Abstract
Tumor lysis syndrome (TLS) represents a constellation of laboratory and clinical derangements that can occur following treatment of malignancies with high cellular turnover. Most commonly noted in hematologic malignancies, TLS has been reported to occur following liver-directed therapy in the form of both ablative therapies and transarterial therapies. Classification schemes exist, as do established diagnostic criteria, to aid in the definitive diagnosis of TLS. In addition, treatment algorithms are reported for patients with the diagnosis of TLS. This manuscript will review the risk factors associated with the development of TLS, the diagnostic criteria used, and treatment and preventative strategies employed. In addition, an algorithm for the diagnosis and treatment of TLS will be provided.
Keywords: interventional radiology, tumor lysis syndrome, complications, chemoembolization
Objectives: Upon completion of this article, the reader will be able to describe the pathophysiology, risk factors, diagnosis, and treatment of tumor lysis syndrome.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Tumor lysis syndrome (TLS) is an oncological emergency caused by the sudden and rapid destruction of proliferating neoplastic cells resulting in significant electrolyte and metabolite imbalance, which if left unchecked can lead to multiorgan failure and possibly patient demise.1 The syndrome is characterized by laboratory and clinical derangements resulting from the release of lysed cellular contents into the surrounding organ system and eventually the systemic circulation. If severe enough, TLS can lead to acute renal failure, cardiac dysrhythmias, seizures, and ultimately death.2 Malignancies with high proliferative rate, large tumor burden, and/or high sensitivity to cytotoxic therapy have a higher association with tumor lysis.3 TLS is most commonly observed in patients with hematological malignancies such as lymphoma and leukemia, particularly after initiation of chemotherapy, or can sometimes occur spontaneously without therapy.4 A variety of therapeutic interventions can lead to TLS including chemotherapy, radiation therapy, surgery, immunotherapy, hormonal therapy, and chemoembolization.5 Successful management of TLS depends on prophylactic treatment based on risk stratification as well as early detection and aggressive intervention when necessary.1 While the incidence is much less common in solid tumors, TLS has been reported in case reports and small case series, and can specifically occur after locoregional therapies for primary hepatic malignancies, as noted in Table 1.6
Table 1. Cases of tumor lysis syndrome and primary hepatic malignancy5 9 23 24 25 26 27 28 29 30 31 32 33 .
| Author | Year | Patient | Diagnosis | TLS etiology | LTLS | CTLS |
|---|---|---|---|---|---|---|
| Burney23 | 1998 | 44 M | HCC | TACE | – | ARF, D |
| Burney23 | 1998 | 46 M | HCC | TACE | – | ARF |
| Sakamoto et al26 | 2007 | 55 M | HCC | TACE | LDH,a UA, K, Ca | ARF, D |
| Shiba et al27 | 2008 | 77 M | HCC | TACE | LDH UA Phos | ARF |
| Hsieh et al9 | 2009 | 79 F | HCC | TACE | UA, K, Ca | ARF, D |
| Hsieh et al9 | 2009 | 56 M | HCC | TACE | UA, Phos, Ca | ARF |
| Wang and Chen34 | 2010 | 54 F | HCCb | TACE | LDH UA K Phos Ca | ARF |
| Chao and Chiang30 | 2012 | 51 M | HCC | TACE | LDH, UA, K, Ca | ARF |
| Nishida et al31 | 2013 | 70 M | HCC | TACE | – | ARF |
| Lehner et al5 | 2004 | 64 M | HCC | RFA | LDH, K, Phos, Ca | ARF CA D |
| Shiozawa et al29 | 2010 | 79 F | HCC | Sorafenib | LDH, UA | ARF |
| Huang and Yang28 | 2009 | 55 M | HCC | Sorafenib | UA, K, Phos | ARF, D |
| Lee et al25 | 2004 | 62 M | HCC | Thalidomide | LDH UA K Phos Ca | ARF CA D |
| Kekre et al33 | 2012 | 76 M | HCC | Spontaneous | LDH, UA, K, Phos | ARF, D |
| Vaisban et al24 | 2003 | 72 M | HCC | Spontaneous | LDH, UA, Phos | ARF, D |
| Ali et al32 | 2014 | 66 M | Cholangio Ca | Spontaneous | LDH, UA, K, Ca | ARF, D |
Abbreviations: ARF, acute renal failure; Ca, calcium (hypocalcemia); CA, cardiac arrhythmia; Cholangio, cholangiocarcinoma; CTLS, clinical tumor lysis syndrome; D, death; F, female; HCC, hepatocellular carcinoma; K, potassium (hyperkalemia); LDH, lactate dehydrogenase; LTLS, laboratory tumor lysis syndrome; M, male; Phos, phosphorus (hyperphosphatemia); RFA, radiofrequency ablation; TACE, transarterial chemoembolization; UA, uric acid (hyperuricemia).
