Abstract
Aims
To evaluate the pharmacokinetics (PK) and pharmacodynamics (PD) of subcutaneous peginterferon beta-1a in patients with relapsing-remitting multiple sclerosis (RRMS) in the phase 3 ADVANCE study (n = 1512).
Methods
During year 1, patients were randomized (1:1:1) to placebo or peginterferon beta-1a 125 μg every 2 or 4 weeks. After year 1, patients randomized to placebo were re-randomized to 125 μg peginterferon beta-1a administered every 2 weeks or every 4 weeks for year 2. Patients randomized to peginterferon beta-1a in year 1 remained on the same dosing regimen in year 2. Intensive blood samples for PK and PD (neopterin elevation; a biomarker of pharmacological activity induced by interferon beta-1a) measurements were collected from 44 patients pre-dosing and at intervals over 240 h post-dosing at weeks 4 and 24. Sparse samples were collected from all patients after each dosing at weeks 4, 12, 24, 56 and 84.
Results
The PK profile of peginterferon beta-1a did not change over time or between dosing regimens. No accumulation was observed. Peak serum concentrations were reached 1–1.5 days post-dosing, with a mono-phasic decline and a median half-life of approximately 2–3 days. Dosing every 2 weeks provided approximately two-fold greater monthly cumulative area under the curve than every 4 weeks. Neopterin elevation was sustained for 10−14 days following each dose, indicating doubled cumulative duration of pharmacological activity for dosing every 2 weeks vs. every 4 weeks.
Conclusions
These PK/PD profiles potentially explain the enhanced efficacy of dosing every 2 weeks in patients with RRMS.
Keywords: interferon beta-1a, multiple sclerosis, pegylation
What is Already Known about this Subject
In relapsing-remitting multiple sclerosis (RRMS) patients, subcutaneous peginterferon beta-1a provides improvements in clinical and magnetic resonance imaging (MRI), endpoints vs. placebo (numerically greater efficacy with dosing every 2 weeks than every 4 weeks).
The pharmacokinetics (PK) and pharmacodynamics (PD) of peginterferon beta-1a have been characterized in healthy subjects. An assessment of these profiles is yet to be determined in RRMS patients.
What this Study Adds
PK and PD were characterized in RRMS patients and the impact of demographics and immunogenicity on PK parameters was explored.
Every 2 weeks dosing provided ∼two-fold greater monthly cumulative AUC and a two-fold longer neopterin elevation vs. every 4 weeks, which may explain the enhanced efficacy of dosing every 2 weeks and informs dosing strategies in RRMS.
Introduction
Subcutaneous peginterferon beta-1a is a pegylated form of interferon (IFN) beta-1a is approved in the US for the treatment of relapsing multiple sclerosis, and in the EU for the treatment of relapsing-remitting multiple sclerosis (RRMS) 1,2. In phase 1 studies, compared with non-pegylated IFN beta-1a, peginterferon beta-1a had a longer terminal half-life (t1/2), greater exposure (peak serum concentration [Cmax] and area under the concentration–time curve [AUC]), and prolonged and higher elevations in well-characterized pharmacodynamic (PD) markers of type 1 interferon receptor activation (serum neopterin and 2′-5′-oligoadenylate synthetase) 3. These characteristics support the notion that pegylation of IFN beta-1a provides potential for increased biologic activity and more convenient, less frequent dosing.
In the ADVANCE study, a phase 3 multicentre, randomized, double-blind study with a 1 year placebo-controlled period, 1512 patients with RRMS were randomized to and received either placebo (n = 500) or peginterferon beta-1a 125 μg administered once every 2 weeks (n = 512) or once every 4 weeks (n = 500) 4. At year 1 of ADVANCE, peginterferon beta-1a dosed every 2 weeks reduced annualized relapse rate [ARR; primary endpoint] by 36%. Risk of relapse, risk of disability progression and the number of new or newly-enlarging T2 lesions (secondary endpoints), along with gadolinium-enhancing (Gd+) lesions (tertiary endpoint) were also reduced, when compared with placebo 4. The safety profile reflected that of established IFN beta-1a therapies. While peginterferon beta-1a dosed every 4 weeks also had a significant effect on the clinical endpoints, the reduction relative to placebo in ARR and magnetic resonance imaging (MRI) endpoints was numerically greater in the every 2 weeks dosing group compared with the every 4 weeks dosing group.
The objectives of these intensive pharmacokinetic (PK) and PD analyses from the ADVANCE study were to estimate PK and PD parameters of peginterferon beta-1a in patients with RRMS, to explore covariates that influenced PK parameters and to assess the impact of treatment-emergent antibodies on PK and PD parameters.
