Abstract
Background
Nephrotoxicity is a serious adverse effect of calcineurin inhibitor therapy in patients after heart transplantation (HTX).
Aim
In this retrospective registry study, renal function within the first 2 years after HTX in patients receiving de novo calcineurin inhibitor treatment, that is, cyclosporine A (CSA) or tacrolimus (TAC), was analyzed. In a consecutive subgroup analysis, renal function in patients receiving conventional tacrolimus (CTAC) was compared with that of patients receiving extended-release tacrolimus (ETAC).
Methods
Data from 150 HTX patients at Heidelberg Heart Transplantation Center were retrospectively analyzed. All patients were continuously receiving the primarily applied calcineurin inhibitor during the first 2 years after HTX and received follow-up care according to center practice.
Results
Within the first 2 years after HTX, serum creatinine increased significantly in patients receiving CSA (P<0.0001), whereas in patients receiving TAC, change of serum creatinine was not statistically significant (P=not statistically significant [ns]). McNemar’s test detected a significant accumulation of patients with deterioration of renal function in the first half year after HTX among patients receiving CSA (P=0.0004). In patients receiving TAC, no significant accumulation of patients with deterioration of renal function during the first 2 years after HTX was detectable (all P=ns). Direct comparison of patients receiving CTAC versus those receiving ETAC detected no significant differences regarding renal function between patients primarily receiving CTAC or ETAC treatment during study period (all P=ns).
Conclusion
CSA is associated with a more pronounced deterioration of renal function, especially in the first 6 months after HTX, in comparison with patients receiving TAC as baseline immunosuppressive therapy.
Keywords: heart transplantation, renal function, extended-release tacrolimus
Introduction
Calcineurin inhibitor (CNI-)-based immunosuppressive therapy is mainly used to prevent acute rejection episodes (AREs) in patients after heart transplantation (HTX).1,2 Referring to current International Society for Heart and Lung Transplantation registry data, the use of cyclosporine A (CSA) decreased in recent years, and accordingly, tacrolimus (TAC) has become the predominant CNI in patients after HTX, as TAC is more effective in avoiding AREs.1,3–7 Given the improved patient mortality during the last decades, possible toxic effects of immunosuppression, especially nephrotoxicity, have to be considered more carefully.8 In addition, prevention of severe renal dysfunction becomes all the more important, as heart transplanted patients receiving long-term dialysis have adverse survival rates.9,10
For this reason, this study was initiated to compare both CNI immunosuppressive regimens regarding renal function within the first 2 years after HTX in patients continuously receiving the primarily applied CNI regimen.
Primary endpoint was change in renal function assessed by Modification of Diet in Renal Disease (MDRD) equation during the first 2 years after HTX. In addition, we compared patients receiving conventional tacrolimus (CTAC) with patients receiving extended-release tacrolimus (ETAC) regarding renal function.
All human studies were reviewed by the ethics committee of the University of Heidelberg and have been performed in accordance with the ethical standards laid down in the 2008 Declaration of Helsinki.
Patients and methods
Patients
A total of 150 patients who were continuously receiving the primary applied CNI within first 2 years after HTX were retrospectively analyzed. Inclusion criteria included CNI-based immunosuppression without change of primarily applied CNI within the first 2 years after HTX (per protocol analysis). Exclusion criteria were external follow-up and death within first 2 years after transplantation.
Patients underwent HTX between May 1998 and October 2010 at the Heidelberg Heart Transplantation Center in Germany. All patients received routine follow-up visits according to center standard, including physical examination, and routine laboratory analysis, including immunosuppressive drug monitoring.
Routine follow-up visits were performed monthly after primary discharge after HTX until month 6, bimonthly from month 6 to month 12, and every 3 months in the second year after transplantation.
Renal function
Renal function was analyzed by MDRD equation and serum creatinine levels.11 For the purpose of the present study, renal function tests immediately after HTX as baseline value and at months 6, 12, and 24 after HTX were analyzed.
Referring to the National Kidney Foundation, patients were divided into three groups, based on estimated glomerular filtration rate (eGFR), assessed by MDRD equation.12 In group 1, eGFR was <30 mL/minute/1.73 m2, in group 2, eGFR was ≤30 and ≥60 mL/minute/1.73 m2, and in group 3, eGFR was >60 mL/minute/1.73 m2. In subgroup analysis, patients’ renal function was analyzed according to primarily applied TAC formulation.
Routine laboratory testing and immunosuppressive drug-level monitoring
Laboratory analysis was performed during routine follow-up visits, including complete blood count, liver function tests, blood glucose levels, and blood lipid profile.
Immunosuppressive medication was applied and monitored according to the center’s routine protocol. In line with the center’s routine protocol, target levels of immunosuppressive drugs were reduced during the study period.13
In general, CNI therapy was part of a dual immunosuppressive regimen. In 2001, azathioprine was replaced by mycophenolate mofetil as a concomitant immunosuppressive drug. Steroids were routinely applied during the first half year after HTX and were tapered whenever possible.
Statistical analysis
Data were statistically analyzed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA). Numerical data were expressed as mean value ± standard deviation or were described as absolute numbers or percentages. Analyses of differences in groups were detected using t-test for continuous data and chi-square-test for categorical data. To detect significant deterioration of renal function within groups between study visits, McNemar’s test was used. A P-value of <0.05 was assessed as statically significant.