While not classified as a marker of LTLS based on the Cairo–Bishop classification, LDH is often used as a marker of high cellular turnover and indirectly a nonspecific marker for risk of TLS.
HCC in this case was well-differentiated HCC with neuroendocrine features.
Pathophysiology
TLS can develop spontaneously from rapid tumor cell death, or more commonly it can occur soon after the delivery of cytotoxic therapy. As tumor cells lyse, cellular contents are released into the host organs. However, extensive damage occurs as electrolytes and metabolic products become systemic and overwhelm the body's homeostatic mechanisms, ultimately resulting in multiorgan failure.3 4 7 Due to this release of cellular contents, TLS is characterized by hyperkalemia, hyperphosphatemia, hypocalcemia, and hyperuricemia.8 This combination of electrolyte derangement and the rapidity with which it occurs is what induces the clinical spectrum of the lysis syndrome described below. Additionally, the cellular destruction and efflux of contents results in release of cytokines, which in turn cause a systemic inflammatory response syndrome, further contributing to multiorgan failure.8
An elevated concentration of uric acid is the most frequently recognized manifestation of TLS and often predisposes to many of the clinical derangements. Hyperuricemia results from the rapid release of intracellular deoxynucleic acids (DNA), which is subsequently metabolized to different purine and pyrimidine products.9 The catabolic pathway of purines (adenine and guanine) leads to the formation of hypoxanthine and xanthine and, ultimately, to uric acid via xanthine oxidase enzyme.1 The accumulation of uric acid is exacerbated by its low urinary excretion rate causing it to linger in the body. Allantoin, on the other hand, is the downstream byproduct of uric acid and is readily excreted in the urine due to its greater solubility.1
Hyperuricemia can lead to the precipitation of uric acid crystals in the renal collecting tubules, which can result in obstructive nephropathy.2 Additionally, elevated uric acid can cause inflammation, renal vasoconstriction, impaired autoregulation, and decreased renal blood flow.8 The available therapies used to treat hyperuricemia are allopurinol and rasburicase. Allopurinol is a purine analog that inhibits xanthine oxidase and prevents oxidation of hypoxanthine to xanthine, ultimately reducing the production of uric acid. Rasburicase is a recombinant uric oxidase that lowers the uric acid level by converting it to allantoin, which is more soluble and better excreted by the kidneys.
Potassium and phosphate are two highly concentrated intracellular ions in normal cells that can have severe deleterious effects on the body when released into the bloodstream in large quantities. In TLS, hyperkalemia usually occurs when the kidneys are unable to clear the massive potassium load generated from the lysed tumor cells. Hyperkalemia has multiple adverse effects including muscle weakness, cramps, paresthesias, and potentially paralysis.10 Most importantly, hyperkalemia can result in cardiac dysrhythmias including ventricular tachycardia, ventricular fibrillation, and ultimately asystole.10 Similar to potassium, malignant cells may contain increased phosphate concentration (as much as four times the amount within a normal cell). Tumor cell death can therefore lead to a large and rapid release of intracellular phosphate.1 Increased phosphate concentration in the blood will then lead to increased calcium binding, and as a result calcium phosphate precipitates within the blood and kidneys. In turn, hyperphosphatemia will cause hypocalcemia and further exacerbate renal insufficiency as calcium phosphate precipitates in the renal tubules, exacerbating the obstructive uropathy. Other clinical manifestations of hyperphosphatemia and hypocalcemia include muscle cramping, tetany, seizure, and cardiac dysrhythmias.8
Maintaining renal function is of utmost importance in successfully treating TLS, since renal excretion is the primary means of clearing uric acid, potassium, and phosphate. When functioning properly, clinical complications of TLS are less likely to develop. Renal impairment may result from the uric acid crystal nephropathy or calcium-phosphate crystal nephrocalcinosis, or a combination of both, and may secondarily lead to urinary acidosis and acute obstructive uropathy.11 This can lead to acute renal failure with concomitant increases in blood urea nitrogen (BUN) and creatinine levels.11 12 If renal function rapidly declines, the patient may experience acute shortness of breath due to pulmonary edema, hypertension, uremia, congestive heart failure, and worsening metabolic derangement.11 12