Methods
Study design and patients
ADVANCE was a 2 year, phase 3, multicentre, randomized, double-blind, parallel group study with a 1 year placebo-controlled period. The full methods of the ADVANCE study have been published previously 4. During year 1 of the study, patients were randomized (1:1:1) to receive subcutaneous injections of placebo, peginterferon beta-1a at a dose of 125 μg every 2 weeks, or peginterferon beta-1a 125 μg every 4 weeks. To maintain the blind, patients received either peginterferon beta-1a or placebo every 2 weeks. Those randomized to the peginterferon beta-1a dosed every 4 weeks group received alternate injections of placebo and peginterferon beta-1a every 2 weeks. At the end of year 1, patients on placebo were re-randomized to peginterferon beta-1a 125 μg dosed every 2 weeks or every 4 weeks. Thus, during year 2, all patients received dose-frequency blinded peginterferon beta-1a. Key eligibility criteria were a diagnosis of RRMS as defined by the McDonald criteria, an age of 18 to 65 years, a score of between 0 to 5 on the Expanded Disability Status Scale (EDSS, with higher scores indicating greater disability 5), and at least two clinically documented relapses in the previous 3 years, with at least one of these relapses having occurred within the 12 months prior to randomization. Patients who had progressive forms of multiple sclerosis (MS), pre-specified laboratory abnormalities and prior interferon treatment for MS exceeding 4 weeks or discontinuation less than 6 months prior to baseline were excluded. The protocol was approved by each site's institutional review board and was conducted according to the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. Every patient provided written informed consent prior to study entry.
Blood sampling
Blood samples were taken from all patients for PK and PD analysis, using a combination of intensive and sparse sampling strategies. The intensive PK and PD samples were targeted to be taken from 5% of the overall population who provided additional consent for the intensive PK study pre-dose, and at 6, 24, 28, 36, 72, 120, 168 and 240 h post-dose during week 4 and week 24 (the first week of the study was designated as week 0).
For sparse sampling, one sample was taken after each dosing at weeks 4, 12, 24, 56 and 84. The window for drawing a sparse sample was defined as at least 1 h after the last dose administered at week 4 and no later than 10 days after the scheduled dosing date at weeks 12, 24, 56 and 84. The rationale for this sampling window was that the serum drug concentration may fall under the lower limit of quantification (LLOQ) by 10 days post-dose, which would render a PK blood draw irrelevant. The sampling window at week 4 was shorter as the patients were in the office during this visit.
Determination of serum concentrations of peginterferon beta-1a
Serum concentrations of peginterferon beta-1a were evaluated using an enzyme-linked immunosorbent assay (ELISA). The quantitative range was 31.3–1500 pg ml−1. The accuracy of the assay was in the range of 100.4–103.6% during study testing and the precision of the assay, expressed as percent coefficient of variation (%CV) and evaluated using assay controls, was less than 7%. Serum neopterin was measured using a validated competition ELISA. The quantitative range was 1.29–101 ng ml−1. The accuracy range of the assay was 97.9–99.7% during study testing and the precision of the assay, expressed as %CV and evaluated using assay controls, was less than 10%.
Immunogenicity assays
Immunogenicity assays, including testing of anti-interferon binding antibodies (anti-IFN BAbs), anti-interferon neutralizing antibodies (anti-IFN NAbs) and antibodies directed against the polyethyleneglycol (PEG) moiety of peginterferon beta-1a (anti-PEG antibodies), were performed using validated assays. Anti-IFN BAbs were detected by a sandwich ELISA. Positive serum samples were subsequently tested for anti-IFN NAbs in a cell-based myxovirus protein A induction assay and then titered to generate a quasi-quantitative assessment of NAbs concentration. Anti-PEG antibodies were tested and titred to generate a quasi-quantitative assessment in an ELISA.
