Abstract
Epicardial fat is a metabolically active fat depot that is strongly associated with obesity, metabolic syndrome and coronary artery disease (CAD). The relationship of epicardial fat and diastolic function is unknown. We sought to: a) understand the relationship of epicardial fat volume (EFV) and diastolic function and b) understand the role of EFV relative to potential risk factors (hypertension, subclinical CAD and metabolic syndrome) of diastolic dysfunction in apparently healthy subjects with preserved systolic function and with no history of CAD. We studied 110 consecutive subjects (65% male, 55±13 years, mean BMI 28±5 kg/m2) who underwent cardiac computed tomography (CCT) and a transthoracic echocardiogram, within 6 months as part of a self-referred health screening program. Exclusion criteria included: history of CAD, significant valvular disease, systolic dysfunction (LVEF<50%). Diastolic function was defined according to American Society of Echocardiography guidelines. EFV was measured using validated CCT software by 2 independent cardiologists blinded to the clinical and echocardiographic data. Hypertension and metabolic syndrome were present in 60% and 45%, respectively. Subclinical CAD was identified in 20% of the cohort. Diastolic dysfunction was present in 45 patients. EFV was an independent predictor of diastolic dysfunction, mean e′ velocities and E/e′ ratio (p=0.01, <0.0001 and 0.001, respectively) with incremental contribution to the other clinical factors. In conclusion, EFV is an independent predictor of impaired diastolic function in apparently healthy overweight individuals, even after accounting for associated comorbidities such as metabolic syndrome, hypertension and subclinical CAD.
Keywords: epicardial fat, diastolic dysfunction, obesity, metabolic syndrome
The association of obesity with myocardial dysfunction is probably mediated through its strong links with hypertension, dyslipidemia and atherosclerotic coronary artery disease (CAD). However, obesity may be associated with structural changes in the myocardium independent of its effects on risk factors or CAD. Subclinical changes of LV structure and function, including abnormal relaxation and strain have been reported in overweight subjects even after adjustment for mean arterial pressure, age, gender and LV mass.1 Obese patients may present with heart failure with normal ejection fraction even in the absence of CAD; each 1 kg/m2 increase in body mass index (BMI) has been shown to increase the risk of heart failure by 5% for men and by 7% for women.2 In this context, it is extremely important to understand the mechanisms by which obesity may cause LV dysfunction with a preserved ejection fraction. Epidemiological studies have shown that epicardial fat, a metabolically active fat depot, is strongly associated with obesity, metabolic syndrome and diabetes.3,4 The proximity of epicardial fat to the coronary arteries has been used to explain the association of epicardial fat volume (EFV) with increased coronary artery calcium, atherosclerotic plaque, and myocardial ischemia.3,5–7 Although these factors may be responsible for diastolic dysfunction, epicardial fat may also have a direct paracrine effect on the myocardium and thereby alter the structural properties of the left ventricle. We sought to: a) understand the relationship of EFV and diastolic dysfunction and b) understand the role of EFV relative to potential risk factors (hypertension, subclinical CAD and metabolic syndrome) of diastolic dysfunction in healthy subjects with preserved systolic function.
METHODS
We studied consecutive individuals who underwent cardiac computed tomography (CCT) and a transthoracic echocardiogram at the Cleveland Clinic, Cleveland, OH, within 6 months as part of a self-referred health screening program. Exclusion criteria were: a) history of coronary artery disease (myocardial infarction and/or percutaneous or surgical revascularization), b) moderate (≥2+) valvular regurgitation or any valvular stenosis, c) systolic dysfunction (EF<50%), d) incomplete echocardiogram data and e) intercurrent event between the two imaging studies. Subjects with pathological Q-waves on electrocardiogram, wall motion abnormalities on echocardiogram or myocardial wall-thinning seen on CCT suggestive of prior myocardial infarction were also excluded. This study was approved by the Institutional Review Board.