Classification
Identifying and grading TLS requires a comprehensive analysis of each patient individually, taking into account both the metabolic abnormalities and clinical manifestations. A classification system first developed by Hande and Garrow,13 and later modified by Cairo and Bishop,14 is now utilized, and categorizes TLS based on laboratory values and clinical status. Laboratory TLS (LTLS) and clinical TLS (CTLS) are the two main categories in this system (Tables 2 and 3). Under the Cairo–Bishop definition, LTLS is considered to be present if two or more abnormal serum values (uric acid, potassium, phosphate, or calcium) are identified to be above or below normal ranges, or if they change by 25% within 3 days before or 7 days after the initiation of treatment.14 CTLS is defined as the presence of one or more clinical criteria that are not believed to be attributable to a therapeutic agent (chemotherapy, radiation therapy), and includes renal insufficiency, cardiac dysrhythmias, seizures, and/or sudden death.14 Under this classification system, LTLS is considered either present or absent, while the CTLS is defined by the maximal grade of each clinical manifestation.14 When LTLS is clinically silent, it should be regarded as a sign of potential risk for developing CTLS.
Table 2. Cairo–Bishop definition of tumor lysis syndrome.
| Laboratory tumor lysis syndrome | |
| Uric acid | ≥8 mg/dL or 25% increase from baseline |
| Potassium | ≥6 mEq/L or 25% increase from baseline |
| Phosphorous | ≥4.5 mg/dL or 25% increase from baseline |
| Calcium | ≤7 mg/dL or 25% decrease from baseline |
| Clinical tumor lysis syndrome | |
| 1. | Creatinine ≥ 1.5× ULN |
| 2. | Cardiac dysrhythmia/sudden death |
| 3. | Seizure |
Abbreviation: ULN, upper limit of normal.
Source: Adapted from Wilson and Berns.15
Table 3. Cairo–Bishop grading system for tumor lysis syndrome.
| Grade 0 | Grade I | Grade II | Grade III | Grade IV | Grade V | |
|---|---|---|---|---|---|---|
| LTLS | − | + | + | + | + | + |
| Creatinine | Normal | 1.5× ULN | > 1.5–3.0× ULN | > 3.0–6.0× ULN | > 6.0× ULN | Death |
| Cardiac arrhythmia | None | Intervention not indicated | Nonurgent medical intervention indicated | Symptomatic and incompletely controlled medically or controlled with device (defibrillator) | Life-threatening (arrhythmia associated with CHF, hypotension, syncope, shock) |
Death |
| Seizure | None | – | One brief generalized seizure; Seizure(s) well controlled by anticonvulsants, or Infrequent focal motor seizures not interfering with activities of daily living |
Seizure in which consciousness is altered Poorly controlled seizure disorder Breakthrough generalized seizures despite medical intervention |
Seizure of any kind which is prolonged, repetitive, or difficult to control (status epilepticus, intractable epilepsy) | None |
Abbreviations: CHF, congestive heart failure; LTLS, laboratory tumor lysis; ULN, upper limits of normal.
Incidence
High-grade lymphoproliferative malignancies comprise the vast majority of TLS cases, particularly non-Hodgkin lymphoma, Burkitt lymphoma, acute lymphoblastic lymphoma, and acute myelogenous leukemia.15 16 17 The incidence of TLS in the treatment of hematologic malignant disease is 4 to 42%,17 although the rate decreases with the use of preventive measures.8
While much less common, TLS can occur in patients with solid cancers, either spontaneously or following therapy. It is thought that solid tumors are less affected by TLS because they are relatively resistant to cytotoxic therapies. For this reason, the diagnosis of TLS is often unsuspected and thus can lead to higher morbidity and mortality.5 8 The prevalence of TLS in patients with solid tumors is difficult to ascertain because it is based primarily on case reports and small case series. Mirrakhimov et al6 presented a review of the literature on TLS occurrence in solid tumors from 1950 to 2014, dividing the review by solid tumor types including pulmonary, breast, gynecological, gastrointestinal, neurological, and sarcomatous tumors. The majority of publications comprised case reports, with total patient numbers for each organ system–based tumors ranging from under 10 to 30 reports in the literature.