PK and PD analyses
The PK and PD parameters were calculated by non-compartmental analysis using Phoenix WinNonLin software (Pharsight, Sunnyvale, CA, USA) based on intensive data. The PK parameters included AUC(0,τ), AUC(0,168 h), Cmax, time to reach Cmax (tmax), apparent total clearance (CL/F), t1/2 and apparent volume of distribution (Vd/F). PD parameters included median peak neopterin concentration (Epeak), area under the effect–time-curve over 336 h from dosing (EAUC(0,336 h)), and time to reach maximum effect (Etmax). Correction for baseline neopterin concentration was applied for calculation of Epeak. EAUC(0,336 h) was estimated because neopterin elevation lasted up to 2 weeks based on a previous study and was calculated by defining partial area in Phoenix WinNonLin. Correlations between AUC(0,τ) (mean of week 4 and 24) and selected baseline demographic characteristics (age, gender, weight, body mass index [BMI] and body surface area [BSA]) were examined, using a linear regression analysis. As renal function has been shown to affect clearance of peginterferon beta-1a 6, correlation between AUC over the dosing period (AUC(0,τ)) and creatinine clearance (CLcr) rates, estimated by the Cockcroft−Gault equation 7, was also examined. The impact of treatment-emergent antibodies on peginterferon beta-1a exposure was assessed by comparing the individual parameters in antibody positive (up to week 24) subjects with the respective group median values, as well as via visual inspection of sparse PK concentration–time plot stratified by antibody status.
Results
Patient demographics
A full description of participant flow in the ADVANCE study and details of baseline demographic and clinical characteristics in each treatment group have been published previously 4. Forty-four patients consented to the intensive PK and PD subgroup at year 1 of the ADVANCE study. Among these, four male and 21 female patients received peginterferon beta-1a (every 2 weeks n = 12; every 4 weeks n = 13) and had sufficient time point samples for evaluation of PK and PD parameters. Of these patients, mean (SD) age was 36.2 (10.6) years, mean (SD) weight was 69.9 (16.0) kg, mean (SD) BMI was 25.7 (5.4) kg m−2, and mean (SD) BSA was 1.78 (0.22) m2.
Pharmacokinetics
In general, PK profiles were similar across treatment groups (every 2 weeks vs. every 4 weeks dosing) and over time (week 4 vs. week 24) (Figure 1 and Table 1). For both every 2 weeks and every 4 weeks dosing groups, no accumulation was observed. Overall, Cmax was reached at approximately 1 to 1.5 days post-dosing, followed by a monophasic decline, with a median t1/2 of approximately 2–3 days for both every 2 weeks and every 4 weeks dosing groups. By doubling the dosing frequency, the every 2 weeks dosing regimen provided approximately two-fold greater median cumulative AUC over a month (58.6 ng ml−1 h vs. 28.1 ng ml−1 h) and similar Cmax as compared with the every 4 weeks dosing regimen. High variability was observed in peginterferon beta-1a PK parameters. Percent coefficient of variation (%CV) ranges were 41–68% for AUC(0,τ), 74–89% for Cmax and 45–93% for t1/2 (Table 1).
Figure 1.
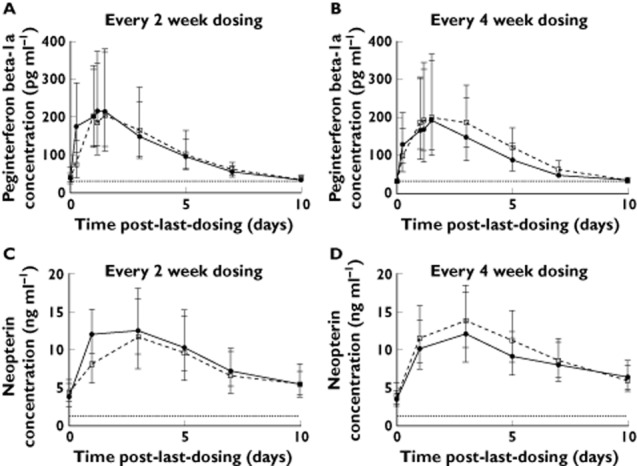
Peginterferon beta-1a concentration-time profiles (A and B) and neopterin concentration−time profiles (C and D) from intensive subjects. Circles represent geometric mean concentration at week 4; squares represent geometric mean concentration at week 24; error bars indicate 95% confidence interval; dotted lines represent lower limit of quantitation, 31.3 pg ml−1 and 1.29 ng ml−1 for peginterferon beta-1a and neopterin, respectively
Table 1.