Hypertension was defined as systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg at the time of the visit (mean of 2 readings), or history of hypertension but not on treatment. Diabetes mellitus was defined as fasting blood glucose ≥ 110 mg/dl or use of diabetes medications. Dyslipidemia was defined as total serum cholesterol ≥ 240 mg/dl or use of lipid lowering treatment. Body mass index (BMI) was calculated as weight (kg) divided by height-squared (m2). According to a standard definition, overweight individuals had a BMI between 25.0 and 29.9 kg/m2, whereas obese had a BMI ≥ 30 kg/m2. Metabolic syndrome was defined by the criteria proposed by the National Cholesterol Education Program, Adult Treatment Panel III 8. At least 3 or more of the following components were needed to meet the metabolic syndrome criteria: a) fasting blood glucose ≥ 100 mg/dl or the patient’s self-reported history of diabetes or use of diabetes medications; b) blood pressure ≥ 130/85 mmHg or the patient’s self-reported history of hypertension or use of antihypertensive medications; c) triglycerides ≥ 150 mg/dl; d) high density lipoprotein <40 mg/dl and e) BMI >30 kg/m2 (where central obesity was assumed as per the International Diabetes Federation guidelines 9). The 10-year Framingham Risk Score (FRS) was calculated according to the guidelines,8 and included the following risk factors: age, gender, systolic blood pressure, total cholesterol, high density lipoprotein cholesterol and smoking history.
LV linear dimensions were measured from a parasternal long-axis view according to the recommendations of the American Society of Echocardiography (ASE) 10. LV mass index (LVMI) was calculated by the corrected ASE simplified cubed equation 10 and indexed for body surface area. The LV ejection fraction (LVEF) and LV volume index were calculated by biplane modified Simpson’s rule. LV diastolic function assessment was obtained according to the ASE guidelines and included peak velocities of the early phase (E) and late phase (A) of the transmitral inflow, with derivation of the E/A ratio. LV myocardial velocities were evaluated by tissue Doppler imaging with pulsed sample volume placed at the level of the lateral and septal mitral valve annulus. Peak early diastolic mitral annular velocities (e′) were measured and averaged over 3 cardiac cycles 11. Diastolic dysfunction was categorized as: 1) E/A <0.8 and deceleration time >200 msec (stage I, impaired relaxation); or 2) E/A ≥0.8 and <1.5, deceleration time of 160–200 msec and mean e′ <8 cm/s (stage II, pseudonormal); or 3) E/A ≥ 1.5, deceleration time <160msec and mean e′ <8 cm/s (stage III, restrictive). All echocardiograms were reviewed by 2 board certified cardiologists blinded to clinical and CCT information.
Non-contrast CT was acquired using a 64-slice CT scanner (Sensation 64, Siemens Medical Solutions, Erlangen, Germany) in the axial mode during a single breath-hold with prospective ECG-triggering and 120 kVp tube voltage. Images were reconstructed using a medium sharp kernel (B35f) with a slice thickness of 3 mm. Coronary calcium was quantified on non-enhanced cardiac CT scans. Coronary calcium was defined as a plaque of at least 3 contiguous pixels (area 1.0 mm2) with a density of >130 Hounsfield Units. A total coronary calcium score was determined by summing the individual Agatston scores from each of 4 anatomic sites (left main, left anterior descending, circumflex, and right coronary) 12. Contrast enhanced coronary CT angiography was performed on the same scanner 13. Non-overlapping images were reconstructed using a medium sharp kernel (B26f) with a thickness of 3-mm for quantification of epicardial fat. Scans were analyzed independently by 2 experienced investigators who were blinded to the clinical and echocardiographic information.
Epicardial fat quantification was performed by QFAT software as previously described 14. Epicardial fat volume (EFV) was defined as adipose tissue enclosed by the visceral pericardium, including fat directly surrounding the coronary arteries. Definition of epicardial contours was based in an upper slice limit (bifurcation of the pulmonary trunk) and lower slice limit (slice just below the posterior descending artery). Contiguous 3D voxels between the Hounsfield Unit limits of −190 to −30 were defined as fat voxels by default. EFV index was calculated dividing the EFV by the body surface area.
