Summary
Chronic liver disease is associated with remarkable alterations in the intra- and extrahepatic vasculature. Because of these changes, the fields of liver vasculature and portal hypertension have recently become closely integrated within the broader vascular biology discipline. As developments in vascular biology have evolved, a deeper understanding of vascular processes has led to a better understanding of the mechanisms of the dynamic vascular changes associated with portal hypertension and chronic liver disease. In this context, hepatic vascular cells, such as sinusoidal endothelial cells and pericyte-like hepatic stellate cells, are closely associated with one another, where they have paracrine and autocrine effects on each other and themselves. These cells play important roles in the pathogenesis of liver fibrosis/cirrhosis and portal hypertension. Further, a variety of signaling pathways have recently come to light. These include growth factor pathways involving cytokines such as transforming growth factor β, platelet derived growth factor, and others as well as a variety of vasoactive peptides and other molecules. An early and consistent feature of liver injury is the development of an increase in intra-hepatic resistance; this is associated with changes in hepatic vascular cells and their signaling pathway that cause portal hypertension. A critical concept is that this process aggregates signals to the extrahepatic circulation, causing derangement in this system’s cells and signaling pathways, which ultimately leads to the collateral vessel formation and arterial vasodilation in the splanchnic and systemic circulation, which by virtue of the hydraulic derivation of Ohm’s law (pressure = resistance × flow), worsens portal hypertension. This review provides a detailed review of the current status and future direction of the basic biology of portal hypertension with a focus on the physiology, pathophysiology, and signaling of cells within the liver, as well as those in the mesenteric vascular circulation. Translational implications of recent research and the future directions that it points to are also highlighted.
Keywords: Endothelial cell, Pericyte, Sinusoid, Pressure, Resistance, Therapy
Introduction
Understanding of the basic elements of general and liver specific vascular biology has grown substantially over the last decade. This work has led to exciting new developments in the field of portal hypertension. This review aims to put these advances into context.
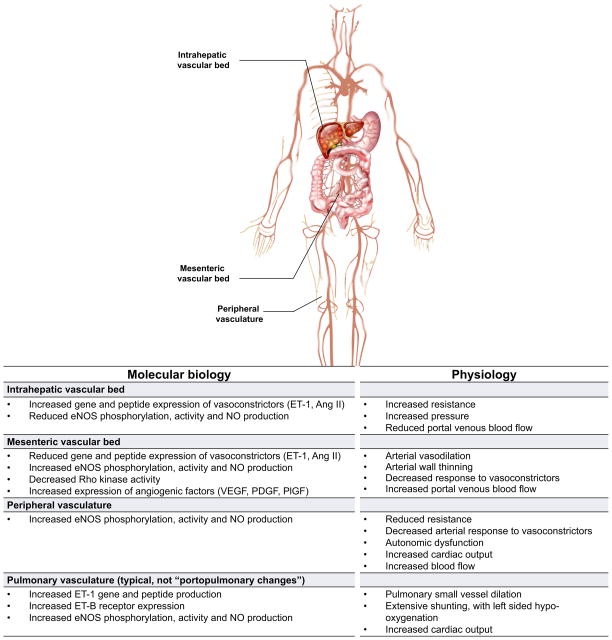
Vascular beds are diverse, each with their own specific functional attributes. Notwithstanding, a number of themes have emerged. In patients with chronic liver disease, the peripheral vasculature, the mesenteric vascular bed, and the intrahepatic microcirculatory unit have received attention (Fig. 1). A recurrent theme is that while the cells and molecules in each vascular structure exhibit many similarities, variability in signaling pathways lead to unique functional attributes in each. For example, in the sinusoid and liver, vasoconstriction and increased resistance to blood flow is prominent. In contrast, in the mesenteric vasculature, vasodilation is prominent. The combination of increased resistance in the liver and increased flow to the portal vein from the mesenteric circulation results in increased portal pressure as indicated by the hydraulic equivalent of Ohm’s Law (pressure = flow × resistance). Additionally, it is important to recognise that factors other than the vascular bed itself may be important in the development of changes in resistance or flow. For example, within the liver, it is likely that fibrosis, particularly in advanced states is critically important in the increased resistance, typical of portal hypertension.