Summary of PK and PD parameters (median; mean [percent coefficient of variation]) from intensive subjects
| Peginterferon beta-1a every 2 weeks | Peginterferon beta-1a every 4 weeks | |||
|---|---|---|---|---|
| Week 4 | Week 24 | Week 4 | Week 24 | |
| PK parameters | ||||
| AUC(0,τ) (ng ml−1 h) | 30.8; 34.5 (68) | 29.28; 34.85 (50) | 34.8; 34.9 (41) | 28.1; 32.9 (60) |
| AUC(0,168 h) (ng ml−1 h) | 25.8; 28.3 (82) | 24.5; 30.0 (59) | 32.0; 30.7 (49) | 23.5; 29.5 (61) |
| CL/F (l h−1) | 4.06; 7.89 (168) | 4.27; 4.13 (32) | 3.60; 5.48 (109) | 4.46; 5.93 (74) |
| Cmax (pg ml−1) | 236; 321 (85) | 221; 280 (89) | 264; 309 (76) | 202; 305 (74) |
| t1/2 (h) | 56.7; 76.4 (93) | 62.8; 77.3 (57) | 46.2; 77.2 (83) | 56.8; 67.7 (45) |
| tmax (h) | 28.5; 26.7 (39) | 35.9; 37.4 (37) | 34.6; 33.9 (45) | 35.1; 43.4 (50) |
| Vd/F (l) | 336; 596 (99) | 403; 479 (66) | 248; 817 (177) | 366; 726 (112) |
| PD parameters | ||||
| Epeak (ng ml−1) | 10.8; 11.3 (44) | 6.53; 11.45 (141) | 8.99; 9.99 (70) | 8.25; 11.6 (72) |
| EAUC(0,336 h) (ng ml−1 h) | 1847; 1698 (50) | 956; 1717 (147) | 1749; 1865 (59) | 1397; 1766 (71) |
| Etmax (h) | 70.3; 59.7 (60) | 72.0; 76.6 (35) | 72.2; 100.3 (74) | 72.0; 79.8 (35) |
AUC(0,τ) = AUC over the dosing period; AUC(0,168 h) = AUC from time 0–168 h post-dose; Cmax = peak serum concentration; CL/F = apparent peginterferon beta-1a clearance; EAUC(0,336 h) = area under the concentration curve after baseline correction through 336 h post-dose; Epeak = peak concentration after correcting for baseline; Etmax = time to reach maximum effect; t1/2 = terminal half-life; tmax = time to reach peak serum concentration; Vd/F = apparent volume of distribution.
Figure 2 shows the correlation between AUC(0,τ) and selected demographic characteristics, and renal function based on estimation of CLcr. BSA, BMI and weight showed weak correlation (r2 ≈ 0.3), indicating an inverse correlation between body size and peginterferon beta-1a exposure. CLcr also correlated inversely with AUC(0,τ) (r2 = 0.24), consistent with our previous report 6. There was almost no correlation between peginterferon beta-1a exposure and age or gender.
Figure 2.
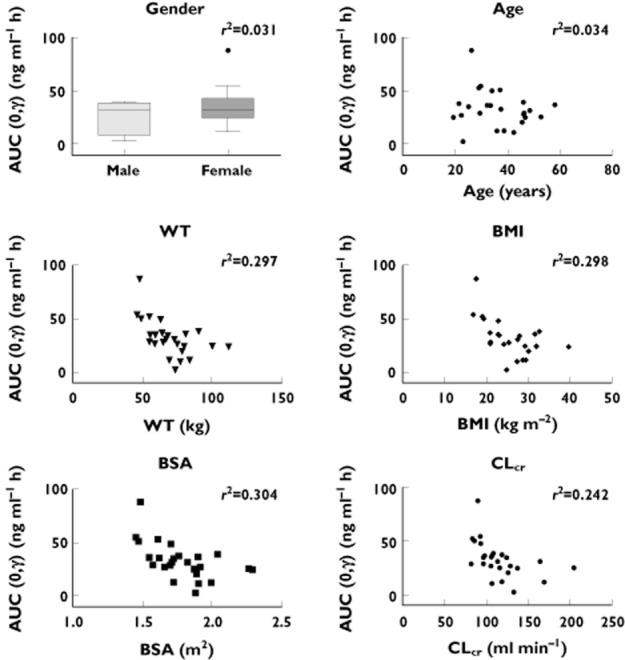
Correlation between peginterferon beta-1a AUC(0,τ) and demographic characteristics and creatinine clearance. AUC(0,τ) (mean of weeks 4 and 24) are shown for intensive subjects. Linear regression lines are shown with r2 values. BMI = body mass index; BSA = body surface area; CLcr = creatinine clearance estimated based on Cockcroft–Gault equation; WT = weight
Presence of anti-PEG antibodies did not have an impact on peginterferon beta-1a exposure. The AUC(0,τ) and Cmax in the two intensive PK subjects who were positive for anti-PEG antibodies were similar to the overall group median. In the sparse PK sampling population, peginterferon beta-1a exposure was similar for patients who were positive and negative for anti-PEG antibodies (Figure 3A, B). The impact of anti-IFN BAbs on peginterferon beta-1a exposure was inconclusive. In one patient from the intensive PK sampling population, AUC(0,τ) and Cmax were comparable with the overall group median at week 4, but much lower at week 24. Another anti-IFN BAbs positive patient showed no detectable drug at week 24 (one patient without anti-IFN BAb or anti-PEG antibodies also had no detectable drug in one time course). When peginterferon beta-1a concentrations in patients with sparse sampling were examined, no consistent trend could be determined, as a result of a small number of patients with positive anti-IFN BAbs (Figure 3C, D). The impact of anti-IFN NAbs on PK parameters could not be assessed as no intensive PK patients and few sparse PK patients were positive for anti-IFN Nabs.