Results
Patient demographic and baseline characteristics
Baseline demographic data and characteristics are shown in Table 1. Seventy-eight patients (52.0%) received a CSA-based immunosuppressive regimen, and 72 patients (48.0%) received a TAC-based immunosuppressive therapy continuously during the first 2 years after HTX. Within the TAC group, 29 patients (40.3%) received a CTAC-based de novo regimen, and 43 (59.7%) patients received an ETAC immunosuppressive regimen (as soon as oral uptake was possible). In each subgroup, five patients changed their primarily applied immunosuppressive regimen from CTAC to ETAC, respectively ETAC to CTAC. Data from these patients were censored for subgroup analysis at the point of switch. Regarding age at HTX, no statistically significant differences between the groups were detectable (P=not statistically significant [ns]). However, in patients receiving a TAC-based immunosuppressive regimen, ischemic time was significantly longer (P<0.0001), donors were older (P<0.0001), percentage of male donors was lower (P=0.0037), and sex mismatch was higher (P=0.038). Further analysis of diagnoses leading to HTX detected a significantly higher percentage of patients transplanted due to coronary heart disease in the CSA group (P=0.0134); all other principal diagnoses leading to HTX did not differ statistically significantly between both groups (all P=ns). Regarding concomitant medication, no statistically significant differences regarding use of statins was seen between groups 2 years after HTX (P=ns).
Table 1.
Patient baseline demographic data
| Characteristics | CSA patients | TAC patients | P-value |
|---|---|---|---|
| Included patients, n | 78 | 72 | na |
| Mean age at HTX, years ± SD | 50.90±10.71 | 50.43±10.10 | ns |
| Mean donor age, years ± SD | 36.10±14.04 | 45.31±12.62 | <0.0001 |
| Male/female recipient, n | 66/12 | 52/20 | ns |
| Male/female donor, n | 42/36 | 22/50 | 0.0037 |
| Sex mismatch, n (%) | 27 (34.6) | 37 (51.4) | 0.038 |
| Ischemic time, minutes ± SD | 202.36±58.01 | 250.17±44.05 | <0.0001 |
| Diagnosis leading to HTX, n | |||
| DCM | 43 | 44 | ns |
| CAD | 28 | 13 | 0.0134 |
| Cardiac vitium | 1 | 1 | ns |
| Cardiac amyloidosis | 4 | 10 | ns |
| Other | 2 | 4 | ns |
Abbreviations: CSA, cyclosporine A; TAC, tacrolimus; na, not applicable; HTX, heart transplantation; SD, standard deviation; DCM, dilated cardiomyopathy; CAD, coronary artery disease; ns, not statistically significant; n, total number.
Renal function and serum electrolytes
Analysis of renal function referring to eGFR by MDRD equation is shown in Figure 1. Renal function during the study period according to serum creatinine levels is described in Figure 2. Analysis of renal function at each visit with regard to eGFR detected no statistically significant differences between patients receiving TAC and patients receiving CSA (all P=ns; Table 2). A significant decrease of eGFR during the study period was detectable in both groups (CSA group, 2 years after HTX vs baseline, P<0.0001; TAC group, 2 years after HTX vs baseline, P=0.0094). McNemar’s test detected a significant accumulation of patients with deterioration of renal function in the first half year after HTX among patients receiving CSA (P=0.0004). In patients receiving TAC, no significant accumulation of patients with deterioration of renal function during the first 2 years after HTX was detectable (all P=ns). Figures 3 and 4 show the composition of both CNI groups referring to eGFR at performed study visits.
Figure 1.
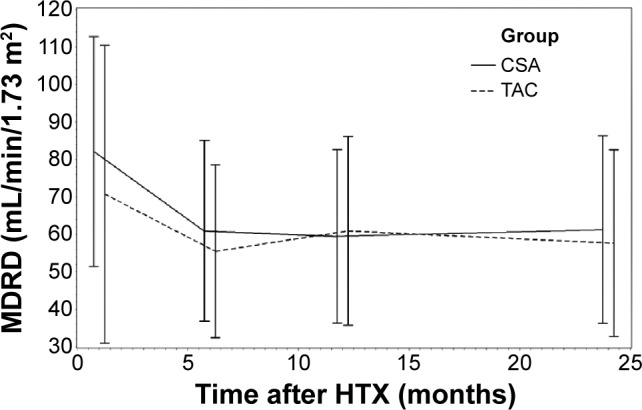
Renal function assessed by MDRD equation.
Abbreviations: MDRD, Modification of Diet in Renal Disease; HTX, heart transplantation; CSA, cyclosporine A; TAC, tacrolimus.
Figure 2.
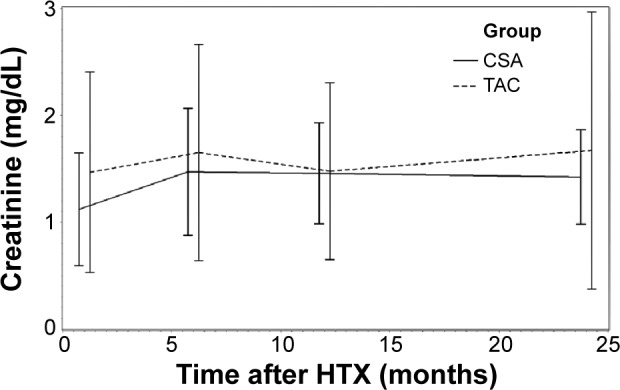
Renal function assessed by serum creatinine.