Fig. 1. Heterogeneity of vascular beds in portal hypertension.
Shown are prominent vascular beds and their associated pathophysiology. The intrahepatic vascular bed is typified by an increase in resistance to flow. Liver injury leads to abnormal endothelial function, with a reduction in the production of vasodilators by sinusoidal endothelial cells such as nitric oxide (NO); concomitantly, there is an increase in the synthesis of vasoconstrictors such as endothelin-1 (ET1) and angiotensin II (Ang II) by other cells in the sinusoid (see also Fig. 2). The mesenteric vascular bed is characterised by vasodilation by reduced resistance caused by upregulation of vasodilators such as NO, leading to increased flow to the portal vein. The net result of this physiology is an increase in intrahepatic resistance and portal blood flow from the splanchnic circulation, leading to increased portal pressure and portal hypertension. In peripheral vascular beds, increased eNOS activity and NO production typically leads to reduced resistance, low systemic pressure, and a hyperdynamic state typical of patients with cirrhosis. Abnormalities also exist in other vascular beds such as the pulmonary, brain, renal, and likely even others. Of note, increased intrahepatic resistance is only for the portal system, and the resistance of the hepatic artery is decreased. Therefore, due to the hepatic arterial buffer response, the total blood flow into the liver may be the same in cirrhosis [130]. ET-1, endothelin-1; Ang II, angiotensin II; NO, nitric oxide; eNOS, endothelial nitric oxide synthase; VEGF, vascular endothelial growth factor; PDGF, platelet derived growth factor; PlGF, placental growth factor.
Intrahepatic vascular pathophysiology
The intrahepatic microvascular unit is made up of several discrete units, including portal venules, hepatic arterioles, sinusoids, central venules, and lymphatics. The cellular elements in these structures include endothelial cells, smooth muscle cells, and in the sinusoid, pericyte-like hepatic stellate cells. It is important to recognise that these cells are intimately associated with one another, where they have paracrine and autocrine effects on each other and themselves. The canonical example of course is the paracrine effect of nitric oxide (NO), released by sinusoidal endothelial cells on smooth muscle cells and on stellate cells.
Hepatic cells in intrahepatic vascular physiology and pathophysiology
Liver sinusoidal endothelial cells (LSEC)
The majority of hepatic endothelial cells reside within the hepatic sinusoids; these cells, known as liver sinusoidal endothelial cells (LSECs), have therefore garnered great attention. The LSEC phenotype is uniquely different, not only from endothelial cells in other portions of the liver, but also from endothelial cells in other organs [1]. Perhaps the most unique feature of the LSEC phenotype is fenestration; fenestrae are organised in typical sieve plates [2]. While the function of fenestrae is controversial, it appears that they help facilitate the transport of macromolecules from the sinusoidal lumen, across the cell, into the space of Disse, providing access of these molecules to hepatocytes. Additionally, in vivo, LSECs lack a typical basement membrane, further facilitating macromolecular transport [1,3].
A key signature of most forms of liver injury/disease is the loss of many of these unique phenotypes. Additionally, it should be emphasised that typical LSEC features are lost in culture, making in vitro study of LSEC phenotypes challenging. It is well appreciated that liver fibrosis is associated with alterations in the diameter and number of fenestrae [3]. A recent innovative structural analysis [4] showed that fenestrae formation could be regulated by membrane lipid rafts, which are cholesterol and sphingolipid rich domains that serve as a platform for many membrane proteins such as caveolin. Interestingly, fenestrae distribution appears to be inversely related to lipid raft regions.
Recent work has begun to untangle the molecular signaling pathways that lead from cell injury to abnormalities in fenestration. For example, exposure of LSECs to vascular endothelial growth factor (VEGF) treatment increases fenestrae formation by decreasing the abundance of lipid raft regions, which may explain an essential role of VEGF for the maintenance of fenestrae observed by others (see below). Furthermore, decreased fenestrae formation may be linked to increased caveolin-1 levels observed in LSEC after liver fibrosis [5]. Further work to better understand the pathways leading from injury and fibrosis to fenestral abnormality is expected.