Figure 3.
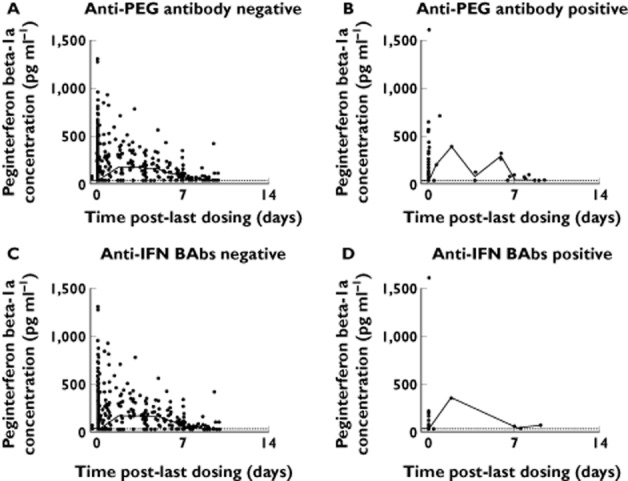
Peginterferon beta-1a concentration−time profiles stratified by antibody status in patients with sparse sampling only. Data from every 2 weeks and every 4 weeks groups at weeks 4, 12, 24, 48, 56 and 84 were pooled. Open circles represent observed data; solid lines represent median values in specified time windows; dashed lines indicate lower limit of quantitation (LLOQ; 31.3 pg ml−1). Concentrations below LLOQ are shown as LLOQ
Pharmacodynamics
Neopterin concentration increased in all intensive PD subjects following dosing, confirming pharmacological activity of peginterferon beta-1a. Unlike PK profiles, which did not change over time, the magnitude of neopterin elevation decreased over time for the every 2 weeks dosing group (Figure 1 and Table 1). In general, the neopterin concentrations peaked at approximately 3 days post-dosing, which was 1.5 to 2 days later than peginterferon beta-1a peak time, and remained above the baseline level at 10 days post-dosing (Figure 1), after peginterferon beta-1a concentration had dropped below the LLOQ. Comparing neopterin pre-dose concentrations at weeks 0, 4, and 24 for both groups, it was evident that neopterin concentrations had returned to baseline within 2 weeks after the previous dose for both the every 2 weeks and every 4 weeks dosing groups (Figure 4). The Epeak and EAUC(0,336 h) of the every 2 weeks dosing group decreased by 40% and 48%, respectively, from week 4 to week 24. No obvious decrease was observed for the every 4 weeks dosing group. Median monthly cumulative EAUC(0,336 h) was 1912 ng ml−1 h for the every 2 weeks dosing group and 1397 ng ml−1 h for the every 4 weeks dosing group, based on week 24 data assuming steady-state. The PD parameters were highly variable with the %CV of Epeak and EAUC(0,336 h) ranging from 44% to 147% (Table 1).
Figure 4.
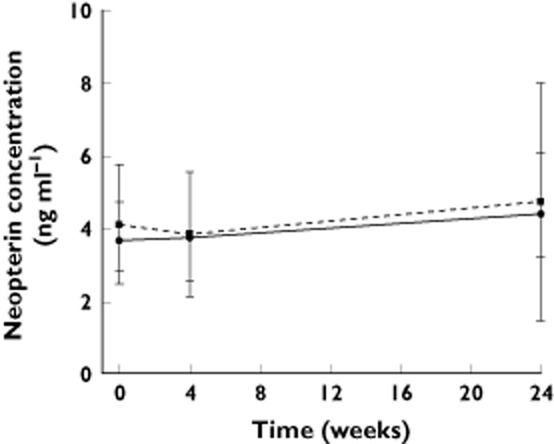
Pre-dose neopterin concentration in week 0, week 4 and week 24 for intensive subjects. Circles represent geometric mean concentration for the every 2 weeks group; squares represent geometric mean concentration for the every 4 weeks group; error bars indicate 95% confidence interval
Anti-PEG antibodies and anti-IFN BAbs did not seem to impact the neopterin response. Intensive PD subjects with positive anti-PEG antibodies or anti-IFN BAbs exhibited similar PD parameters, as compared with group median values. Neopterin concentration–time profiles did not suggest any trend of decrease in antibody-positive subjects (Figure 5).