Abbreviations: HTX, heart transplantation; CSA, cyclosporine A; TAC, tacrolimus.
Table 2.
Renal function and serum electrolytes
| Characteristics and month | CSA patients, mean ± SD | TAC patients, mean ± SD | P-value |
|---|---|---|---|
| eGFR, mL/minute/1.73 m2 | |||
| Baseline | 82.13±30.72 | 70.75±39.77 | ns |
| 6 | 60.89±24.14* | 55.46±23.10* | ns |
| 12 | 59.48±23.18* | 60.91±25.17* | ns |
| 24 | 61.28±25.04* | 57.61±24.92* | ns |
| Serum creatinine, mg/dL | |||
| Baseline | 1.12±0.53 | 1.47±0.94 | 0.0066 |
| 6 | 1.47±0.59* | 1.65±1.01† | ns |
| 12 | 1.46±0.47* | 1.48±0.83† | ns |
| 24 | 1.42±0.44* | 1.67±1.30† | ns |
| Serum sodium, mmol/L | |||
| Baseline | 136.65±3.86 | 137.72±3.43 | ns |
| 6 | 138.82±3.75* | 137.99±3.90† | ns |
| 12 | 138.84±2.94* | 139.31±2.94* | ns |
| 24 | 138.60±3.47* | 139.18±2.80* | ns |
| Serum potassium, mmol/L | |||
| Baseline | 4.23±0.50 | 4.27±0.54 | ns |
| 6 | 4.05±0.42* | 4.05±0.46* | ns |
| 12 | 4.28±0.41† | 4.34±0.53† | ns |
| 24 | 4.28±0.36† | 4.36±0.54† | ns |
Notes:
P<0.05 vs baseline, statistically significant
P<0.05 vs baseline, ns.
Abbreviations: CSA, cyclosporine A; SD, standard deviation; TAC, tacrolimus; eGFR, estimated glomerular filtration rate; ns, not statistically significant.
Figure 3.
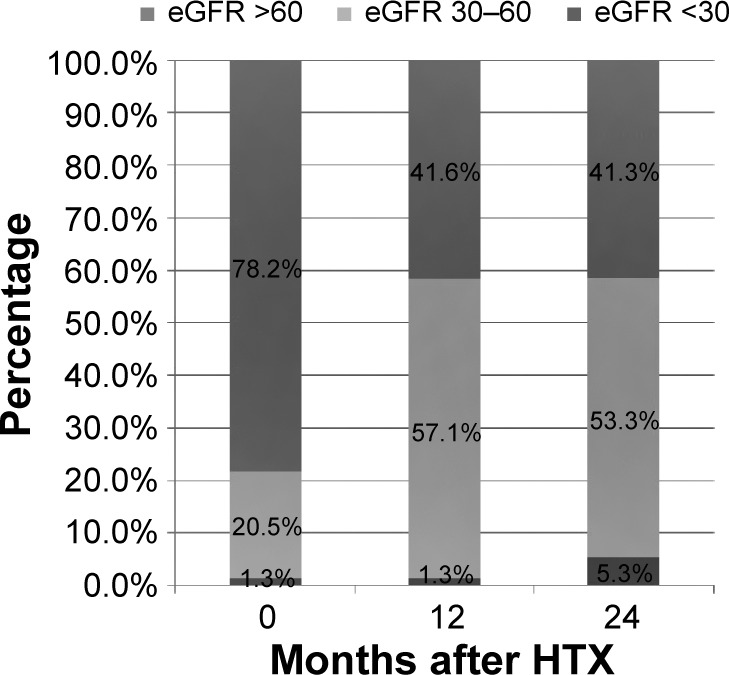
Patient distribution percentage regarding MDRD in CSA patients at performed study visits.
Abbreviations: eGFR, estimated glomerular filtration rate; HTX, heart transplantation; MDRD, Modification of Diet in Renal Disease; CSA, cyclosporine A.
Figure 4.
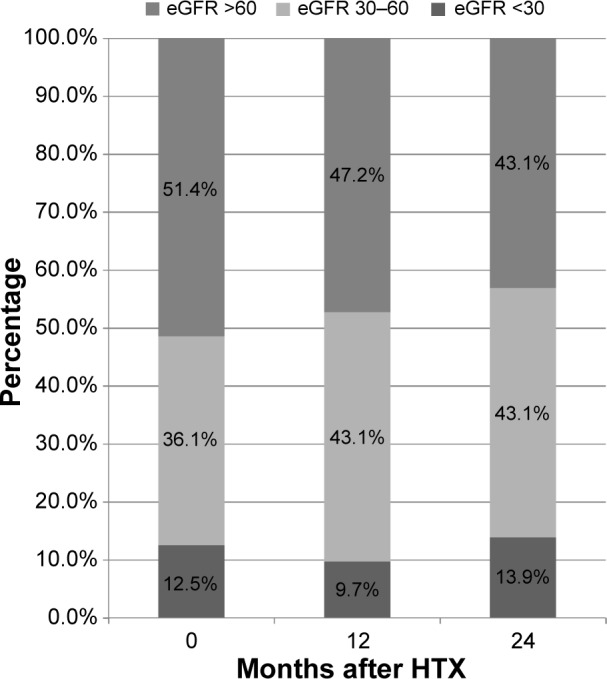
Patient distribution percentage regarding MDRD in TAC group at performed study visits.