The exclusion criteria for the current study removed any subjects with either known CAD or any prior evaluation for CAD. Subclinical CAD was defined using the CCT data as either total Agatston score ≥ 400 and/or presence of any non-calcified or mixed plaque encompassing > 25% of the luminal diameter of a coronary artery on contrast coronary computed angiography. An Agatston score of 400 is currently accepted in the guidelines as being sufficient to justify a functional study in an asymptomatic patient 15 who is at an increased risk for coronary artery disease and adverse clinical events. The upper 90th percentile of total Agatston score was obtained from the MESA cohort population and used as reference value 16.
Continuous variables were described as mean±standard deviation. Differences between groups were assessed by 1-way analysis of variance and post-hoc multiple comparisons were performed using the Bonferroni correction when appropriate. Spearman correlation was used for non-parametric distributions. Multivariate binary logistic regression models were constructed to identify the relationship between diastolic dysfunction vs. EFV, 10yr FRS, subclinical CAD and metabolic syndrome. Multiple linear regression models were constructed to assess the independent association of these parameters and other continuous diastolic function parameters (mean e′ and mean E/e′ ratio). Nonstandardized (B) coefficient estimates with relative standard errors were reported. Statistical analyses were performed using SPSS software version 19.0 (SPSS, Inc., Chicago, Illinois), and a 2-tailed p <0.05 was considered significant.
RESULTS
Clinical and demographic characteristics of 110 subjects who met the inclusion criteria are shown in Table 1. The calculated mean 10-year Framingham Risk Score was 10% for men and 4% for women (p<0.0001), both of which still considered low-risk thresholds for major adverse cardiovascular events. There was a correlation between metabolic syndrome and diabetes and metabolic syndrome with dyslipidemia, (r=0.30, p=0.002 for both).
Table 1.
Clinical baseline characteristics of the subjects (n=110)
| Age (years) | 55 ± 13 |
|
| |
| Men | 72 (65%) |
|
| |
| Body mass index (kg/m²) | 28 ± 5 |
|
| |
| Systolic Blood Pressure (in mmHg) | 124 ± 19 |
|
| |
| Diastolic Blood Pressure (in mmHg) | 76 ± 10 |
|
| |
| Diabetes mellitus | 13 (12%) |
|
| |
| Dyslipidemia | 56 (50%) |
|
| |
| Hypertension | 65 (59%) |
|
| |
| Metabolic Syndrome | 44 (40%) |
|
| |
| 10 year Framingham Risk Score* | 6% (0.5 – 34%) |
| (W) 4 ± 3% | |
| (M) 10 ± 7% | |
Values are expressed as mean ± SD, number of subjects (percentage), or median (range). W stands for women, M for men.
Likelihood of coronary heart disease in 10-years.
Most subjects had normal echocardiograms including, left atrial volume index (LAVI), LVMI, LVEF, and diastolic function parameters (Table 2). According to the ASE guidelines, there were 40 subjects with abnormal diastolic function further classified as stage 1 (29 subjects, 72%) and stage 2 (11 subjects, 28%). The median duration between the two imaging studies was 1 day. Most patients (73%) underwent non-contrast CCT for coronary calcium scoring for screening of CAD. Subclinical CAD, as defined before, was present in 20% of the cohort (20 males vs. 2 females, p=0.005). EFV followed a non-normal distribution and was associated with metabolic disturbance, LV mass index and diastolic dysfunction (Table 3). There was no correlation between calcium score and EFV.
Table 2.