Figure 5.
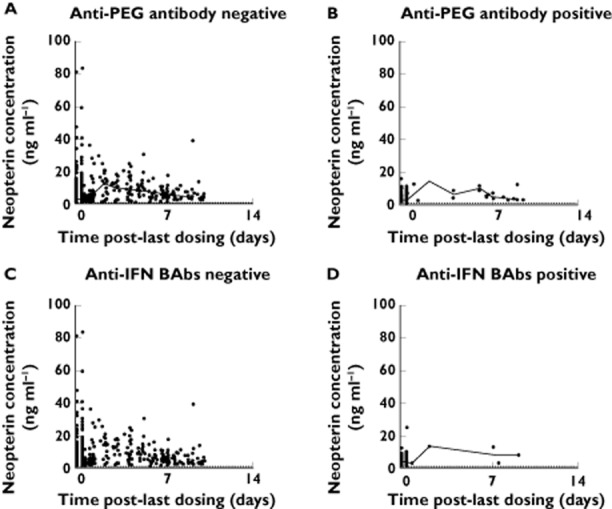
Neopterin concentration−time profiles stratified by antibody status in patients with sparse sampling only. Data from every 2 weeks and every 4 weeks groups at weeks 4, 12, 24, 48, 56 and 84 were pooled. Open circles represent observed data; solid lines represent median values in specified time windows; dashed lines indicate lower limit of quantitation (LLOQ; 1.29 pg ml−1). Concentrations below LLOQ are shown as LLOQ
Discussion
The PK profile of subcutaneous peginterferon beta-1a 125 μg in the present study in RRMS patients is consistent with that in healthy subjects. Median AUC from time 0 to 168 h post-dose (AUC(0,168 h)) was reported to be 27.2 ng ml−1 h in healthy volunteers 3, which compares with the median AUC(0,168 h) that ranged from 23.5 to 32.0 ng ml−1 h in the present study in RRMS patients. The PK parameters exhibited high variability in both populations. These results suggest that RRMS does not alter disposition of peginterferon beta-1a. Therefore, previous PK analyses in healthy subjects, which showed that peginterferon beta-1a 125 μg provided a nine-fold higher AUC relative to intramuscular administration of non-pegylated IFN beta-1a 30 μg (based on a cytopathic effect assay) 3, could be extrapolated to the RRMS population. Corrected for dosing frequency, peginterferon beta-1a every 2 weeks and every 4 weeks dosing regimens provided 4.5-fold and 2.3-fold higher cumulative AUC, as compared with non-pegylated IFN beta-1a administered weekly. Although definitive exposure−efficacy relationships are yet to be established, the increased cumulative exposure potentially explains the maintained efficacy of peginterferon beta-1a despite its reduced dosing frequency. The AUC from time 0 to 96 h post-dose (AUC(0,96 h)) of another non-pegylated IFN beta-1a is reported to be 294 ± 81 IU ml−1 h 8 following a single 60 μg dose. When considering a 240% accumulation at steady-state, correcting for dose, IFN beta-1a 44 μg three times weekly provides a cumulative monthly (12 doses) AUC of 23.0 ng ml−1 h (units converted based on in vitro specific activity of 270 mIU mg−1. In comparison, peginterferon beta-1a dosing every 2 weeks and every 4 weeks provided 2.5- and 1.2-fold higher monthly cumulative AUC, respectively. It should be noted that a head-to-head comparison study would provide a more reliable comparison of the cumulative exposure of pegylated and non-pegylated IFN beta-1a products. Nevertheless, AUC comparisons for peginterferon beta-1a and non-pegylated IFN beta-1a confirm the desired PK modification by pegylation and support the concept of pegylation 9.
For peginterferon beta-1a, no accumulation was observed. The accumulation observed with other subcutaneous pegylated IFNs such as for pegylated IFN alpha-2b (PEG-Intron®) 10 and peginterferon alfa-2a (Pegasys®) 11, which are dosed at 1.5 μg kg−1 and 180 μg weekly, respectively, compared with peginterferon beta-1a could be due to the more frequent weekly dosing schedules.