Abbreviations: eGFR, estimated glomerular filtration rate; HTX, heart transplantation; MDRD, Modification of Diet in Renal Disease; TAC, tacrolimus.
Within the first 2 years after transplantation, serum creatinine increased significantly in CSA patients (2 years after HTX vs baseline P<0.0001), whereas in patients receiving TAC, serum creatinine did not differ significantly from baseline values (P=ns). At baseline, serum creatinine in patients receiving CSA was significantly lower (CSA, 1.12±0.53 mg/dL; TAC, 1.47±0.94 mg/dL; P=0.0066). Starting from month 6 after HTX, serum creatinine levels in patients receiving CSA and patients receiving TAC did not differ statistically significantly (all P=ns).
Analysis of serum electrolytes, that is, serum sodium and serum potassium, detected no statistically significant differences between both CNI regimens (all P=ns). Changes within both study groups regarding eGFR, serum creatinine, and electrolytes versus baseline values are given in Table 2.
Analyzing eGFR in TAC subgroups detected no statistically significant differences between patients primarily receiving an ETAC or patients receiving a CTAC immunosuppressive regimen at all performed study visits (all P=ns). At baseline, eGFR in patients receiving CTAC treatment was 71.62±41.17 mL/minute/1.73 m2 versus 70.16±39.28 mL/minute/1.73 m2 (P=ns). After the study period, eGFR was 57.46±27.64 mL/minute/1.73 m2 in patients receiving CTAC versus 57.71±23.25 mL/minute/1.73 m2 in patients receiving ETAC (P=ns). Analysis of serum creatinine detected no statistically significant differences between patients receiving CTAC and patients receiving ETAC at performed study visits (all P=ns).
Laboratory evaluation and physical data
No statistically significant differences between patients receiving CSA- and TAC-based therapy were found regarding serum triglycerides and high-density lipoprotein at baseline and during the study period (all P=ns). In contrast to patients receiving CSA, within the patients receiving TAC, a significant reduction of serum triglyceride levels during the study period was observed (2 years after HTX vs baseline, P=0.0031). Analysis of serum cholesterol levels detected no significant differences within the first year after HTX. Beginning at month 12 after transplantation, statistically significant differences regarding blood cholesterol levels were seen between both groups (month 12 after HTX, P=0.0002; month 24 after HTX, P=0.0252). However, a significantly lower blood cholesterol level was detected in both CNI groups 2 years after HTX versus baseline values (patients receiving CSA: 2 years after HTX vs baseline, P=0.0010; patients receiving TAC: 2 years after HTX vs baseline, P=0.0003). Two years after HTX, high-density lipoprotein levels were significantly lower in both groups (CSA: 2 years vs baseline, P=0.0004; TAC: 2 years vs baseline, P=0.0002), low-density lipoprotein was continuously higher in patients receiving CSA at all performed study visits. Except from month 6 after HTX, serum glucose did not differ significantly between patients receiving CSA and patients receiving TAC (P=0.0169).
Analyzing liver function enzymes detected continuously higher values of aspartate aminotransferase and alanine aminotransferase at all performed study visits in patients receiving TAC. In addition, gamma-glutamyl-transferase was significantly higher in patients receiving a TAC-based immunosuppressive regimen, and a higher serum bilirubin level was detected in patients with de novo CSA therapy during study period (all P<0.0001).
With regard to blood pressure profile, no statistically significant differences between groups were seen during the first year after HTX (all P=ns). Beginning in month 12 after HTX, systolic blood pressure was significantly higher in patients receiving CSA. Detailed laboratory findings are shown in Table 3.
Table 3.
