Abstract
Aims
To establish the dose−response for pharmacodynamics (bronchodilatation), safety and pharmacokinetics for a nebulized formulation of the long acting muscarinic antagonist glycopyrrolate (EP-101) with a high efficiency nebulizer in patients with chronic obstructive pulmonary disease (COPD).
Methods
Patients with moderate to severe COPD (GOLD II/III), with reversible lung function, were enrolled into this randomized, double-blind, placebo-controlled, six period crossover study (n = 42). Patients received single doses of EP-101 (12.5–400 μg) and placebo via a high efficiency nebulizer (eFlow® PARI nebulizer), with washout between treatments. Plasma pharmacokinetics were assessed in a subset of patients (n = 11).
Results
All treatments were well tolerated with similar adverse event rates reported with placebo and at all doses. There were no clinically relevant changes in heart rate, systolic and diastolic blood pressure or in ECG parameters including QTc interval. Following treatment with EP-101 at all doses there was a rapid bronchodilator response within 5 min. Significant improvements in mean change from baseline FEV1 at 24 h were reported at doses ≥50 μg compared with placebo, with a clear dose−response relationship. Mean changes in FEV1 were 0.10 l (95% CI 0.06, 0.14) and 0.12 l (95% CI 0.08, 0.16) for 100 μg and 200 μg, respectively.
Conclusion
Single doses of EP-101 ranging from 12.5 μg to 400 μg were well tolerated. EP-101 delivered by high efficiency nebulizer device produced a rapid onset of bronchodilatation with clinically meaningful improvements in lung function maintained over a 24 h period at all doses >50 μg.
Keywords: COPD, EP-101, glycopyrrolate, LAMA, muscarinic antagonist, nebulizer
What is already known about this subject
Long acting muscarinic antagonists, including tiotropium and glycopyrrolate, are a cornerstone of therapy in chronic obstructive pulmonary disease (COPD). EP-101 is a novel soluble glycopyrrolate formulation that can be delivered using an efficient nebulizer device.
What this study adds
This paper demonstrates dose related bronchodilatation following single dose administration of EP-101 in COPD patients, using a novel high efficiency nebulizer to deliver glycopyrrolate safely and effectively.
Introduction
Muscarinic receptor antagonists (anticholinergics) play a central role in the management of chronic obstructive pulmonary disease (COPD) 1–3. Long acting muscarinic antagonists (LAMAs) provide effective bronchodilatation with a convenient dosing schedule. Glycopyrrolate is a LAMA that is kinetically selective for M1- and M3-receptors 4, with a slow receptor dissociation time and has a quaternary ammonium structure 4. It has long been approved for intravenous (i.v.) and intramuscular (i.m.) injection and oral formulations are also available. The safety of systemic glycopyrrolate bromide administration has been well documented over a 30 year period 5,6. The safety of inhaled glycopyrrolate is also supported by extensive clinical studies with a dry powder formulation of this drug 7.
A soluble formulation of glycopyrrolate bromide has been developed (EP-101) which can be delivered by nebulizer. Nebulizer delivery is an effective way to deliver drugs to COPD patients who have difficulty using inhalers, such as those with low inspiratory flow rates which make it difficult to use dry powder inhalers. The eFlow® PARI device (see Figure 1) is a high efficiency nebulizer, which can be used to deliver EP-101 for the long term, maintenance treatment of patients with COPD. The eFlow® nebulizer system is able to deliver a wide range of drug volumes (0.5 ml to 5 ml) and dosages (0.01 mg to 1000 mg), allowing patients to take their treatment during consecutive breaths. The hole sizes can be adjusted from just below 2 μm upwards.
Figure 1.
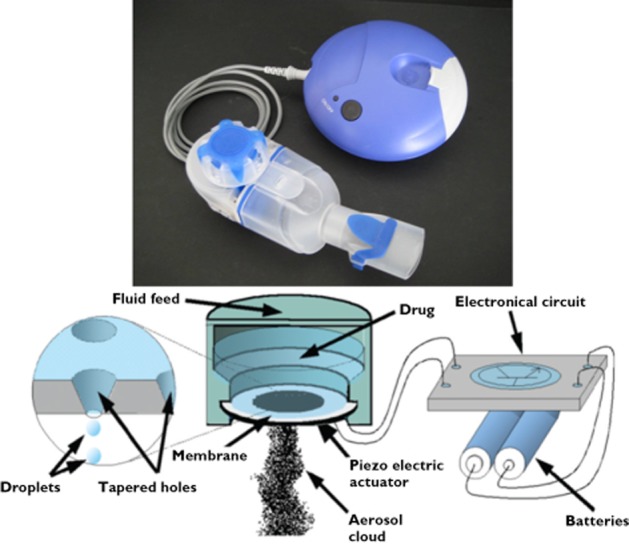
A schematic of the aerosol production within the eFlow® nebulizer system
This study investigated the dose−response effects and duration of bronchodilatation of EP-101 administered using the eFlow® PARI device in patients with moderate to severe COPD. We also assessed the tolerability and pharmacokinetics of this formulation.