Regulation of the LSEC phenotype
A variety of molecular signaling pathways regulate the LSEC phenotype; these are both soluble factors and mechanical forces. Among the soluble factors, growth factors appear to be the most prominent. As referred to above, VEGF appears to be the most critical molecule in the modulation of the size and number of LSEC fenestrae [5–8]. Removal of VEGF from the cell culture medium results in loss of fenestrae, which can be restored by resupply of VEGF [6]. Similarly, disruption of VEGF signaling by a conditional deletion of Vegfr1 in mice led to loss of fenestrae, while restitution of VEGFR1 led to refenestration [8]. A number of growth factors other than VEGF also regulate the LSEC phenotype, with most of these being activators of receptor tyrosine kinases and include angiopoietins, ephrins, and fibroblast growth factors [9,10].
The LSEC phenotype is also regulated by biomechanical forces such as shear stress. The most prominent effect of shear stress appears to be in the modulation of endothelial nitric oxide synthase (eNOS) activity in LSECs, thereby regulating flow and vascular tone in the sinusoids [11]. Exposure of cultured LSECs to varying degrees of flow leads to different degrees of eNOS activation and NO release [11]. Similarly, isolated perfused rat livers increased NO release as a result of shear stress [11].
LSEC-mediated paracrine regulation
Not only do exogenous factors play an important role in the regulation of the LSEC phenotype, but recent evidence indicates that LSECs themselves play an important role in the function of neighbouring cells and, therefore, the microenvironment. For example, LSECs produce angiocrine growth factors and regulate liver regeneration and fibrosis. Wnt2 and hepatocyte growth factor (HGF) induced by LSECs promote hepatocyte proliferation [12]. It has also been reported that bone marrow-derived LSEC progenitor cells are important for liver regeneration because of the large portion of HGF they induce [13]. Interestingly, however, LSECs isolated from biliary cirrhotic mice exhibit enhanced pro-fibrotic growth factors and cytokines, such as transforming growth factor (TGF)-β, bone morphogenetic protein 2(BMP2) and platelet derived growth factor (PDGF)-C, with decreased anti-fibrotic factors such as follistatin and apelin [14]. Furthermore, LSECs may release vesicles, including “microvesicles” (also referred to as “microparticles”) and exosomes; these structures appear to contain signaling molecules that regulate other cell types in a paracrine fashion [15]. Our understanding of both structures is at a nascent state but increasing information indicates a role in paracrine signaling. Interestingly, recent studies indicate that growth factor stimulation of endothelial cells may stimulate release of these “signaling vesicles.” One such growth factor may be the fibroblast growth factor (FGF). While less studied than VEGF in the hepatic microcirculation, FGF signaling through its cognate receptor FGFR is important for LSEC stimulatory signaling and release of paracrine molecules [9]. These features are pertinent not only in physiologic conditions but also in pathophysiologic situations, such as cirrhosis and portal hypertension as discussed below.
LSECs also appear to be an important source of certain types of extracellular matrix. For example, LSECs produce the cellular isoform of fibronectin in response to injury [16]. Fibronectin in turn has potent paracrine effects on hepatic stellate cells (HSCs) [16], stimulating their activation early in the injury process. Additionally, fibronectin appears to stimulate HSC synthesis of endothelin-1 (ET-1), which in turn has paracrine effects on HSCs [17].
LSEC phenotype in disease
During liver injury, the LSEC phenotype changes dramatically [1]. One of the most remarkable phenotypic changes is “capillarisation”, characterised by loss of fenestrae and abnormal deposition of a basement membrane matrix on the abluminal surface of LSECs [1]. In addition to these anatomical changes, a number of biochemical changes also occur in the LSEC phenotype. For example, it is now well established that eNOS activity is diminished in LSECs after liver injury, consistent with an endothelialopathy in liver disease [5,18]. This has a number of important effects on portal hypertension, including that a reduction in intrahepatic NO appears to be a critical component of the intense vasoconstrictive nature of the injured liver [19].