Clinical and laboratory parameters
| Characteristics and month | CSA patients, mean ± SD | TAC patients, mean ± SD | P-value |
|---|---|---|---|
| Triglycerides, mg/dL | |||
| Baseline | 191.53±103.56 | 195.97±78.64 | ns |
| 6 | 164.76±88.70† | 170.67±82.26* | ns |
| 12 | 166.03±92.08† | 161.79±93.56* | ns |
| 24 | 163.19±135.50† | 158.76±84.17* | ns |
| Blood cholesterol, mg/dL | |||
| Baseline | 221.29±57.97 | 204.52±49.41 | ns |
| 6 | 190.20±38.21* | 180.13±37.03* | ns |
| 12 | 194.59±47.49* | 168.10±34.14* | 0.0002 |
| 24 | 193.72±46.61* | 178.14±35.50* | 0.0252 |
| HDL, mg/dL | |||
| Baseline | 60.13±23.61 | 62.28±19.42 | ns |
| 6 | 50.04±15.66* | 51.20±16.87* | ns |
| 12 | 50.50±18.13* | 49.14±18.11* | ns |
| 24 | 48.74±15.63* | 50.07±16.34* | ns |
| LDL, mg/dL | |||
| Baseline | 128.56±49.66 | 108.16±31.50 | 0.0068 |
| 6 | 109.11±29.47* | 94.71±31.76† | 0.0056 |
| 12 | 111.65±35.65* | 85.82±28.22* | <0.0001 |
| 24 | 114.04±30.51* | 95.77±31.42† | 0.0006 |
| Serum glucose, mg/dL | |||
| Baseline | 112.49±45.06 | 118.51±43.11 | ns |
| 6 | 100.91±20.56* | 116.99±51.96† | 0.0169 |
| 12 | 103.60±23.03† | 109.24±31.39† | ns |
| 24 | 109.15±30.15† | 114.00±37.04† | ns |
| ASAT, U/L | |||
| Baseline | 16.53±9.07 | 23.87±12.37 | 0.0003 |
| 6 | 15.21±7.71† | 24.49±11.04† | <0.0001 |
| 12 | 15.50±7.95† | 28.77±16.78* | <0.0001 |
| 24 | 16.39±8.29† | 29.83±32.79† | 0.0014 |
| ALAT, U/L | |||
| Baseline | 36.85±33.43 | 48.39±36.08 | 0.0444 |
| 6 | 15.63±10.84* | 26.04±20.75* | 0.0003 |
| 12 | 14.62±8.02* | 26.44±19.67* | <0.0001 |
| 24 | 17.62±13.55* | 24.33±15.24* | 0.0061 |
| GGT, U/L | |||
| Baseline | 104.96±90.20 | 273.93±242.54 | <0.0001 |
| 6 | 45.49±60.70* | 123.54±186.62* | 0.0013 |
| 12 | 32.69±34.99* | 122.28±199.35* | 0.0003 |
| 24 | 30.56±29.70* | 83.31±99.36* | <0.0001 |
| Serum bilirubin, mg/dL | |||
| Baseline | 1.58±1.16 | 0.88±0.62 | <0.0001 |
| 6 | 0.81±0.36* | 0.46±0.22* | <0.0001 |
| 12 | 0.81±0.37* | 0.54±0.27* | <0.0001 |
| 24 | 0.77±0.34* | 0.56±0.21* | <0.0001 |
| Hemoglobin, g/dL | |||
| Baseline | 11.28±1.18 | 11.21±1.10 | ns |
| 6 | 11.63±1.25* | 10.84±1.37* | 0.0004 |
| 12 | 12.03±1.39* | 11.68±1.61* | ns |
| 24 | 12.73±1.58* | 12.81±1.83* | ns |
| Thrombocytes, 1/nL | |||
| Baseline | 264.79±115.41 | 318.33±143.55 | 0.0134 |
| 6 | 238.88±71.73* | 230.41±74.87* | ns |
| 12 | 240.21±67.64* | 238.59±122.86* | ns |
| 24 | 244.29±66.47† | 215.83±67.27* | 0.0109 |
| Leukocytes, 1/nL | |||
| Baseline | 9.48±4.59 | 10.34±4.64 | ns |
| 6 | 5.33±2.11* | 4.63±1.99* | 0.0406 |
| 12 | 6.06±1.78* | 5.26±2.04* | 0.0131 |
| 24 | 6.67±1.97* | 6.26±2.03* | ns |
| Systolic blood pressure, mmHg | |||
| Baseline | 126.10±14.27 | 121.86±16.86 | ns |
| 6 | 127.40±15.91† | 122.72±15.13† | ns |
| 12 | 127.17±13.74† | 121.90±14.20† | 0.0230 |
| 24 | 126.15±15.77† | 121.06±14.44† | 0.0405 |
Notes:
P<0.05 vs baseline, statistically significant
P<0.05 vs baseline, ns.
Abbreviations: CSA, cyclosporine A; SD, standard deviation; TAC, tacrolimus; ns, not statistically significant; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ASAT, aspartate aminotransferase; ALAT, alanine aminotransferase; GGT, gamma-glutamyltransferase.
Immunosuppressive regimen and measured drug levels
Immunosuppressive drug trough levels are described in Table 4, and applied doses of immunosuppression are described in Table 5. No statistically significant differences regarding immunosuppressive drug trough levels were found between the CTAC and ETAC groups (all P=ns). Only at baseline, patients receiving ETAC received significantly higher TAC doses (ETAC: 11.71±4.71 mg; CTAC: 8.14±3.13 mg; P=0.0002).
Table 4.
Immunosuppressive drug trough levels
| Month and characteristics, all parameters μg/L | CSA patients, mean ± SD | TAC patients, mean ± SD | P-value |
|---|---|---|---|
| Baseline | |||
| CNI | 266.1±102.0 | 12.6±4.4 | na |
| +MPA | 2.1±1.9 | 2.4±1.5 | ns |
| +mTOR | 7.4±3.9 | 6.2±2.7 | ns |
| Month 6 | |||
| CNI | 202.3±71.6* | 10.1±3.1* | na |
| +MPA | 2.3±1.2 | 2.9±1.4 | 0.0207 |
| +mTOR | 6.7±1.9 | 7.6±2.6 | ns |
| Month 12 | |||
| CNI | 174.5±83.7* | 8.9±4.2* | na |
| +MPA | 2.5±1.7 | 2.3±1.2 | ns |
| +mTOR | 7.9±5.4 | 6.1±3.1 | ns |
| Month 24 | |||
| CNI | 130.8±56.7* | 7.2±2.2* | na |
| +MPA | 2.5±1.5 | 2.2±1.2 | ns |
| +mTOR | 6.8±3.9 | 6.3±2.1 | ns |
Notes:
P<0.05 vs baseline, statistically significant.