Methods
This was a two centre, randomized, placebo-controlled, double-blind, dose ranging, single dose, six way crossover study in patients with COPD of 40–75 years of age. The study was approved by the North West 7 Research Ethics Committee – Greater Central Manchester (10/H1008/36) and all subjects provided written informed consent.
Study design
Subjects were eligible for the study if they had a clinical diagnosis of moderate to severe COPD with a post-bronchodilator FEV1 30–70% of predicted normal at the screening visit. Patients were also required to demonstrate at least 12% and 150 ml reversibility to inhaled ipratropium bromide. Eligible subjects participated in six treatment visits separated by washout periods of 5–12 days. Subjects were randomized to receive a single dose of EP-101 (12.5 μg, 25 μg, 50 μg, 100 μg and 200 μg) or placebo (between 08.00 h and 09.00 h) at each treatment visit. The study treatments (both active and placebo) were administered using the eFlow® nebulizer (PARI, Starnberg, Germany).
During each treatment visit the subject's pre-dose FEV1 value was required to be within 15% of the pre-dose value at the first treatment visit, otherwise the visit was rescheduled. Study procedures during each treatment visit included serial spirometry at pre-dose, immediately following dosing (within 5 min) and 15, 30 min and 1, 2, 4, 6, 8, 10, 12, 14, 20, 23.5, 24, 27 and 30 h post-dose. Blood samples were obtained to determine plasma glycopyrrolate concentrations at pre-dose and 5, 15, 30, 45 and 60 min and 2, 4, 6, 8 and 12 h post-dose in a subset of patients (n = 11). Heart rate, blood pressure and 12-lead electrocardiogram were obtained at pre-dose and 30 and 60 min and 2, 6, 12 and 24 h post-dose. Blood for biochemistry screening was taken at the screening visit and within 7 days following the last study treatment (safety follow-up visit).
Subjects were required to withhold any long acting β2-adrenoceptor agonists (LABA) bronchodilators for at least 24 h and short acting bronchodilators (e.g. salbutamol, ipratropium or a combination) for at least 8 h prior to receiving each study treatment. Inhaled LAMA (e.g. tiotropium bromide) was not permitted within 7 days prior to the screening visit and for the duration of the study and was replaced by inhaled ipratropium bromide. Inhaled corticosteroid use was permitted during the study provided that the steroid dose was stabilized for at least 6 weeks prior to the screening visit and throughout the study period. Rescue salbutamol was allowed for as-needed use any time during the study. However, if rescue salbutamol was used within 8 h of the treatment visit, then the visit was rescheduled.
Lung function
Spirometry was performed in accordance with guidelines according to American Thoracic Society/European Respiratory Society standards 8. For Caucasians of non-European descent and non-Caucasians, predicted values for FEV1 and FVC were to be adjusted for race as per the European Coal and Steel Community (ECSC) guidelines 9. Recordings were made with Vitalograph equipment (Buckingham, UK).
Laboratory analysis
Pharmacokinetic measurements of EP-101 were performed by Simbec Research Limited (Merthyr Tydfil, UK) using a validated liquid chromatography-tandem mass spectrometry (LC-MS-MS) method 10. Data acquisition and integration were achieved using Applied Biosystems MDS Sciex Analyst software. The upper and lower limits of quantification were 10 443.56 pg ml−1 and 26.11 pg ml−1, respectively.
Statistical analysis
Analysis was performed on the intention-to-treat population. An analysis of covariance (ancova) with fixed effects for centre, treatment, period, sequence and a random effect for subject within sequence was used to calculate least square means using pre-dose FEV1 as a covariate. For comparisons point estimates, 95% confidence intervals (CI) and P values for the difference between each EP-101 treatment vs. placebo were constructed using the residual mean square error obtained from the ancova, standardized FEV1 and AUC were calculated using the linear trapezoidal rule (WinNonlin). The incidence of adverse events (AE) was compared between treatment groups with summary tables by treatment group, with descriptive statistics to summarize data.
Results
Patient characteristics
Patient demographics are shown in Table 1. The patients had moderate to severe COPD (mean FEV1 54% predicted). Of the 42 subjects enrolled 35 completed the study, seven subjects were withdrawn, four due to adverse events including COPD exacerbations, two subjects were withdrawn for protocol non-compliance and one subject withdrew consent.
Table 1.