Correlations between PK parameters and demographic characteristics were explored in the present study, using a two stage approach 12. The two stage approach has been applied in new drug development and evaluation processes for more than 30 years 13–16. The main advantage of the two stage approach is minimal data manipulation and the mean estimates of parameters are usually unbiased. However, rich PK data are required for each subject. Multivariate analysis or non-linear regression is rarely carried out using this approach. Additionally, the random effects are likely to be overestimated. An alternative approach would be population PK analysis, which could combine information from subjects with intensive and sparse PK sampling and test for multiple covariates on multiple PK parameters simultaneously. A population PK analysis is currently underway and relationships analyzed in this report will be further examined.
Similar pharmacological activity was observed for peginterferon beta-1a 125 μg administered every 2 weeks and every 4 weeks, based on neopterin concentrations, which peaked at approximately 3 days after each dosing. The neopterin response decreased over time in the present study (week 4 vs. week 24), especially in the peginterferon beta-1a every 2 weeks dosing group. Previous studies in humans and monkeys have shown that continuous exposure to interferon beta-1a reduced the magnitude of neopterin response 17,18 It is thought that this may be a result of the down regulation of the type 1 IFN receptor 19 or inhibitory feedback by prolonged elevation of neopterin concentrations 20. However, neopterin did remain elevated for 10−14 days following each dose. These results suggested that neopterin and the monthly cumulative duration of IFN pharmacological activity of the every 2 weeks dosing group is therefore doubled relative to the every 4 weeks dosing group.
Treatment-emergent anti-PEG antibodies did not seem to impact peginterferon beta-1a PK or PD. The impact of treatment-emergent anti-IFN BAbs and NAbs was inconclusive due to low incidence in patients in the ADVANCE study. In the phase 3 ADVANCE study, administration of peginterferon beta-1a every 2 weeks consistently provided statistically significant differences vs. placebo for all clinical measures, with numerically greater treatment effects across relapse activity outcomes and MRI endpoints vs. peginterferon beta-1a administered every 4 weeks 4. The approximate two-fold higher monthly cumulative exposure and longer monthly cumulative duration of PD response in the peginterferon beta-1a every 2 weeks dosing group, compared with the every 4 weeks dosing group, seen in this study was expected based on the dose regimens used and the results of previous studies with peginterferon beta-1a 3, and may partly explain the better clinical efficacy with peginterferon beta-1a dosed every 2 weeks vs. every 4 weeks in the phase 3 study 4.
In conclusion, peginterferon beta-1a dosed every 2 weeks provided approximately two-fold greater cumulative AUC over a month relative to the every 4 weeks regimen, with a similar Cmax. No accumulation was observed. Neopterin elevations were sustained for approximately 2 weeks post-dosing and dosing every 2 weeks provided a two-fold longer duration of pharmacological activity relative to dosing every 4 weeks. Results from this PK and PD study may provide an explanation for the enhanced efficacy observed with peginterferon beta-1a dosed every 2 weeks compared with every 4 weeks in patients with RRMS.
Competing Interests
The study was sponsored by Biogen Idec Inc (Cambridge, MA, USA). All authors have completed the Unified ICJME Competing Interest form and declare that they are employees of Biogen Idec Inc., own stocks and have stock options in Biogen Idec Inc.
We wish to thank the patients who volunteered for this study and the site staff members who help to conduct the study. The authors were assisted in the preparation of the manuscript by Shelley Davies PhD, a professional medical writer contracted to CircleScience, part of KnowledgePoint360, an Ashfield Company. Writing support was funded by the study sponsor.
Contributors
All authors provided critical review of all drafts of the manuscript, had full editorial control of the manuscript and provided their final approval of all content. XH and IN contributed to the design and execution of the study, and analysis and interpretation of data. JW provided oversight of bioanalysis (PK, PD, anti-drug antibodies) and scientific discussion of impact on PK/PD. YC performed statistical analyses and contributed to interpretation of data. YZ contributed to the design of the study, and analysis and interpretation of data. SH and AD contributed to the design of the study, and interpretation of data.
XH, YC, JW, YZ, AD, IN and SH had support from Biogen Idec Inc. for the submitted work. XH, YC, JW, YZ, AD, IN and SH are employees of Biogen Idec Inc., own stocks and have stock options in Biogen Idec Inc in the previous 3 years. There are no other relationships or activities that could appear to have influenced the submitted work.