Abbreviations: CSA, cyclosporine A; SD, standard deviation; TAC, tacrolimus; CNI, calcineurin inhibitor; na, not applicable; MPA, mycophenolic acid; ns, not statistically significant; mTOR, mammalian target of rapamycin inhibitor.
Table 5.
Immunosuppressive drug doses
| Visit and characteristics, all parameters mg | CSA patients, mean ± SD (n) | TAC patients, mean ± SD (n) | P-value |
|---|---|---|---|
| Baseline | |||
| CNI | 323.0±101.5 (78) | 10.3±4.5 (72) | na |
| +MMF | 2803.0±918.0 (33) | 2300.7±902.0 (69) | 0.0116 |
| +mTOR | 1.3±0.4 (2) | 4.0±2.0 (3) | ns |
| +AZA | 88.4±25.1 (41) | — (0) | na |
| +steroids | 19.3±12.1 (77) | 30.4±13.7 (72) | <0.0001 |
| Month 6 | |||
| CNI | 260.6±72.4 (78) | 7.1±4.1 (72) | na |
| +MMF | 2750.0±908.7 (34) | 1810.3±680.7 (58) | <0.0001 |
| +Mycophenolate sodium | — (0) | 840.0±680.7 (3) | na |
| +mTOR | — (0) | 3.6±1.5 (11) | na |
| +AZA | 82.8±24.1 (32) | — (0) | na |
| +steroids | 6.4±3.1 (68) | 5.9±3.4 (63) | ns |
| Month 12 | |||
| CNI | 219.4±69.6 (78) | 5.7±3.4 (72) | na |
| +MMF | 2700.0±848.8 (45) | 1804.4±739.6 (46) | <0.0001 |
| +Mycophenolate sodium | — (0) | 1170.0±877.2 (5) | na |
| +mTOR | 3.2±4.1 (4) | 3.0±1.4 (20) | ns |
| +AZA | 74.0±34.2 (25) | — (0) | na |
| +steroids | 7.8±13.1 (54) | 3.8±2.9 (16) | 0.0411 |
| Month 24 | |||
| CNI | 193.1±62.3 (78) | 4.5±2.5 (72) | na |
| +MMF | 2779.4±869.8 (51) | 1625.0±670.5 (50) | <0.0001 |
| +Mycophenolate sodium | — (0) | 1260.0±496.2 (6) | na |
| +mTOR | 1.1±0.1 (4) | 3.0±1.3 (14) | <0.0001 |
| +AZA | 57.1±15.3 (14) | 50.0±35.4 (2) | ns |
| +steroids | 5.2±2.7 (34) | 2.6±1.5 (8) | 0.0017 |
Note: — indicates that there is no measured drug level.
Abbreviations: CSA, cyclosporine A; TAC, tacrolimus; SD, standard deviation; CNI, calcineurin inhibitor; na, not applicable; MMF, mycophenolat mofetil; mTOR, mammalian target of rapamycin inhibitor; ns, not statistically significant; AZA, azathioprine; n, number of patients.
Discussion
The primary endpoint of our study was to evaluate the nephrotoxic effects of a TAC- or CSA-based immunosuppressive regimen in patients after HTX. To detect possible effects caused by CNI, we only analyzed the data of patients who received the same CNI, that is, CSA or TAC, for 2 years after HTX. Analyzing renal function by means of serum creatinine detected a significant deterioration of renal function in patients receiving CSA as their baseline immunosuppressive regimen within the first 2 years after HTX (2 years after HTX vs baseline, P<0.0001). In patients receiving TAC, no statistically significant deterioration of renal function measured by serum creatinine was detectable (2 years after HTX vs baseline, P=ns).
As the MDRD equation is a more appropriate marker to assess renal function than serum creatinine, we used the MDRD equation to analyze renal function more precisely in our study cohort.11 Analyzing renal function according to the MDRD equation detected a significant decrease of eGFR, both in patients receiving CSA and in patients receiving TAC.
On the basis of eGFR, we divided both CNI groups into three subgroups to analyze the composition of both groups at performed study visits. To detect a significant shift of patients with deterioration of renal function between study visits, we used McNemar’s test. In contrast to recently published data reporting similar nephrotoxic effects of both CNIs, we detected significant deterioration of renal function in patients receiving CSA.6,14–16 Our data underline data reporting better renal function of TAC-based immunosuppression, as a significant accumulation of patients with deterioration of renal function was only seen in patients receiving CSA immunosuppressive treatment within the first 6 months after HTX.4,17 Between all other study visits, a significant shift of patients receiving CSA with deterioration of renal function was not seen (all P=ns). Analyzing patients receiving TAC by McNemar’s test detected no statistically significant transition during the study period.