Patient demographics and summary of baseline characteristics
| Demographics baseline (n = 42) | Mean |
|---|---|
| Age (years) (SD) | 62.0 (7.0) |
| Age range (years) | 44–74 |
| Male, n (%) | 27 (64) |
| Female, n (%) | 15 (36) |
| Duration of COPD (years) (SD) | 7.5 (6.1) |
| Tobacco pack-years, mean (SD) | 42.9 (17.9) |
| Current smoker, n (%) | 24 (57) |
| Ex-smoker, n (%) | 18 (43) |
| Currently using ICS, n (%) | 29 (69) |
| No ICS use, n (%) | 13 (31) |
| Currently using ICS/LABA, n (%) | 27 (64) |
| FEV1 pre-bronchodilator (l) (SD) | 1.21 (0.40) |
| FEV1 pre-bronchodilator (% predicted), (SD) | 42.7 (11.5) |
| FEV1 post-bronchodilator (l) (SD) | 1.52 (0.46) |
| FEV1 post-bronchodilator (% predicted) (SD) | 53.8 (12.7) |
| FEV1/FVC post-bronchodilator (%) (SD) | 44.9 (11.0) |
| FEV1 reversibility (ml) (SD) | 306 (119) |
| FEV1 reversibility (%) (SD) | 27.3 (11.8) |
Pharmacodynamic response
At all tested doses there was rapid onset of response (at 5 min, the first assessment) in FEV1 and the response followed the normal circadian rhythm over the 24 h period (Figure 2). There was a dose−response relationship in the time normalized FEV1 (0–24 h; Figure 3). The placebo-adjusted mean FEV1 change from baseline at 24 h post-dose (Figure 4) also showed a dose−response relationship, with statistically significant improvements observed at doses ≥50 μg. Clinically meaningful improvements >100 ml for the placebo-adjusted FEV1 improvements at 24 h post-dose were observed at the 100 μg and 200 μg doses, suggesting sustained 24 h bronchodilatation at these doses. Table 2 shows the placebo adjusted FEV1 mean difference from pre-dose across the different doses, demonstrating dose–response effects.
Figure 2.
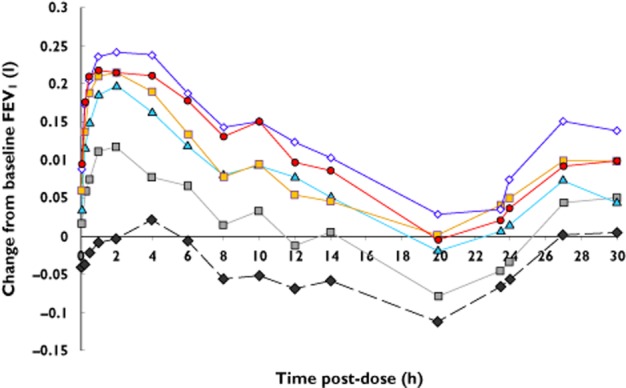
Mean change from baseline FEV1 after different doses of nebulized glycopyrrolate. The first assessment at 5 min post-dose is displayed at 0 h.  , placebo;
, placebo;  , 12.5 μg;
, 12.5 μg;  , 50 μg;
, 50 μg;  , 100 μg;
, 100 μg;  , 200 μg;
, 200 μg;  , 400 μg
, 400 μg
Figure 3.
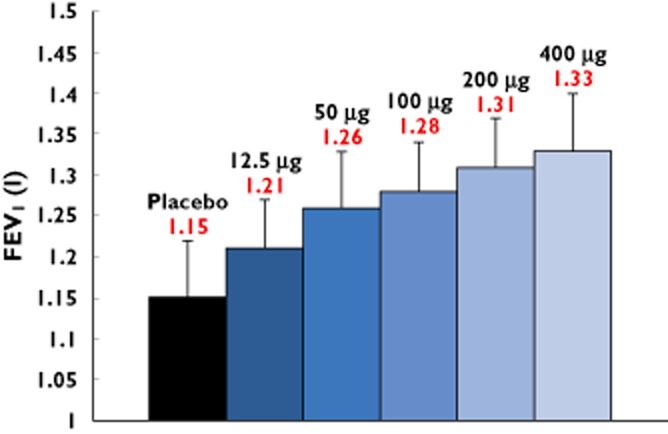
Standardized (time normalized) FEV1 (0–24 h) (ITT population; mean ± SEM). n = 37–39
Figure 4.
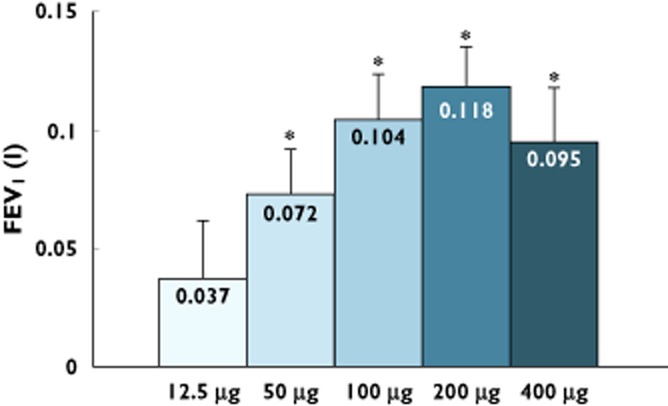
Placebo-adjusted FEV1 at 24 h (ITT population; mean ± SEM) after different doses of nebulized glycopyrrolate. *P < 0.001 ancova. n = 37–39
Table 2.