Imaging characteristics of the subjects.
| VARIABLE | OVERALL (n=110) | NORMAL VALUES 4, 12, 13, 19 | % Subjects with | |
|---|---|---|---|---|
| < 2 SD | > 2 SD | |||
| Left Atrial Volume Index (cm3/m2) | 26 ± 9 | 22 ± 6 | 0% | 15% |
| Left Ventricular Mass Index (g/m2) | (W) 74 ± 14 | (W) 44–88 | 0 | 24% (F) |
| (M) 91 ± 11 | (M) 50–102 | 16% (M) | ||
| LV ejection fraction (%) | 58 ± 4 | ≥ 55% | - | - |
| Diastolic Mitral Inflow Ratio = E/A | 1.23 ± 0.5 | 1.28 ± 0.25 | 13% | 10% |
| Deceleration time (msec) | 226 ± 57 | 181 ± 19 | 2% | 45% |
| Average Tissue Doppler Velocity = e′ (cm/sec) | 10 ± 3 | ≥ 8 | 21% | - |
| Average LV Filling Pressures = E/e′ | 8 ± 3 | ≤ 8 | - | 36% |
| Epicardial Fat Volume (cm3) | (W) 67 ± 40 | (W) 110 ± 41 | (W) 8% | (W) 0% |
| (M) 101 ± 51 | (M) 137 ± 53 | (M) 4% | (M) 3% | |
| Epicardial Fat Volume Index (cm3/m2) | (W) 32.8 (18.7–53.7) | (W) 31.8 (24.2–41.3) | - | 14 (13%)* |
| (M) 46.6 (30.0–59.4) | (M) 34.2 (24.8–45.5) | |||
| Agatston Score (n=65) | (W) 40 ± 110 | (W) 126 (upper 90th perc) | - | - |
| (M) 556 ± 1109 | (M) 324 (upper 90th perc) | |||
Values are expressed as mean±standard deviation. LAVI stands for left atrial volume index, LVMI – left ventricular mass index, E/A – transmitral inflow ratio where E represents early diastolic filling, A late diastolic filling at time of atrial systole, e′ – tissue Doppler velocity averaged from septal and lateral annulus, EFV – epicardial fat volume, (W) – women; (M) - men.
EFV index > 68 cm3/m2.
Table 3.
Univariate Correlations of Epicardial Fat Volume
| VARIABLES | R | P Value |
|---|---|---|
| Age | 0.34 | <0.0001 |
| Male Gender | −0.35 | <0.0001 |
| Body Mass Index | 0.43 | <0.0001 |
| Systolic Blood Pressure (mmHg) | 0.22 | 0.02 |
| Waist Circumference (n=35) | 0.43 | 0.009 |
| 10-year Framingham Risk Score (*) | 0.41 | <0.0001 |
| Metabolic Syndrome | 0.13 | 0.15 |
| Subclinical Coronary Artery Disease | 0.21 | 0.03 |
| Hyperlipidemia | 0.25 | 0.007 |
| Left Ventricular Mass Index | 0.41 | <0.0001 |
| E, A, E/A, Deceleration Time | −0.05 to 0.08 | NS|| |
| e′ | 0.44 | <0.0001 |
| E/e′ | 0.34 | <0.0001 |
| Agatston score (n=65) | 0.08 | 0.49 |
Values are expressed as mean ± SD or median (range). W stands for women, M for men. BMI stands for body mass index, LAVI stands for left atrial volume index, LVMI – left ventricular mass index, E/A – transmitral inflow ratio where E represents early diastolic filling, A late diastolic filling at time of atrial systole, e′ – tissue Doppler velocity averaged from septal and lateral annulus.
Framingham Risk Score included: age, gender, systolic blood pressure, total cholesterol, high density lipoprotein, smoking status.
p=non-significant for each one of the 4 diastolic parameters.
To answer how these 3 parameters related to diastolic dysfunction, a multivariate binary logistic regression model was constructed. EFV was the only independent predictor (HR=1.09, 95% CI 1.03 – 1.15, p=0.003), whereas BMI (p=0.36) and waist circumference (p=0.09) where not. When we considered BMI > 30 kg/m2 as a surrogate for visceral adiposity and equivalent risk factor for metabolic syndrome development, EFV again was the only predictor of diastolic dysfunction (HR=1.02, 95% CI 1.007–1.03, p=0.001).