Risk Factors
The risk of developing TLS is based on two factors: the type of malignancy and patient characteristics.8 14 17 18 First, malignancy-related factors revolve around the tumor cell burden and the rate of cellular proliferation and turnover. Large-sized masses, the presence of metastases, rapid tumor proliferation, and sensitivity to therapy can predispose the patient to developing lysis syndrome.18 Second, predisposing patient characteristics include preexisting renal insufficiency, dehydration, exposure to nephrotoxic substances, and baseline metabolic derangements, particularly elevations in uric acid or phosphorous.6 15 Increased lactate dehydrogenase (LDH) levels are a nonspecific marker of cell turnover, and have been used to try and identify patients susceptible to TLS prior to therapy.6
In 2010, an expert consensus panel on TLS developed guidelines for risk stratification and associated TLS prophylaxis recommendations.18 A decision tree was developed to assign low-, intermediate-, and high-risk categories to patients with cancer at risk for TLS. Factors integrated include tumor bulk and stage, and biological evidence of laboratory abnormalities and/or renal impairment.18 Nearly all the recommended intermediate- and high-risk scenarios were slated for hematological malignancies. The guidelines indicate that all solid tumors are regarded as “low-risk” for lysis, unless the disease is bulky or highly sensitive to chemotherapy, in which case the tumor is classified as intermediate risk. If in addition the patient has risk factors of severe renal insufficiency, oliguria, dehydration, or hypotension, the patient may be potentially stratified as high risk for TLS. The prophylactic recommendations for both low- and intermediate-risk include monitoring and hydration, with allopurinol administration optional for low-risk disease and recommended for intermediate-risk disease. As for high-risk disease, prophylactic rasburicase is recommended in place of allopurinol.
Management
The most important aspect of management is identifying patients at risk for developing TLS so that prophylactic measures may be implemented before the initiation of therapy. Most clinical manifestations can be managed when recognized early; delay in recognition and treatment of TLS complications can be potentially life threatening. Serum levels of creatinine, BUN, potassium, calcium, phosphorous, LDH, and uric acid levels should be determined prior to therapy and then serially drawn over the first few days after initiation of tumor therapy. Additionally, patients should have a baseline electrocardiogram for comparison, with continuous cardiac monitoring while therapy is administered. Before the initiation of therapy, attempts should be made to correct dehydration, fluid overload, and electrolyte abnormalities as well as establish adequate urinary output.
The three main principles in treating TLS include hydration, correction of metabolic abnormalities, and treatment of acute renal failure.2 3 4 7 In high-risk patients without a known contraindication (e.g., congestive heart failure), aggressive fluid administration is a fundamental strategy in the prevention and treatment of TLS.11 19 Ideally, when a risk for TLS is suspected, these patients should receive intravenous hydration 24 to 48 hours prior to initiating tumor therapy. This serum volume expansion promotes increased renal perfusion, glomerular filtration rate, and urine output, as well as minimizing urinary acidosis, ultimately decreasing the concentrations of solutes in the renal tubules and reducing the likelihood of precipitation.8 17 Hydration is the preferred method of increasing urine output, but diuretics may also be necessary.8 The use of sodium bicarbonate to alkalinize the urine to promote excretion of uric acid has been traditionally recommended for prevention and management of TLS.20 However, the use of sodium bicarbonate to alkalinize the urine is not currently recommended due to a lack of evidence of benefit and due to potential complications including metabolic alkalosis and further worsening of calcium phosphate precipitation.18