Statistical analysis of change from pre-dose in FEV1 through 24 h post-dose compared with placebo group (ITT population); Least square mean difference from pre-dose FEV1 (95% CI) all values P < 0.001; *P < 0.01; NS not significant. n = 37 for placebo, n values for each dose are stated in the table
| Time | Dose (μg 0.5 ml–1) | ||||
|---|---|---|---|---|---|
| 12.5 – placebo (n = 39) | 50 – placebo (n = 38) | 100 – placebo (n = 37) | 200 – placebo (n = 37) | 400 – placebo (n = 37) | |
| 5 min | 0.06 (0.03–0.09) | 0.08 (0.04–0.11) | 0.10 (0.07–0.13) | 0.13 (0.1–0.16) | 0.14 (0.11–0.17) |
| 15 min | 0.10 (0.06–0.14) | 0.15 (0.11–0.19) | 0.17 (0.13–0.21) | 0.21 (0.17–0.25) | 0.21 (0.17–0.25) |
| 30 min | 0.10 (0.07–0.14) | 0.17 (0.14–0.21) | 0.21 (0.17–0.24) | 0.22 (0.18–0.26) | 0.23 (0.20–0.27) |
| 1 h | 0.13 (0.09–0.17) | 0.20 (0.16–0.24) | 0.21 (0.17–0.25) | 0.23 (0.20–0.27) | 0.23 (0.19–0.27) |
| 2 h | 0.13 (0.08–0.17) | 0.20 (0.16–0.25) | 0.21 (0.17–0.26) | 0.24 (0.19–0.28) | 0.22 (0.18–0.26) |
| 4 h | 0.07 (0.02–0.11)* | 0.15 (0.10–0.19) | 0.16 (0.12–0.21) | 0.21 (0.16–0.26) | 0.19 (0.15–0.24) |
| 8 h | 0.08 (0.03–0.12) | 0.14 (0.10–0.18) | 0.13 (0.09–0.18) | 0.19 (0.15–0.24) | 0.20 (0.15–0.24) |
| 12 h | 0.07 (0.02–0.11)* | 0.15 (0.10–0.19) | 0.12 (0.07–0.17) | 0.19 (0.14–0.23) | 0.17 (0.13–0.22) |
| 24 h | 0.04 (−0.01–0.08) NS | 0.07 (0.03–0.11) | 0.10 (0.06–0.14) | 0.12 (0.08–0.16) | 0.10 (0.06–0.14) |
Safety
Single doses of EP-101 were well tolerated (Table 3) with comparable AE profiles to placebo. There were no clinically significant differences from placebo in heart rate, blood pressure and ECG parameters, including QTc.
Table 3.
Number and frequency of spontaneously reported adverse events. The percentage of subjects who reported adverse events was similar comparing EP-101 and placebo treatment
| EP-101 doses | ||||||
|---|---|---|---|---|---|---|
| Patients | Placebo | 12.5 μg | 50 μg | 100 μg | 200 μg | 400 μg |
| n (%) | n = 37 | n = 39 | n = 38 | n = 37 | n = 37 | n = 37 |
| Total | 14 (38) | 16 (41) | 14 (37) | 17 (46) | 15 (41) | 13 (35) |
| Skin injury | 2 (6) | 0 | 0 | 0 | 0 | 0 |
| Headache | 4 (11) | 6 (3) | 0 | 0 | 0 | 0 |
| COPD | 0 | 2 (5) | 1 (3) | 0 | 0 | 0 |
| Cough | 5 (14) | 2 (5) | 2 (5) | 5 (14) | 3 (8) | 3 (8) |
| Dyspnoea | 2 (5) | 2 (5) | 2 (5) | 1 (4) | 1 (3) | 1 (3) |
Pharmacokinetics
The mean delivery time for all doses of EP-101 was less than 2 min (range 1.6−1.9) and EP-101 was rapidly absorbed with peak plasma concentrations within 15–30 min. Systemic exposure of EP-101 showed a dose-proportional increase in Cmax over the dose range 12.5–400 μg. There were only two measurable plasma glycopyrrolate concentrations available at the 12.5 μg dose level. Therefore, 12.5 μg was not included in the dose proportionality analysis (Figure 5, Table 4).
Figure 5.
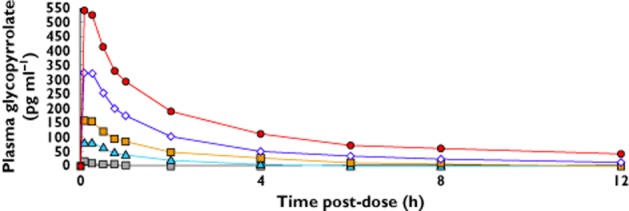
Plasma glycopyrrolate concentrations after different doses.  , 12.5 μg;
, 12.5 μg;  , 50 μg;
, 50 μg;  , 100 μg;
, 100 μg;  , 200 μg;
, 200 μg;  , 400 μg
, 400 μg
Table 4.