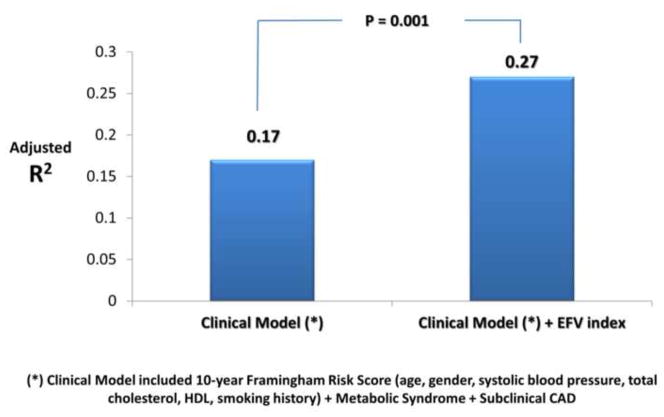
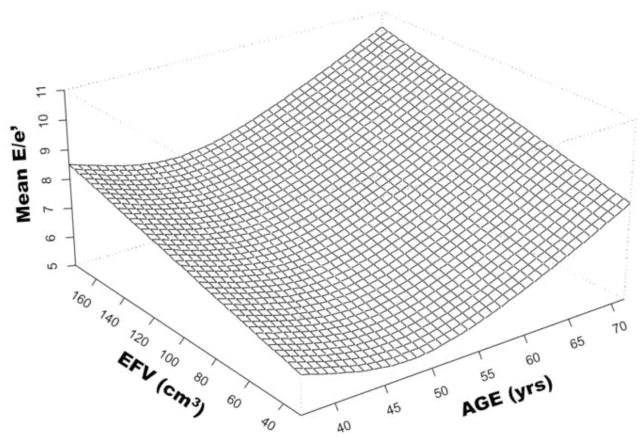
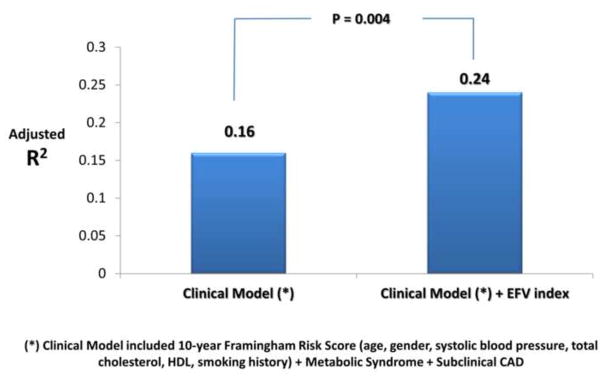
Age, systolic blood pressure, FRS, LVMI, EFV and EFV index were independently associated with diastolic dysfunction. The independent and incremental value of EFV in addition to these traditional cardiovascular risk factors was sought in a multivariable binary logistic regression model; only FRS (p=0.048) and EFV (p=0.02) were independent predictors of diastolic dysfunction (Stage ≥1). Furthermore, EFV also had incremental prognostic value (R2 change from 0.16 to 0.21, p=0.02) when added to the clinical model which included FRS, metabolic syndrome, LVMI and subclinical CAD. Better model fit was seen when EFV index was used instead of EFV (model R2 increased from 0.16 to 0.24, p=0.004). EFV index remained to sole independent predictor of diastolic dysfunction (HR=1.03, p=0.01) after adjusting for FRS, metabolic syndrome, LVMI and subclinical CAD (Table 4 and Figure 1). The association of with mean e′ with FRS, metabolic syndrome, subclinical CAD, LVMI and EFV index was sought in a separate multiple linear regression model (Table 4). EFV index was not only independently associated with mean e′, but was also incremental to the association with clinical and echocardiographic data (model R2 increased from 0.17 to 0.27, p=0.001) (Figure 2). In contrast, only EFV index was independently associated with mean E/e′ (Table 4). Again, EFV index was not only an independent predictor of mean E/e′ (p=0.001) but also incrementally added to the model (R2 of the model increased from 0.04 to 0.14, p=0.001). The relationship between EFV and age in the determination of LV filling pressures (mean E/e′ ratio) is shown in Figure 3. Note that the age-associated increase in LV filling pressures (higher mean E/e′) is amplified by the increase in EFV.