An important therapy in the treatment of TLS includes the reduction of uric acid within the blood. While both allopurinol and rasburicase are the main hypouricemic agents used for TLS, they differ in their mechanisms of action and therapeutic strategy. Allopurinol is a potent inhibitor of xanthine oxidase, and blocks the conversion of xanthine and hypoxanthine (catabolic products of purine DNA) to uric acid (citation). Oral or intravenous allopurinol needs to be initiated a few days prior to initiation of cytotoxic therapy, because it only prevents new uric acid formation and does not reduce the amount of uric acid already present. Rasburicase, a recombinant urate oxidase, is a newer therapy, and as opposed to inhibiting uric acid formation, promotes the breakdown of uric acid into allantoin via the uric acid oxidase enzyme.21 Allantoin is 5 to 10 times more soluble in the urine than uric acid, and as such, a favorable byproduct of uric acid. In leukemia patients with TLS and requiring rasburicase, a median of three daily doses were administered in the published North American compassionate use trial.22 Of note, patients with glucose-6-phosphate dehydrogenase deficiency (a rare genetic defect) should avoid rasburicase because hydrogen peroxide, a breakdown product of uric acid, can cause methemoglobinemia and, in severe cases, lead to hemolytic anemia.6 8 Patients at high risk for TLS are recommended to receive rasburicase prophylactically because it is the only agent currently available to reduce the level of uric acid in the blood.18
Once TLS occurs, electrolyte and acid–base abnormalities may need to be corrected. Hyperkalemia is a dangerous component of TLS as it can cause sudden death due to cardiac dysrhythmia. The serum potassium level should be monitored closely (every 4– 6 hours), along with continuous cardiac monitoring, and possibly treated per established protocols with glucose plus insulin to induce cellular uptake of potassium, and with calcium gluconate to reduce the risk of dysrhythmia.10 Hemodialysis and hemofiltration should be considered for refractory hyperkalemia, especially in the setting of oliguria.8 10 While lacking proven efficacy, the use of oral phosphate binders such as aluminum salts or sevelamer for treating hyperphosphatemia is optional and is often administered for established TLS.8 A potential role for controlling the serum phosphorus level is to prevent hypocalcemia, although treatment of asymptomatic hypocalcemia is generally not recommended. If symptomatic hypocalcemia is present, intravenous calcium gluconate (50–100 mg/kg per dose) may be administered to correct the clinical symptoms (e.g., neuromuscular irritability, paresthesias, petechiae).1 However, administration of IV calcium must be weighed against the risk of increasing calcium phosphate deposition and causing or worsening acute obstructive uropathy.
In patients with persistent or worsening obstructive nephropathy leading to acute renal failure, or those with significant uremia or severe electrolyte abnormalities, hemodialysis should be initiated.17 Continuous hemofiltration can be used for those patients with fluid overload and TLS requiring hemodialysis.1 A delay in initiating hemodialysis for acute renal failure may turn a potentially reversible clinical situation into an irreversible one.14
Successful management and treatment of TLS is highly dependent on anticipation and prevention in at-risk patients, followed by early recognition and prompt treatment of the clinical signs and symptoms. Establishing vascular access and initiating prophylactic measures, especially hydration and possibly administering allopurinol or rasburicase, are vital. Early recognition of metabolic abnormalities usually prevents any severe or life-threatening complications associated with TLS. Patients at low to intermediate risk for TLS can be managed with hydration, allopurinol, and close monitoring. Patients with high risk or clinically established TLS should be managed with aggressive hydration, rasburicase, and possibly with hemodialysis.
Tumor Lysis Syndrome in Primary Liver Malignancy
TLS has been reported in several different solid tumor types, including hepatocellular carcinoma (HCC) and cholangiocarcinoma. There are 13 published reports of primary hepatic malignancy presenting with TLS and acute kidney injury5 9 23 24 25 26 27 28 29 30 31 32 33 (see Table 1), not including 2 publications of pediatric hepatoblastoma6; 12 of these publications are of HCC and 1 is of cholangiocarcinoma.32 TLS may occur spontaneously, as occurred in the case of cholangiocarcinoma,32 or it may occur after various treatments of HCC including sorafenib,28 29 oral thalidomide,25 radiofrequency ablation (RFA),5 and transarterial chemoembolization (TACE).9 23 26 27 30 31 34 When reported, most cases of TLS occurring after TACE or RFA had an onset within 72 hours of the locoregional therapy, with the longest interval to onset of TLS being 5 days.25 34 The TLS-related mortality in primary hepatic malignancy from these cases is 56% (9 of 16 reported patients), with 40% of patients treated in this cohort with locoregional therapy resulting in death.