Pharmacokinetics summary table. Data are mean (SD) except for tmax (min−max)
| Doses | Cmax (pg ml−1) | tmax (h) | t1/2 (h) | AUC(0,t) (pg mL−1 h) | AUC(0,∞) (pg mL−1 h) |
|---|---|---|---|---|---|
| 50 μg (n = 13) | 87 (52) | 0.32 (0.015-0.35) | 0.9 (0.02) | 101 (81) | 255 (91) |
| 100 μg (n = 12) | 162 (82) | 0.26 (0.015-0.33) | 3.3 (1.2) | 306 (194) | 659 (188) |
| 200 μg (n = 12) | 347 (155) | 0.28 (0.15-0.38) | 3.4 (1.3) | 797 (505) | 796 (217) |
| 400 μg (n = 13) | 566 (269) | 0.18 (0.15-0.32) | 4.8 (3.0) | 1805 (1008) | 1642 (1077) |
The elimination half-life (t1/2) of glycopyrrolate was calculated for the 0–1 h and 0–12 h intervals. The median t1/2,0−1h ranged from 1.10−1.15 h and median t1/2, 0–12 h ranged from 2.30−7.45 h following administration of 50, 100, 200 and 400 μg, respectively.
Discussion
COPD is characterized by chronic inflammation of the airways and lung parenchyma, which causes chronic airflow limitation due to a mixture of small airway disease and parenchymal destruction, resulting in air trapping and hyperinflation. Unlike asthma, airflow limitation is progressive and is not fully reversible 11,12. LAMA alone 13 or in combination with LABA 7,14,15 or in triple therapy with inhaled corticosteroids (LABA/LAMA/ICS) 16 are well established treatments for COPD. Vagal cholinergic tone has a greater effect on airway resistance in airways narrowed by fixed obstruction and blocking of this cholinergic bronchoconstriction reduces airway resistance and in peripheral airways reduces air trapping 1. The bronchoconstrictor effect of acetylcholine is mediated mainly through M3-muscarinic receptors on airway smooth muscle cells, whereas M1-receptors enhance cholinergic reflexes. By contrast M2-receptors on cholinergic nerve endings inhibit acetylcholine release and should not be blocked. Anticholinergics, such as tiotropium and glycopyrrolate, which selectively block M1 and M3 receptors are therefore preferable to non-selective muscarinic antagonists, such as atropine and ipratropium bromide, that also block M2 receptors 17,18. Both glycopyrrolate and tiotropium are kinetically selective for M1 and M3 receptors and have a similar duration of action with slow dissociation for these receptor subtypes 4,19,20. The slow dissociation profile of glycopyrrolate contributes to the long duration of action seen in this and other clinical studies 21. By contrast there is a much more rapid dissociation from M2 receptors and this kinetic selectivity avoids prolonged blockade of pre-synaptic M2 receptors thus reducing the increased release of acetylcholine which may antagonize the bronchodilator effect of blocking the M3 receptors.
A dose-related and clinically significant improvement in FEV1 following nebulized EP-101 at 24 h indicates that this drug causes lung function improvements that are present for up to 24 h after dosing. The bronchodilatation observed is consistent with a local site specific effect 1. The magnitude of the placebo-adjusted FEV1 improvement at 24 h post-dose in this study was >100 ml at both the 100 μg and 200 μg doses (Figure 4). Extrapolating from clinical trial data 22 >100 ml change is felt to be clinically meaningful. The bronchodilator effect persists at 24 h despite low trough concentrations of drug, explained previously by the receptor profile of glycopyrrolate.
Single doses of EP-101 delivered via the investigational eFlow® nebulizer were safe and well tolerated. There were no clinically relevant significant changes in vital signs and ECG parameters, and there was an absence of the typical muscarinic side effects, such as dry mouth and increased heart rate, although this was a single dose study and these data need to be interpreted with caution. Currently available anti-cholinergic drugs all contain a quaternary ammonium, which is why these drugs do not penetrate the blood brain barrier and have a lower systemic absorption and more favourable adverse event profile then their predecessor atropine, which had considerable CNS side effects 18.
The first reported study using nebulized glycopyrrolate described bronchodilatation following methacholine challenge in asthma 21. Subsequently glycopyrrolate has been developed as a single agent 21,23,24, in combination with LABA 25–27, or triple therapy in combination with LABA, LAMA and ICS 28,29 for the treatment of both asthma and COPD. A potential disadvantage of fixed dose combination therapies may be that the fixed daily dose of glycopyrrolate is 50 μg or less. The dose−response to FEV1 suggests that the optimal dose of glycopyrrolate should be at least 100 μg, which corresponds to ΔFEV1 > 100 ml 7,30.