References
- Cambridge, MA: Biogen Idec Inc; 2014. Plegridy (peginterferon beta-1a) Package Insert. [Google Scholar]
- Maidenhead, Berkshire, UK: Biogen Idec Inc; 2014. Plegridy (Peginterferon beta-1a) Package Insert. [Google Scholar]
- Hu X, Miller L, Richman S, Hitchman S, Glick G, Liu S, Zhu Y, Crossman M, Nestorov I, Gronke RS, Baker DP, Rogge M, Subramanyam M, Davar G. A novel PEGylated interferon beta-1a for multiple sclerosis: safety, pharmacology, and biology. J Clin Pharmacol. 2012;52:798–808. doi: 10.1177/0091270011407068. [DOI] [PubMed] [Google Scholar]
- Calabresi PA, Kieseier BC, Arnold DL, Balcer LJ, Boyko A, Pelletier J, Liu S, Zhu Y, Seddighzadeh A, Hung S, Deykin A. Pegylated interferon beta-1a in relapsing-remitting multiple sclerosis: results from ADVANCE, a randomized, phase 3, double-blind study. Lancet Neurol. 2014;13:657–665. doi: 10.1016/S1474-4422(14)70068-7. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Hu X, Seddighzadeh A, Stecher S, Miller L, Zhu Y, Hung S. PEGylated interferon beta-1a pharmacokinetics, pharmacodynamics, and safety in subjects with normal or impaired renal function. Neurology. 2012;78:P06.165. [Google Scholar]
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Rebif® (IFN Beta-1a) Package Insert. Rockland, MA: EMD Serono, Inc; 2013. [Google Scholar]
- Fishburn CS. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J Pharm Sci. 2008;97:4167–4183. doi: 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- PegIntron® (Peginterferon alfa-2b) Package Insert. Whitehouse Station, NJ: Merck; 2003. [Google Scholar]
- PEGASYS (Peginterferon alfa-2a) Package Insert. South San Francisco, CA: Genentech, Inc; 2013. [Google Scholar]
- FDA [Internet] Guidance for Industry Population Pharmacokinetics. Rockville, MD: U.S. Department of Health and Human Services Food and Drug Administration; 1999. [updated 1999 February]. Available at http://www.fda.gov/downloads/Drugs/Guidances/UCM072137.pdf (last accessed 22 July 2014) [Google Scholar]
- Sheiner LB, Beal SL. Evaluation of methods for estimating population pharmacokinetic parameters. I. Michaelis-Menten model: routine clinical pharmacokinetic data. J Pharmacokinet Biopharm. 1980;8:553–571. doi: 10.1007/BF01060053. [DOI] [PubMed] [Google Scholar]
- Sheiner LB, Beal SL. Evaluation of methods for estimating population pharmacokinetic parameters. II. Biexponential model and experimental pharmacokinetic data. J Pharmacokinet Biopharm. 1981;9:635–651. doi: 10.1007/BF01061030. [DOI] [PubMed] [Google Scholar]
- Sheiner LB, Beal SL. Evaluation of methods for estimating population pharmacokinetic parameters. III. Monoexponential model: routine clinical pharmacokinetic data. J Pharmacokinet Biopharm. 1983;11:303–319. doi: 10.1007/BF01061870. [DOI] [PubMed] [Google Scholar]
- Steimer JL, Mallet A, Golmard JL, Boisvieux JF. Alternative approaches to estimation of population pharmacokinetic parameters: comparison with the nonlinear mixed-effect model. Drug Metab Rev. 1984;15:265–292. doi: 10.3109/03602538409015066. [DOI] [PubMed] [Google Scholar]
- Rothuizen LE, Buclin T, Spertini F, Trinchard I, Munafo A, Buchwalder PA, Ythier A, Biollaz J. Influence of interferon beta-1a dose frequency on PBMC cytokine secretion and biological effect markers. J Neuroimmunol. 1999;99:131–141. doi: 10.1016/s0165-5728(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Mager DE, Neuteboom B, Efthymiopoulos C, Munafo A, Jusko WJ. Receptor-mediated pharmacokinetics and pharmacodynamics of interferon-beta1a in monkeys. J Pharmacol Exp Ther. 2003;306:262–270. doi: 10.1124/jpet.103.049502. [DOI] [PubMed] [Google Scholar]
- Pestka S, Langer JA, Zoon KC, Samuel CE. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Liberati AM, Fizzotti M, Proietti MG, Di Marzio R, Schippa M, Biscottini B, Senatore M, Berruto P, Canali S, Peretti G. Biochemical host response to interferon-beta. J Interferon Res. 1988;8:765–777. doi: 10.1089/jir.1988.8.765. [DOI] [PubMed] [Google Scholar]