Table 4.
Predictors of Diastolic Dysfunction
| Stage ≥ 1 (Model R2 = 0.24) | Mean e′ (Model R2 = 0.27) | Mean E/e′ (Model R2 = 0.14) | ||||
|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | p | |
| 10 year Framingham Risk Score (*) | 0.07 (0.04) | 0.09 | −0.11 (0.04) | 0.01 | 0.02 (0.04) | 0.59 |
| Metabolic Syndrome | −0.01 (0.46) | 0.98 | −0.07 (0.54) | 0.90 | 0.59 (0.54) | 0.28 |
| Subclinical Coronary Artery Disease | −0.11 (0.58) | 0.84 | −0.36 (0.69) | 0.60 | −0.03 (0.69) | 0.96 |
| Left Ventricular Mass Index | 0.009 (0.01) | 0.41 | 0.005 (0.01) | 0.70 | −0.006 (0.01) | 0.63 |
| Epicardial Fat Volume Index | 0.03 (0.01) | 0.01 | −0.02 (0.006) | <0.0001 | 0.04 (0.01) | 0.001 |
Values in table are nonstandardized correlation coefficient (B) with standard error (SE) and p values after adjusting for all the covariates listed.
Framingham Risk Score included: age, gender, systolic blood pressure, total cholesterol, high density lipoprotein, smoking status. LVMI stands for left ventricular mass index, EFV – epicardial fat volume index.
Figure 1.
Incremental value of Epicardial Fat Volume Index (EFVI) for prediction of diastolic function class (stage ≥ 1).
Figure 2.
Incremental value of Epicardial Fat Volume Index (EFVI) for prediction of mean e′ velocities.
Figure 3.
Relationship among Age, Epicardial Fat Volume and mean LV filling pressures (mean E/e′). EFV indicates epicardial fat volume in cm3, age is indicated in years.
DISCUSSION
This study defines several important associations of CCT-based quantification of EFV. First, our results indicate that EFV is an independent correlate of impaired diastolic function in apparently healthy overweight individuals, after accounting for associated comorbidities such as CAD risk factors, hypertension, metabolic syndrome, and subclinical CAD. Two other important additional findings are: 1) measurement of EFV adds incrementally to the prediction of diastolic dysfunction, mean e′ and mean E/e′; 2) EFV is a stronger correlate of mean e′ and mean E/e′ than are BMI and metabolic syndrome. We interpret these findings to suggest that changes in myocardial function may arise from the paracrine effects of epicardial fat (maybe even more than the metabolic effects of visceral adiposity), and that these effects are related to myocardial dysfunction, as measured by early/sensitive markers of impaired diastolic function such as e′ velocity and E/e′ ratio.
Diastolic dysfunction is widely prevalent in the general population and its severity is prognostically important. A large longitudinal data from a study from Olmsted County, showed that individuals with advanced diastolic dysfunction had a 10-fold higher risk of all cause death compared to subjects with normal diastolic function after adjustment for age, sex and EF 17. Abhayaratna et al have also shown that diastolic dysfunction severity is associated with cardiovascular risk factors including hypertension, diabetes, obesity and metabolic syndrome 18. These findings were confirmed in a recent study by Russo et al who found a progressively worsening of individual diastolic parameters with worsening BMI 19. The diastolic dysfunction seen in diabetes mellitus and metabolic syndrome has been a matter of extensive investigation with several mechanisms proposed including, but limited to: metabolic disturbances, myocardial fibrosis, microvascular dysfunction, autonomic neuropathy and insulin resistance 20–22.