Tiotropium (Spiriva) is available as a single dose dry powder inhaler (DPI; Handihaler) and a soft mist inhaler (Respimat), whereas glycopyrrolate (Seebri) is available (in the EU only) as a dry powder inhaler. Neither of these marketed drugs is available for nebulized treatment. Tiotropium is only poorly soluble and not suitable for nebulization. While metered dose inhalers (MDIs) and DPIs are the most widely prescribed medications for COPD patients, up to 75% of COPD patients do not receive an optimal dose from inhalers due to poor coordination, an inability to inhale rapidly and forcefully, an inability to hold their breath for the allotted time (up to 10 s) after the dose, and exhalation into the device before or after the dose delivery 31,32. Delivery by nebulization provides an effective alternative for those patients who experience difficulties using an MDI or DPI device. Generally these patients tend to be older, more debilitated and more severely obstructed patients. These patients may find nebulizer delivery of their medications to be more effective since an optimal dose is delivered by normal (tidal) breathing, regardless of disease state 33,34.
Currently available bronchodilatation medication for nebulization are short acting muscarinic antagonists (ipratropium bromide) 35, short acting β2-adrenoceptor agonists (salbutamol, terbutaline) and long acting β2-adrenoceptor agonists (formoterol, salmeterol, indacaterol) 35, as well as a combination of ipratropium bromide and salbutamol (DuoNeB®). The short acting bronchodilators require two or four treatments a day when administered via a general purpose jet nebulizer. These medications also require 10–15 min administration time per dose, which is likely to result in lower compliance. Patients are also burdened with large, non-portable general purpose nebulizers that can take up to 10–15 min to deliver each dose. These frequent, long treatments are inconvenient, making it difficult for the patient to accept and be compliant with treatment. Therefore, COPD patients who rely on nebulized treatment would benefit from a LAMA delivered by a portable nebulizer over a shorter treatment time. This form of delivery may also be appropriate for the treatment of acute exacerbations in the future. The mean delivery time for all doses of EP-101 was less than 2 min (range 1.6–1.9 min), a rapid delivery that compares very favourably with general purpose nebulizers. This delivery using a high efficiency nebulizer (eFlow® system, PARI) also facilitates for the long term, maintenance treatment. The nebulizer device may be used once daily or if preferred as a twice daily regimen to enhance bronchodilatation and reduce side effect profile (by lowering Cmax). The eFlow® nebulizer (see Figure 1) provides glycopyrrolate as fine-sized droplets with a mass median diameter (MMD) of approximately 3.5 microns and a smaller geometric standard deviation (GSD) of approximately 1.5 with better delivery to peripheral airways and more homogenous distribution in the lungs than with standard nebulizer delivery systems. The in vitro lung dose is also increased more than three-fold by using the eFlow® nebulizer compared with general purpose nebulizers.
Mucus secretion is largely mediated by M3- receptors and M3-receptor blockade may also reduce mucus hypersecretion 18,36. Recent studies have demonstrated an anti-inflammatory action of muscarinic antagonists which includes abrogation of animal models of allergen challenge 4, reduction in neutrophil elastase activity 37 and modification of growth factors, such as EGF and TGFβ 38,39. Airway remodelling in COPD correlates with disease severity 40 and both neuronal and non-neuronal acetylcholine and muscarinic receptors appear to be involved in inflammation which suggests a potential long term benefit for LAMA therapy in COPD 41–43.
In conclusion EP-101 delivered via the investigational eFlow® nebulizer was safe and well tolerated, with no significant changes in cardiovascular signs and ECG parameters. There was a dose-related and clinically significant improvement in FEV1 following nebulized EP-101. EP-101 showed linear pharmacokinetics with dose proportionality over the 12.5–400 μg dose range. This study together provides support for development of EP-101 as a convenient nebulized LAMA bronchodilator for COPD patients.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare the study received funding from Elevation Pharmaceuticals Inc., now incorporated with Sunovion Pharmaceuticals Inc. BRL has received research funding from AstraZeneca and Pfizer in the previous 3 years. PJB has served on Scientific Advisory Boards of AstraZeneca, Boehringer-Ingelheim, Chiesi, Daiichi-Sankyo, GlaxoSmithKline, Novartis, Nycomed, Pfizer, Teva and UCB and has received research funding from Aquinox Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Chiesi, Daiichi-Sankyo, GlaxoSmithKline, Novartis, Nycomed, Pfizer and Prosonix. He is also a cofounder of RespiVert (now part of Johnson & Johnson) which has discovered novel inhaled anti-inflammatory treatments for asthma and COPD. CRJ has had no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years. AT was an employee of Elevation Pharmaceuticals when the study was conducted. DS has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards and research grants from various pharmaceutical companies including Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, CIPLA, Forest, Genetech, GlaxoSmithKline, Merck, Novartis, Pfizer and Takeda in the previous 3 years. There are no other relationships or activities that could appear to have influenced the submitted work.