Data comparing ETAC and CTAC are rarely published; however, in contrast to recently published data in patients after renal transplantation, no significant differences regarding renal function in patients receiving either ETAC or CTAC therapy were found in our study cohort.18 To clarify the effects on renal function of both TAC regimes, randomized controlled studies should be initiated. The importance of local renal factors, for example, variability in P-glycoprotein and CYP3A4/5 expression or activity, older kidney age, salt depletion, the use of nonsteroidal anti-inflammatory drugs, and genetic polymorphisms in genes such as TGF-β and ACE, in comparison with systemic exposure to CNIs, for susceptibility to CNI-induced nephrotoxicity has been previously published.19 In addition, the effects of comedication (eg, ACE-inhibitors, amlodipine), CNI avoidance and dose minimization, and simultaneous injection of anti-TGF-β antibodies have to be taken into account.20–26
Cholestasis and hepatotoxic effects in patients receiving a TAC-based medication have already been detected in previously published case reports; however, both CNIs are suspected to cause cholestasis.27–29
In line with previously published data, blood cholesterol levels and systolic blood pressure values were significantly higher in patients receiving CSA.7,14,15,30 Higher systolic blood pressure may partly be explained by a higher sympathetic tone caused by vasoconstriction of afferent and efferent glomerular arterioles.31,32
Limitations
Our retrospective study was performed as a single-center study, and patients were followed up according to the center’s specific routine follow-up care. Moreover, we only considered patients without change of CNI immunosuppressive regimen to analyze specific CNI effects on renal function. A possible era effect cannot be ruled out, given the long follow-up period.
Conclusion
The superior rejection profile of the TAC-based immunosuppressive regimen had already been demonstrated previously.7 In addition, our data detect the favorable effects of a TAC-based immunosuppression with respect to nephrotoxicity, as CSA immunosuppressive therapy was associated with a significant deterioration of renal function (McNemar’s test), especially in the first 6 months after HTX, when high immunosuppressive drug trough levels are needed to prevent AREs. Given the increasingly older HTX recipients, with a multitude of comorbidities, preservation of renal function is of eminent interest. Therefore, in our opinion, a TAC-based immunosuppressive regimen is favorable after HTX.
Footnotes
Disclosure
AOD has applied for a publication grant from Deutsche Forschungsgemeinschaft and Ruprecht-Karls-Universität Heidelberg within the funding programme Open Access Publishing. The other authors report no conflicts of interest in this work.
References
- 1.Lund LH, Edwards LB, Kucheryavaya AY, et al. International Society for Heart and Lung Transplantation The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report–2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):951–964. doi: 10.1016/j.healun.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Lund LH, Edwards LB, Kucheryavaya AY, et al. International Society of Heart and Lung Transplantation The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report–2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33(10):996–1008. doi: 10.1016/j.healun.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Guethoff S, Meiser BM, Groetzner J, et al. Ten-year results of a randomized trial comparing tacrolimus versus cyclosporine a in combination with mycophenolate mofetil after heart transplantation. Transplantation. 2013;95(4):629–634. doi: 10.1097/TP.0b013e318277e378. [DOI] [PubMed] [Google Scholar]
- 4.Kobashigawa JA, Miller LW, Russell SD, et al. Study Investigators Tacrolimus with mycophenolate mofetil (MMF) or sirolimus vs cyclosporine with MMF in cardiac transplant patients: 1-year report. Am J Transplant. 2006;6(6):1377–1386. doi: 10.1111/j.1600-6143.2006.01290.x. [DOI] [PubMed] [Google Scholar]
- 5.Mehra MR, Uber PA, Scott RL, Park MH. Ethnic disparity in clinical outcome after heart transplantation is abrogated using tacrolimus and mycophenolate mofetil-based immunosuppression. Transplantation. 2002;74(11):1568–1573. doi: 10.1097/00007890-200212150-00014. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez-Lázaro IJ, Almenar L, Martínez-Dolz L, et al. A prospective randomized study comparing cyclosporine versus tacrolimus combined with daclizumab, mycophenolate mofetil, and steroids in heart transplantation. Clin Transplant. 2011;25(4):606–613. doi: 10.1111/j.1399-0012.2010.01309.x. [DOI] [PubMed] [Google Scholar]
- 7.Helmschrott M, Beckendorf J, Akyol C, et al. Superior rejection profile during the first 24 months after heart transplantation under tacrolimus as baseline immunosuppressive regimen. Drug Des Devel Ther. 2014;8:1307–1314. doi: 10.2147/DDDT.S68542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castel MA, Farrero M, Vallejos I, Cardona M, Regueiro A, Pérez-Villa F. Primary immunosuppression and outcome differences after heart transplantation: tacrolimus versus cyclosporine. Transplant Proc. 2011;43(6):2244–2246. doi: 10.1016/j.transproceed.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Alam A, Badovinac K, Ivis F, Trpeski L, Cantarovich M. The outcome of heart transplant recipients following the development of end-stage renal disease: analysis of the Canadian Organ Replacement Register (CORR) Am J Transplant. 2007;7(2):461–465. doi: 10.1111/j.1600-6143.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 10.Villar E, Boissonnat P, Sebbag L, et al. Poor prognosis of heart transplant patients with end-stage renal failure. Nephrol Dial Transplant. 2007;22(5):1383–1389. doi: 10.1093/ndt/gfl811. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2(suppl 1)):S1–S266. [PubMed] [Google Scholar]