Epicardial fat is a metabolically active fat depot that, is in anatomic proximity to the myocardium, shares the same microcirculation and may have important paracrine effects 23. Epicardial fat correlates with BMI and is increased in obese subjects 3. The association of increased EFV with diastolic dysfunction may be confounded by factors such as metabolic syndrome 4,24 and hypertension 25 which are often present in obese individuals. Furthermore, increased epicardial fat appears to be related to increased atherosclerotic plaque burden, concomitant coronary artery disease, myocardial ischemia and subclinical microvascular dysfunction which is commonly seen in diabetics and in individuals with metabolic syndrome 3,5,20. Our results indicate that EFV has an independent, albeit modest (8–10% of variance), role in effecting myocardial dysfunction after adjusting for age, gender, systolic blood pressure, lipids, metabolic syndrome, LVMI and subclinical CAD. Furthermore, measurement of EFV adds incrementally to the prediction of impaired LV diastolic function.
Epicardial fat thickness can be readily visualized and measured by echocardiography 26. Several thresholds have been proposed to indicate increased cardiovascular risk 4. However, the reproducibility of this method is imperfect, probably because these measurements only represent one planar dimension which is subject to the probe angulation, adequate visualization of the cardiac structure and the given phase of the cardiac cycle chosen to be measured. A recent study showed poor reproducibility of echocardiographic measurements, and low concordance when compared with CCT, a geometry-independent technique 27.
There are several limitations. Waist circumference was only available in the minority of subjects (< 10%), but BMI > 30 kg/m2 has been endorsed as a surrogate for visceral adiposity and equivalent risk factor for metabolic syndrome development by the International Diabetes Federation guidelines 9. Definition of diabetes chosen (fasting blood sugar ≥ 110 mg/dl) might have included subjects with impaired fasting glucose, although an important proportion of these pre-diabetic individuals (12–37%) carries a risk for future cardiovascular disease 28. Our evaluation of LV diastolic function by Doppler flow analysis did not include parameters such as isovolumic relaxation time or pulmonary venous flow. However, those parameters suffer from high load dependence, and the use of tissue Doppler parameters allowed us to detect diastolic abnormalities even when a pseudonormalized flow pattern was present. Lastly the retrospective nature of the study and small sample size might have contributed to the smaller estimations of the predictive models, but are certainly consistent in conveying the message that EFV might play an important role in the impairment of diastolic function.
Epicardial fat volume (EFV) is an independent correlate of impaired LV diastolic function in apparently healthy overweight individuals, even after accounting for associated comorbidities such as the metabolic syndrome, hypertension and subclinical CAD. Two important additional findings are: 1) CCT-based measurement of EFV adds incrementally to the prediction of diastolic functional class (stage ≥ 1), mean e′ and E/e′ ratio; 2) EFV is a stronger correlate of mean e′ velocities and E/e′ ratio than are BMI and metabolic syndrome. These findings suggest that EFV may exert changes in myocardial function by a paracrine mechanism independent of subclinical CAD, metabolic syndrome or hypertension.