This work was funded by Elevation Pharmaceuticals Inc., now incorporated with Sunovion Pharmaceuticals Inc.
References
- Gross NJ. Anticholinergic agents in COPD. Chest. 1987;91:52S–57. doi: 10.1378/chest.91.5_supplement.52s. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. The role of anticholinergics in chronic obstructive pulmonary disease. Am J Med. 2004;117(Suppl. 12A):24S–32S. doi: 10.1016/j.amjmed.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Koarai A, Traves SL, Fenwick PS, Brown SM, Chana KK, Russell RE, Nicholson AG, Barnes PJ, Donnelly LE. Expression of muscarinic receptors by human macrophages. Eur Respir J. 2012;39:698–704. doi: 10.1183/09031936.00136710. [DOI] [PubMed] [Google Scholar]
- Haddad EB, Patel H, Keeling JE, Yacoub MH, Barnes PJ, Belvisi MG. Pharmacological characterization of the muscarinic receptor antagonist, glycopyrrolate, in human and guinea-pig airways. Br J Pharmacol. 1999;127:413–420. doi: 10.1038/sj.bjp.0702573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter Healthcare Corporation. Robinul® Injection [Package Insert] Deerfield, IL: Baxter Healthcare Corporation; 2006. [Google Scholar]
- 1974. NDA 17-558 Pharmacology Review: FDA centre for drug evaluation and research.
- van Noord JA, Buhl R, Laforce C, Martin C, Jones F, Dolker M, Overend T. QVA149 demonstrates superior bronchodilation compared with indacaterol or placebo in patients with chronic obstructive pulmonary disease. Thorax. 2010;65:1086–1091. doi: 10.1136/thx.2010.139113. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, Force AET. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- Tutuncu A, Leaker B, Singh D. Dose-ranging study to assess the safety and pharmacokinetic profile of nebulized glycopyrrolate (EP-101) using high efficiency nebulizer in COPD patients. Am J Respir Crit Care Med. 2012;185:A2914. [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease. 2007. Global strategy for the diagnosis, management and prevention of COPD.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J Disease GIfCOL. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- Donohue JF. Therapeutic responses in asthma and COPD. Bronchodilators. Chest. 2004;126:125S–137. doi: 10.1378/chest.126.2_suppl_1.125S. discussion 159S–161S. [DOI] [PubMed] [Google Scholar]
- Rennard S, Bantje T, Centanni S, Chanez P, Chuchalin A, D'Urzo A, Kornmann O, Perry S, Jack D, Owen R, Higgins M. A dose-ranging study of indacaterol in obstructive airways disease, with a tiotropium comparison. Respir Med. 2008;102:1033–1044. doi: 10.1016/j.rmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Pearle J, Iezzoni D, Varghese ST. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD. 2009;6:17–25. doi: 10.1080/15412550902724073. [DOI] [PubMed] [Google Scholar]
- Singh D, Brooks J, Hagan G, Cahn A, O'Connor BJ. Superiority of ‘triple’ therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax. 2008;63:592–598. doi: 10.1136/thx.2007.087213. [DOI] [PubMed] [Google Scholar]
- Hansel TT, Barnes PJ. Tiotropium bromide: a novel once-daily anticholinergic bronchodilator for the treatment of COPD. Drugs Today (Barc) 2002;38:585–600. doi: 10.1358/dot.2002.38.9.696535. [DOI] [PubMed] [Google Scholar]
- Restrepo RD. Use of inhaled anticholinergic agents in obstructive airway disease. Respir Care. 2007;52:833–851. [PubMed] [Google Scholar]
- Koumis T, Samuel S. Tiotropium bromide: a new long-acting bronchodilator for the treatment of chronic obstructive pulmonary disease. Clin Ther. 2005;27:377–392. doi: 10.1016/j.clinthera.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Villetti G, Bergamaschi M, Bassani F, Bolzoni PT, Harrison S, Gigli PM, Janni A, Geppetti P, Civelli M, Patacchini R. Pharmacological assessment of the duration of action of glycopyrrolate vs. tiotropium and ipratropium in guinea-pig and human airways. Br J Pharmacol. 2006;148:291–298. doi: 10.1038/sj.bjp.0706724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel TT, Neighbour H, Erin EM, Tan AJ, Tennant RC, Maus JG, Barnes PJ. Glycopyrrolate causes prolonged bronchoprotection and bronchodilatation in patients with asthma. Chest. 2005;128:1974–1979. doi: 10.1378/chest.128.4.1974. [DOI] [PubMed] [Google Scholar]
- Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189:250–255. doi: 10.1164/rccm.201310-1863PP. [DOI] [PubMed] [Google Scholar]
- Walker FB, Kaiser DL, Kowal MB, Suratt PM. Prolonged effect of inhaled glycopyrrolate in asthma. Chest. 1987;91:49–51. doi: 10.1378/chest.91.1.49. [DOI] [PubMed] [Google Scholar]