- 13.Gueler I, Mueller S, Helmschrott M, et al. Effects of vildagliptin (Galvus®) therapy in patients with type 2 diabetes mellitus after heart transplantation. Drug Des Devel Ther. 2013;7:297–303. doi: 10.2147/DDDT.S43092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye F, Ying-Bin X, Yu-Guo W, Hetzer R. Tacrolimus versus cyclosporine microemulsion for heart transplant recipients: a meta-analysis. J Heart Lung Transplant. 2009;28(1):58–66. doi: 10.1016/j.healun.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Penninga L, Møller CH, Gustafsson F, Steinbrüchel DA, Gluud C. Tacrolimus versus cyclosporine as primary immunosuppression after heart transplantation: systematic review with meta-analyses and trial sequential analyses of randomised trials. Eur J Clin Pharmacol. 2010;66(12):1177–1187. doi: 10.1007/s00228-010-0902-6. [DOI] [PubMed] [Google Scholar]
- 16.Meiser BM, Groetzner J, Kaczmarek I, et al. Tacrolimus or cyclosporine: which is the better partner for mycophenolate mofetil in heart transplant recipients? Transplantation. 2004;78(4):591–598. doi: 10.1097/01.tp.0000129814.52456.25. [DOI] [PubMed] [Google Scholar]
- 17.Hornum M, Andersen M, Gustafsson F, et al. Rapid decline in glomerular filtration rate during the first weeks following heart transplantation. Transplant Proc. 2011;43(5):1904–1907. doi: 10.1016/j.transproceed.2011.02.065. [DOI] [PubMed] [Google Scholar]
- 18.Kolonko A, Chudek J, Wiecek A. Improved kidney graft function after conversion from twice daily tacrolimus to a once daily prolonged-release formulation. Transplant Proc. 2011;43(8):2950–2953. doi: 10.1016/j.transproceed.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 20.Höcker B, Tönshoff B. Treatment strategies to minimize or prevent chronic allograft dysfunction in pediatric renal transplant recipients: an overview. Paediatr Drugs. 2009;11(6):381–396. doi: 10.2165/11316100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Gleissner CA, Doesch A, Ehlermann P, et al. Cyclosporine withdrawal improves renal function in heart transplant patients on reduced-dose cyclosporine therapy. Am J Transplant. 2006;6(11):2750–2758. doi: 10.1111/j.1600-6143.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- 22.Cornu C, Dufays C, Gaillard S, et al. Impact of the reduction of calcineurin inhibitors on renal function in heart transplant patients: a systematic review and meta-analysis. Br J Clin Pharmacol. 2014;78(1):24–32. doi: 10.1111/bcp.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González-Vílchez F, Vázquez de Prada JA. Chronic renal insufficiency in heart transplant recipients: risk factors and management options. Drugs. 2014;74(13):1481–1494. doi: 10.1007/s40265-014-0274-9. [DOI] [PubMed] [Google Scholar]
- 24.Leenen FH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100(3):531–535. doi: 10.1016/j.amjcard.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 25.Halimi JM, Giraudeau B, Buchler M, et al. Enalapril/amlodipine combination in cyclosporine-treated renal transplant recipients: a prospective randomized trial. Clin Transplant. 2007;21(2):277–284. doi: 10.1111/j.1399-0012.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- 26.Formica RN, Jr, Friedman AL, Lorber MI, Smith JD, Eisen T, Bia MJ. A randomized trial comparing losartan with amlodipine as initial therapy for hypertension in the early post-transplant period. Nephrol Dial Transplant. 2006;21(5):1389–1394. doi: 10.1093/ndt/gfk058. [DOI] [PubMed] [Google Scholar]
- 27.Ganschow R, Albani J, Grabhorn E, Richter A, Burdelski M. Tacrolimus-induced cholestatic syndrome following pediatric liver transplantation and steroid-resistant graft rejection. Pediatr Transplant. 2006;10(2):220–224. doi: 10.1111/j.1399-3046.2005.00413.x. [DOI] [PubMed] [Google Scholar]
- 28.Sacher VY, Bejarano PA, Pham SM. Tacrolimus induced hepatotoxicity in a patient with bilateral lung transplant. Transpl Int. 2012;25(10):e111–e112. doi: 10.1111/j.1432-2277.2012.01546.x. [DOI] [PubMed] [Google Scholar]
- 29.Oto T, Okazaki M, Takata K, et al. Calcineurin inhibitor-related cholestasis complicating lung transplantation. Ann Thorac Surg. 2010;89(5):1664–1665. doi: 10.1016/j.athoracsur.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 30.Grimm M, Rinaldi M, Yonan NA, et al. Superior prevention of acute rejection by tacrolimus vs cyclosporine in heart transplant recipients–a large European trial. Am J Transplant. 2006;6(6):1387–1397. doi: 10.1111/j.1600-6143.2006.01300.x. [DOI] [PubMed] [Google Scholar]
- 31.Lanese DM, Conger JD. Effects of endothelin receptor antagonist on cyclosporine-induced vasoconstriction in isolated rat renal arterioles. J Clin Invest. 1993;91(5):2144–2149. doi: 10.1172/JCI116440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693–699. doi: 10.1056/NEJM199009133231101. [DOI] [PubMed] [Google Scholar]


