] [Google Scholar]
- Zulian F, Balzarin M, Falcini F, Martini G, Alessio M, Cimaz R, Ciminoz L, Zannin ME. Abatacept for severe anti-tumor necrosis factor alpha refractory juvenile idiopathic arthritis-related uveitis. Arthritis Care Res. 2010;62:821–825. doi: 10.1002/acr.20115. [DOI] [PubMed] [Google Scholar]
- Runner HI, Ruperto N, Zuber Z, Keane C, Harari O, Kenwright A, Cuttica R. Efficacy and safety of tocilizumab in patients with polyarticular juvenile idiopathic arthritis: data from a phase 3 trial. Arthritis Rheum. 2012;64:S682. [Google Scholar]
- Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Perez N, Silva CA, Abud-Mendoza C, Burgos-Vargas R, Gerloni V, Melo-Gomes JA, Saad-Magalhaes C, Sztajnbok F, Goldenstein-Schainberg C, Scheinberg M, Penades IC, Fischbach M, Orozco J, Hashkes PJ, Hom C, Jung L, Lepore L, Oliveira S, Wallace CA, Sigal LH, Block AJ, Covucci A, Martini A, Giannini EH. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383–391. doi: 10.1016/S0140-6736(08)60998-8. [DOI] [PubMed] [Google Scholar]
- Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Perez N, Silva CA, Abud-Mendoza C, Burgos-Vargas R, Gerloni V, Melo-Gomes JA, Saad-Magalhaes C, Chavez-Corrales J, Huemer C, Kivitz A, Blanco FJ, Foeldvari I, Hofer M, Horneff G, Huppertz HI, Job-Deslandre C, Loy A, Minden K, Punaro M, Nunez AF, Sigal LH, Block AJ, Nys M, Martini A, Giannini EH. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum. 2010;62:1792–1802. doi: 10.1002/art.27431. [DOI] [PubMed] [Google Scholar]
- Borker A, Choudhary N. Rituximab. Indian Pediatr. 2011;48:627–632. doi: 10.1007/s13312-011-0098-6. [DOI] [PubMed] [Google Scholar]
- Podolskaya A, Stadermann M, Pilkington C, Marks SD, Tullus K. B cell depletion therapy for 19 patients with refractory systemic lupus erythematosus. Arch Dis Child. 2008;93:401–406. doi: 10.1136/adc.2007.126276. [DOI] [PubMed] [Google Scholar]
- Willems M, Haddad E, Niaudet P, Kone-Paut I, Bensman A, Cochat P, Deschenes G, Fakhouri F, Leblanc T, Llanas B, Loirat C, Pillet P, Ranchin B, Salomon R, Ulinski T, Bader-Meunier B. Rituximab therapy for childhood-onset systemic lupus erythematosus. J Pediatr. 2006;148:623–627. doi: 10.1016/j.jpeds.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Alexeeva EI, Valieva SI, Bzarova TM, Semikina EL, Isaeva KB, Lisitsyn AO, Denisova RV, Chistyakova EG. Efficacy and safety of repeat courses of rituximab treatment in patients with severe refractory juvenile idiopathic arthritis. Clin Rheumatol. 2011;30:1163–1172. doi: 10.1007/s10067-011-1720-7. [DOI] [PubMed] [Google Scholar]
- Jansson AF, Sengler C, Kuemmerle-Deschner J, Gruhn B, Kranz AB, Lehmann H, Kleinert D, Pape L, Girschick HJ, Foeldvari I, Haffner D, Haas JP, Moebius D, Foell D, Peitz J, Grote V. B cell depletion for autoimmune diseases in paediatric patients. Clin Rheumatol. 2011;30:87–97. doi: 10.1007/s10067-010-1630-0. [DOI] [PubMed] [Google Scholar]
- Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, Leon MG, Tanasescu C, Nasonov E, Lan JL, Pineda L, Zhong ZJ, Freimuth W, Petri MA. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW, Stohl W, Ginzler EM, Hough DR, Zhong ZJ, Freimuth W, van Vollenhoven RF. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman TJ. Should the Food and Drug Administration warning of malignancy in children receiving tumor necrosis factor alpha blockers change the way we treat children with juvenile idiopathic arthritis? Arthritis Rheum. 2010;62:2183–2184. doi: 10.1002/art.27506. [DOI] [PubMed] [Google Scholar]
- Simard JF, Neovius M, Hagelberg S, Askling J. Juvenile idiopathic arthritis and risk of cancer: a nationwide cohort study. Arthritis Rheum. 2010;62:3776–3782. doi: 10.1002/art.27741. [DOI] [PubMed] [Google Scholar]
- Horneff G, Foeldvari I, Minden K, Moebius D, Hospach T. Report on malignancies in the German juvenile idiopathic arthritis registry. Rheumatol (Oxford) 2011;50:230–236. doi: 10.1093/rheumatology/keq361. [DOI] [PubMed] [Google Scholar]
- Cleary AG, McDowell H, Sills JA. Polyarticular juvenile idiopathic arthritis treated with methotrexate complicated by the development of non-Hodgkin's lymphoma. Arch Disease Child. 2002;86:47–49. doi: 10.1136/adc.86.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart JF, de Roock S, Wulffraat NM. What are the immunological consequences of long-term use of biological therapies for juvenile idiopathic arthritis? Arthritis Res Ther. 2013;15:213. doi: 10.1186/ar4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore L, Marchetti F, Facchini S, Leone V, Ventura A. Drug-induced systemic lupus erythematosus associated with etanercept therapy in a child with juvenile idiopathic arthritis. Clin Exp Rheumatol. 2003;21:276–277. [PubMed] [Google Scholar]
- Kanakoudi-Tsakalidou F, Tzimouli V, Pratsidou-Gertsi P, Chronopoulou E, Trachana M. The significance of persistent newly developed autoantibodies in JIA patients under long-term anti-TNF treatment. Cytokine. 2008;42:293–297. doi: 10.1016/j.cyto.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Hashkes PJ, Uziel Y, Laxer RM. The safety profile of biologic therapies for juvenile idiopathic arthritis. Nat Rev Rheumatol. 2010;6:561–571. doi: 10.1038/nrrheum.2010.142. [DOI] [PubMed] [Google Scholar]
- Bout-Tabaku S, Rivas-Chacon R, Restrepo R. Systemic lupus erythematosus in a patient treated with etanercept for polyarticular juvenile rheumatoid arthritis. J Rheumatol. 2007;34:2503–2504. [PubMed] [Google Scholar]
- Zannin ME, Birolo C, Gerloni VM, Miserocchi E, Pontikaki I, Paroli MP, Bracaglia C, Shardlow A, Parentin F, Cimaz R, Simonini G, Falcini F, Corona F, Viola S, De Marco R, Breda L, La Torre F, Vittadello F, Martini G, Zulian F. Safety and efficacy of infliximab and adalimumab for refractory uveitis in juvenile idiopathic arthritis: 1-year followup data from the Italian Registry. J Rheumatol. 2013;40:74–79. doi: 10.3899/jrheum.120583. [DOI] [PubMed] [Google Scholar]