- Tzelepis G, Komanapolli S, Tyler D, Vega D, Fulambarker A. Comparison of nebulized glycopyrrolate and metaproterenol in chronic obstructive pulmonary disease. Eur Respir J. 1996;9:100–103. doi: 10.1183/09031936.96.09010100. [DOI] [PubMed] [Google Scholar]
- Buhl R, Banerji D. Profile of glycopyrronium for once-daily treatment of moderate-to-severe COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:729–741. doi: 10.2147/COPD.S36001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin E, Hébert J, Gallagher N, Martin C, Overend T, Alagappan VK, Lu Y, Banerji D. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study. Eur Respir J. 2012;40:1106–1114. doi: 10.1183/09031936.00040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeh KM, Singh D, Di Scala L, Drollmann A. Once-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trial. Int J Chron Obstruct Pulmon Dis. 2012;7:503–513. doi: 10.2147/COPD.S32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Register ECT. 2011. EudraCT 2011-003588-31. Available at https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-003588-31/GB (last accessed 9 October 2014)
- Register ECT. 2012. EudraCT 2011-004759-37. Available at https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-004759-37/GB (last accessed 9 October 2014)
- Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandström T, Taylor AF, D'Andrea P, Arrasate C, Chen H, Banerji D. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1:199–209. doi: 10.1016/S2213-2600(13)70052-3. [DOI] [PubMed] [Google Scholar]
- Sestini P, Cappiello V, Aliani M, Martucci P, Sena A, Vaghi A, Canessa PA, Neri M, Melani AS Group AIPOE. Prescription bias and factors associated with improper use of inhalers. J Aerosol Med. 2006;19:127–136. doi: 10.1089/jam.2006.19.127. [DOI] [PubMed] [Google Scholar]
- Rau JL. Practical problems with aerosol therapy in COPD. Respir Care. 2006;51:158–172. [PubMed] [Google Scholar]
- Hanania NA, Donohue JF. Pharmacologic interventions in chronic obstructive pulmonary disease: bronchodilators. Proc Am Thorac Soc. 2007;4:526–534. doi: 10.1513/pats.200701-016FM. [DOI] [PubMed] [Google Scholar]
- Barta SK, Crawford A, Roberts CM. Survey of patients' views of domiciliary nebuliser treatment for chronic lung disease. Respir Med. 2002;96:375–381. doi: 10.1053/rmed.2001.1292. [DOI] [PubMed] [Google Scholar]
- Dahl R, Greefhorst LA, Nowak D, Nonikov V, Byrne AM, Thomson MH, Till D, Della Cioppa G Group FiCOPDIS. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:778–784. doi: 10.1164/ajrccm.164.5.2007006. [DOI] [PubMed] [Google Scholar]
- Moulton BC, Fryer AD. Muscarinic receptor antagonists, from folklore to pharmacology; finding drugs that actually work in asthma and COPD. Br J Pharmacol. 2011;163:44–52. doi: 10.1111/j.1476-5381.2010.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai N, Kondo M, Izumo T, Tamaoki J, Nagai A. Inhibition of neutrophil elastase-induced goblet cell metaplasia by tiotropium in mice. Eur Respir J. 2010;35:1164–1171. doi: 10.1183/09031936.00040709. [DOI] [PubMed] [Google Scholar]
- Kong KC, Billington CK, Gandhi U, Panettieri RA, Penn RB. Cooperative mitogenic signaling by G protein-coupled receptors and growth factors is dependent on G(q/11) FASEB J. 2006;20:1558–1560. doi: 10.1096/fj.05-5622fje. [DOI] [PubMed] [Google Scholar]
- Gosens R, Dueck G, Rector E, Nunes RO, Gerthoffer WT, Unruh H, Zaagsma J, Meurs H, Halayko AJ. Cooperative regulation of GSK-3 by muscarinic and PDGF receptors is associated with airway myocyte proliferation. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1348–1358. doi: 10.1152/ajplung.00346.2007. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- Kistemaker LE, Oenema TA, Meurs H, Gosens R. Regulation of airway inflammation and remodeling by muscarinic receptors: perspectives on anticholinergic therapy in asthma and COPD. Life Sci. 2012;91:1126–1133. doi: 10.1016/j.lfs.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Gosens R, Bos IS, Zaagsma J, Meurs H. Protective effects of tiotropium bromide in the progression of airway smooth muscle remodeling. Am J Respir Crit Care Med. 2005;171:1096–1102. doi: 10.1164/rccm.200409-1249OC. [DOI] [PubMed] [Google Scholar]
- Grainge CL, Lau LC, Ward JA, Dulay V, Lahiff G, Wilson S, Holgate S, Davies DE, Howarth PH. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364:2006–2015. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]