Footnotes
DISCLOSURES: None of the authors has any conflict of interest related to the content of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 2.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 3.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 4.Mookadam F, Goel R, Alharthi MS, Jiamsripong P, Cha S. Epicardial fat and its association with cardiovascular risk: a cross-sectional observational study. Heart Views. 2010;11:103–108. doi: 10.4103/1995-705X.76801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamarappoo B, Dey D, Shmilovich H, Nakazato R, Gransar H, Cheng VY, Friedman JD, Hayes SW, Thomson LE, Slomka PJ, Rozanski A, Berman DS. Increased pericardial fat volume measured from noncontrast CT predicts myocardial ischemia by SPECT. JACC Cardiovasc Imaging. 2010;3:1104–1112. doi: 10.1016/j.jcmg.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng VY, Dey D, Tamarappoo B, Nakazato R, Gransar H, Miranda-Peats R, Ramesh A, Wong ND, Shaw LJ, Slomka PJ, Berman DS. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3:352–360. doi: 10.1016/j.jcmg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O’Donnell CJ, Fox CS, Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic medicine: a journal of the British Diabetic Association. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 13.Tamarappoo BK, Dey D, Nakazato R, Shmilovich H, Smith T, Cheng VY, Thomson LE, Hayes SW, Friedman JD, Germano G, Slomka PJ, Berman DS. Comparison of the extent and severity of myocardial perfusion defects measured by CT coronary angiography and SPECT myocardial perfusion imaging. JACC Cardiovasc Imaging. 2010;3:1010–1019. doi: 10.1016/j.jcmg.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Dey D, Suzuki Y, Suzuki S, Ohba M, Slomka PJ, Polk D, Shaw LJ, Berman DS. Automated quantitation of pericardiac fat from noncontrast CT. Invest Radiol. 2008;43:145–153. doi: 10.1097/RLI.0b013e31815a054a. [DOI] [PubMed] [Google Scholar]
- 15.Brindis RG, Douglas PS, Hendel RC, Peterson ED, Wolk MJ, Allen JM, Patel MR, Raskin IE, Bateman TM, Cerqueira MD, Gibbons RJ, Gillam LD, Gillespie JA, Iskandrian AE, Jerome SD, Krumholz HM, Messer JV, Spertus JA, Stowers SA. ACCF/ASNC appropriateness criteria for single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI): a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group and the American Society of Nuclear Cardiology endorsed by the American Heart Association. J Am Coll Cardiol. 2005;46:1587–1605. doi: 10.1016/j.jacc.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 16.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 17.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 18.Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006;92:1259–1264. doi: 10.1136/hrt.2005.080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–1374. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell DJ, Somaratne JB, Jenkins AJ, Prior DL, Yii M, Kenny JF, Newcomb AE, Schalkwijk CG, Black MJ, Kelly DJ. Impact of type 2 diabetes and the metabolic syndrome on myocardial structure and microvasculature of men with coronary artery disease. Cardiovasc Diabetol. 2011;10:80. doi: 10.1186/1475-2840-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25:543–567. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- 22.van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbely A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 23.Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev. 2007;8:253–261. doi: 10.1111/j.1467-789X.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 24.Iacobellis G, Willens HJ, Barbaro G, Sharma AM. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity (Silver Spring) 2008;16:887–892. doi: 10.1038/oby.2008.6. [DOI] [PubMed] [Google Scholar]
- 25.Sironi AM, Pingitore A, Ghione S, De Marchi D, Scattini B, Positano V, Muscelli E, Ciociaro D, Lombardi M, Ferrannini E, Gastaldelli A. Early hypertension is associated with reduced regional cardiac function, insulin resistance, epicardial, and visceral fat. Hypertension. 2008;51:282–288. doi: 10.1161/HYPERTENSIONAHA.107.098640. [DOI] [PubMed] [Google Scholar]
- 26.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–1319. doi: 10.1016/j.echo.2009.10.013. quiz 1417–1318. [DOI] [PubMed] [Google Scholar]
- 27.Saura D, Oliva MJ, Rodriguez D, Pascual-Figal DA, Hurtado JA, Pinar E, de la Morena G, Valdes M. Reproducibility of echocardiographic measurements of epicardial fat thickness. Int J Cardiol. 2010;141:311–313. doi: 10.1016/j.ijcard.2008.11.127. [DOI] [PubMed] [Google Scholar]
- 28.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310–1317. doi: 10.1016/j.jacc.2009.10.060. [DOI] [PubMed] [Google Scholar]