For solid liver malignancies, the risk factors for developing TLS include large hepatic tumor burden, rapid expansion or infiltration, and large areas of tumor necrosis.9 Except for the single case of RFA-induced TLS, all the cases of primary hepatic malignancy leading to TLS had large index tumor size (more than 5 cm) and many had areas of tumor necrosis. When such high-risk patients are encountered, intensive and persistent monitoring of the patients renal function and serum potassium, uric acid, calcium, phosphate, and LDH levels is important for early recognition of TLS. If initial therapeutic measures are insufficient, further management may be required, as was the case in Chao et al, where the patient developed TLS post-TACE and was initially unresponsive to hydration, allopurinol, and urine alkalinization, but drastically improved after administering rasburicase.
The clinical presentation of TLS in a patient following TACE can appear very similar to contrast-induced renal insufficiency, as seen in the case report by Hsieh et al.9 Oliguria is an early clinical sign of both etiologies, leading to the initiation of intravenous hydration and assessment of renal function. Assuming the etiology as contrast-induced renal insufficiency is not unreasonable given the much higher incidence than TLS, and in most cases adequate hydration is sufficient to overcome either complication.35 In the case by Hsieh et al, and typical for TLS, the acute renal impairment did not improve with fluid resuscitation, necessitating further investigation and identification of hyperuricemia, hyperkalemia, and hypocalcemia. In this patient, oral allopurinol and urine alkalinization was initiated, and combined with hemodialysis resulted in stabilization of renal function and improved urine output.9 Laboratory analysis can be crucial in differentiating the two etiologies, particularly uric acid and phosphate levels; this is crucial to diagnosing TLS early and treating appropriately. As demonstrated in publications by Burney et al and Hsieh et al, their first encounters with TLS post-TACE were not anticipated and diagnosed late resulting in the patients demise, while their second encounters with TLS resulted in early detection and treatment with clinical improvement.9 23 These cases, while few in number, demonstrate TLS as a possible and potentially lethal complication of TACE for large HCC.
As demonstrated, TLS is a rare complication of locoregional therapy and thus an easily neglected complication. However, as the spectrum and stage of hepatic malignancy treated by interventional oncology continues to expand, it is important to be aware and anticipate the development of this potentially devastating complication; interventionalists performing liver-directed therapy should be informed on the preventative and therapeutic approaches. Fig. 1 represents the authors' algorithm for diagnosing and treating TLS in patients undergoing transarterial therapies.
Fig. 1.
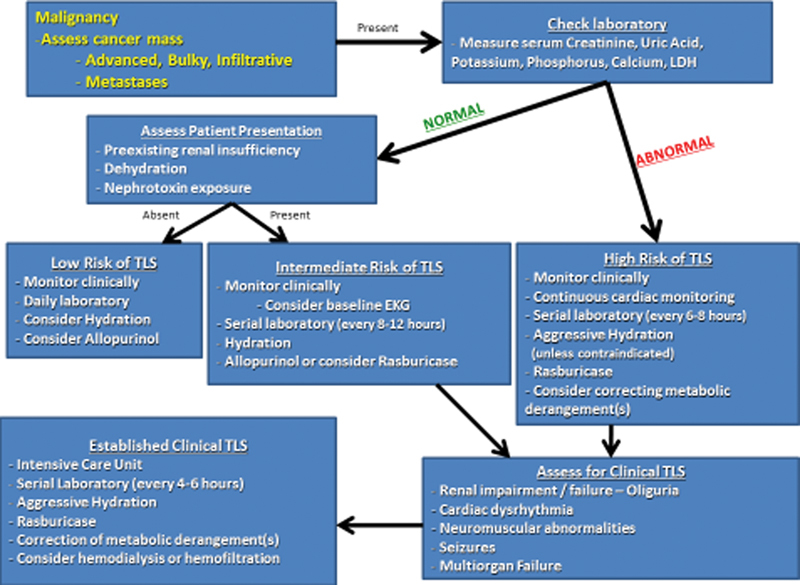
Algorithm for the diagnosis and treatment of tumor lysis syndrome in patients undergoing liver-directed therapy.
Summary
TLS is an oncological emergency and comprises a biochemical derangement of cellular metabolism related to high cellular proliferation and turnover, which can lead to acute renal impairment, cardiac rhythm disturbances, central nervous system toxicity, and death due to multiorgan failure. Stratifying cancer patients based on the risk of TLS is essential in preventing this complication. Vigorous hydration and the use of uric acid lowering agents are the mainstay of management.
References
- 1.Hochberg J, Cairo M S. Tumor lysis syndrome: current perspective. Haematologica. 2008;93(1):9–13. doi: 10.3324/haematol.12327. [DOI] [PubMed] [Google Scholar]
- 2.Davidson M B, Thakkar S, Hix J K, Bhandarkar N D, Wong A, Schreiber M J. Pathophysiology, clinical consequences, and treatment of tumor lysis syndrome. Am J Med. 2004;116(8):546–554. doi: 10.1016/j.amjmed.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 3.Mughal T I, Ejaz A A, Foringer J R, Coiffier B. An integrated clinical approach for the identification, prevention, and treatment of tumor lysis syndrome. Cancer Treat Rev. 2010;36(2):164–176. doi: 10.1016/j.ctrv.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Del Toro G, Morris E, Cairo M S. Tumor lysis syndrome: pathophysiology, definition, and alternative treatment approaches. Clin Adv Hematol Oncol. 2005;3(1):54–61. [PubMed] [Google Scholar]
- 5.Lehner S G, Gould J E, Saad W E, Brown D B. Tumor lysis syndrome after radiofrequency ablation of hepatocellular carcinoma. AJR Am J Roentgenol. 2005;185(5):1307–1309. doi: 10.2214/AJR.04.1265. [DOI] [PubMed] [Google Scholar]
- 6.Mirrakhimov A E, Ali A M, Khan M, Barbaryan A. Tumor lysis syndrome in solid tumors: an up to date review of the literature. Rare Tumors. 2014;6(2):5389. doi: 10.4081/rt.2014.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McBride A, Westervelt P. Recognizing and managing the expanded risk of tumor lysis syndrome in hematologic and solid malignancies. J Hematol Oncol. 2012;5(75):75. doi: 10.1186/1756-8722-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard S C, Jones D P, Pui C H. The tumor lysis syndrome. N Engl J Med. 2011;364(19):1844–1854. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh P-M, Hung K C, Chen Y S. Tumor lysis syndrome after transarterial chemoembolization of hepatocellular carcinoma: case reports and literature review. World J Gastroenterol. 2009;15(37):4726–4728. doi: 10.3748/wjg.15.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott M J, Ronksley P E, Clase C M, Ahmed S B, Hemmelgarn B R. Management of patients with acute hyperkalemia. CMAJ. 2010;182(15):1631–1635. doi: 10.1503/cmaj.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Alfa A K Younes A Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management Am J Kidney Dis 201055503S1–S13., quiz S14–S19 [DOI] [PubMed] [Google Scholar]
- 12.El-Husseini A, Sabucedo A, Lamarche J, Courville C, Peguero A. Acute kidney injury associated with tumor lysis syndrome: a paradigm shift. Am J Emerg Med. 2012;30(2):390000–3.9E8. doi: 10.1016/j.ajem.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Hande K R, Garrow G C. Acute tumor lysis syndrome in patients with high-grade non-Hodgkin's lymphoma. Am J Med. 1993;94(2):133–139. doi: 10.1016/0002-9343(93)90174-n. [DOI] [PubMed] [Google Scholar]
- 14.Cairo M S, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127(1):3–11. doi: 10.1111/j.1365-2141.2004.05094.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilson F P, Berns J S. Onco-nephrology: tumor lysis syndrome. Clin J Am Soc Nephrol. 2012;7(10):1730–1739. doi: 10.2215/CJN.03150312. [DOI] [PubMed] [Google Scholar]
- 16.Will A, Tholouli E. The clinical management of tumour lysis syndrome in haematological malignancies. Br J Haematol. 2011;154(1):3–13. doi: 10.1111/j.1365-2141.2011.08697.x. [DOI] [PubMed] [Google Scholar]
- 17.Coiffier B, Altman A, Pui C H, Younes A, Cairo M S. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767–2778. doi: 10.1200/JCO.2007.15.0177. [DOI] [PubMed] [Google Scholar]
- 18.Cairo M S Coiffier B Reiter A Younes A; TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus Br J Haematol 20101494578–586. [DOI] [PubMed] [Google Scholar]
- 19.Rampello E, Fricia T, Malaguarnera M. The management of tumor lysis syndrome. Nat Clin Pract Oncol. 2006;3(8):438–447. doi: 10.1038/ncponc0581. [DOI] [PubMed] [Google Scholar]
- 20.Ten Harkel A D, Kist-Van Holthe J E, Van Weel M, Van der Vorst M M. Alkalinization and the tumor lysis syndrome. Med Pediatr Oncol. 1998;31(1):27–28. doi: 10.1002/(sici)1096-911x(199807)31:1<27::aid-mpo6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Sood A R, Burry L D, Cheng D K. Clarifying the role of rasburicase in tumor lysis syndrome. Pharmacotherapy. 2007;27(1):111–121. doi: 10.1592/phco.27.1.111. [DOI] [PubMed] [Google Scholar]
- 22.Jeha S, Kantarjian H, Irwin D. et al. Efficacy and safety of rasburicase, a recombinant urate oxidase (Elitek), in the management of malignancy-associated hyperuricemia in pediatric and adult patients: final results of a multicenter compassionate use trial. Leukemia. 2005;19(1):34–38. doi: 10.1038/sj.leu.2403566. [DOI] [PubMed] [Google Scholar]
- 23.Burney I A. Acute tumor lysis syndrome after transcatheter chemoembolization of hepatocellular carcinoma. South Med J. 1998;91(5):467–470. doi: 10.1097/00007611-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Vaisban E, Braester A, Mosenzon O, Kolin M, Horn Y. Spontaneous tumor lysis syndrome in solid tumors: really a rare condition? Am J Med Sci. 2003;325(1):38–40. doi: 10.1097/00000441-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Lee C C Wu Y H Chung S H Chen W J Acute tumor lysis syndrome after thalidomide therapy in advanced hepatocellular carcinoma Oncologist 200611187–88., author reply 89 [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto N, Monzawa S, Nagano H, Nishizaki H, Arai Y, Sugimura K. Acute tumor lysis syndrome caused by transcatheter oily chemoembolization in a patient with a large hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2007;30(3):508–511. doi: 10.1007/s00270-005-0240-8. [DOI] [PubMed] [Google Scholar]
- 27.Shiba H, Ishida Y, Wakiyama S, Sakamoto T, Misawa T, Yanaga K. Acute tumor lysis syndrome after transarterial chemoembolization for hepatocellular carcinoma. Cancer Sci. 2008;99(10):2104–2105. doi: 10.1111/j.1349-7006.2008.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang W-S, Yang C H. Sorafenib induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol. 2009;15(35):4464–4466. doi: 10.3748/wjg.15.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiozawa K, Watanabe M, Takenaka H. et al. Tumor lysis syndrome after sorafenib for hepatocellular carcinoma: a case report. Hepatogastroenterology. 2010;57(101):688–690. [PubMed] [Google Scholar]
- 30.Chao C T, Chiang C K. Rasburicase for huge hepatocellular carcinoma with tumor lysis syndrome: case report. Medical principles and practice: international journal of the Kuwait University. Health Science Centre. 2012;21(5):498–500E. doi: 10.1159/000339083. [DOI] [PubMed] [Google Scholar]
- 31.Nishida Y, Fujii H, Hagihara A. et al. [Tumor lysis syndrome after transarterial embolization for hepatocellular carcinoma] Nippon Shokakibyo Gakkai Zasshi. 2013;110(3):441–448. [PubMed] [Google Scholar]
- 32.Ali A M, Barbaryan A, Zdunek T, Khan M, Voore P, Mirrakhimov A E. Spontaneous tumor lysis syndrome in a patient with cholangiocarcinoma. J Gastrointest Oncol. 2014;5(2):E46–E49. doi: 10.3978/j.issn.2078-6891.2014.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kekre N, Djordjevic B, Touchie C. Spontaneous tumour lysis syndrome. CMAJ. 2012;184(8):913–916. doi: 10.1503/cmaj.111251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang K, Chen Z. Acute tumor lysis syndrome after transarterial chemoembolization for well-differentiated hepatocellular carcinoma with neuroendocrine features. Onkologie. 2010;33(10):532–535. doi: 10.1159/000319695. [DOI] [PubMed] [Google Scholar]
- 35.Huo T I, Wu J C, Lee P C, Chang F Y, Lee S D. Incidence and risk factors for acute renal failure in patients with hepatocellular carcinoma undergoing transarterial chemoembolization: a prospective study. Liver Int. 2004;24(3):210–215. doi: 10.1111/j.1478-3231.2004.00911.x. [DOI] [PubMed] [Google Scholar]