The mechanism for the reduction in eNOS activity and NO synthesis after injury is tied to extensive post-translational dysregulation of eNOS. For example, it has been established that eNOS function is tied to a series of events that regulate the phosphorylation status of eNOS, including by interacting and/or binding to calmodulin, caveolin-1, HSP90, Akt, and a variety of other intracellular proteins [20,21]. In the liver increased expression of caveolin-1 in LSECs appears to be important in the reduced eNOS activity described [5]. More recent work suggests that a series of complex molecular events, involving molecules that regulate and/or dampen G protein coupled receptor signaling, potently modulate eNOS [22,23].
Reduced NO from LSECs may also play a role in progression of fibrosis. NO has been shown to maintain quiescence of hepatic stellate cells (HSCs) and reduced exposure of HSCs to NO may facilitate their activation [24,25].
As mentioned above, VEGF is important in the maintenance of LSEC fenestrae and may prevent LSECs from undergoing capillarisation [6–8]. The mechanism of this effect is currently unknown. However, there may be a role for VEGF in NO signaling in LSECs, and it is possible that VEGF’s downstream NO signaling plays an important role in the maintenance of LSEC fenestrae [26].
Neighbouring cells also appear to change the LSEC phenotype in disease. For example, in response to a treatment with saturated free fatty acids in vitro, which mimics lipid accumulation in steatosis, hepatocytes release microvesicles that have pro-angiogenic activity [27]. Microvesicles collected from conditioned media from these lipid-challenged hepatocytes enhanced migration and tube formation of endothelial cells in vitro. Although the effects of these hepatocyte-derived microvesicles on LSECs have not been clearly specified, these observations suggest that the hepatocyte-LSEC communication induces angiogenesis.
Pericytes, stellate cells, and myofibroblasts
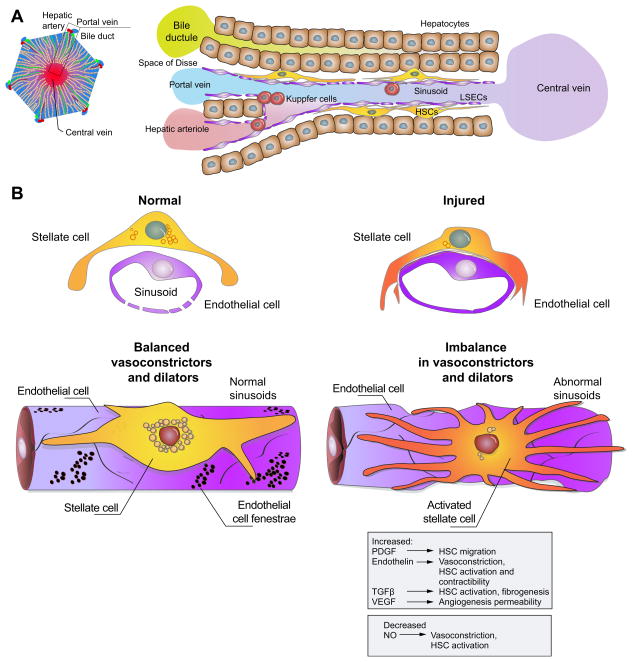
By virtue of their anatomic position in the sinusoid (Fig. 2), stellate cells have also been coined liver specific pericytes. Pericytes are found throughout the body in small calibre blood vessels, typically capillaries [28]. They exhibit many features of smooth muscle cells and are believed to play a role in blood flow regulation. Recent work has suggested that their role may be more complex, and that they have more pleiotropic roles, in particular in wound healing; here they differentiate into cells with other functions [29,30]. For example, fate mapping experiments in several organs, including the liver, indicate that pericytes are the cellular precursors of myofibroblasts (see below) and transform in response to injury [29,30].
Fig. 2. Changes in the hepatic sinusoid in response to liver injury.
(A) shows a simplified version of a portal tract (left), central vein (right), and hepatic sinusoidal morphology with associated cell types within the sinusoid. (B) shows the sinusoid in response to liver injury; the upper panel depicts a cross sectional image, while the lower panel shows a 3-dimensional view. In the normal state (left), sinusoidal endothelial cells produce NO supporting a low resistance state. In response to liver injury, extensive changes occur in the sinusoid. Liver injury (right) leads to both morphological as well as molecular abnormalities in sinusoidal endothelial cells and stellate cells. For example, endothelial cells become defenestrated, develop a defect in NO production, and concurrently increase production of other proteins such as endothelin and fibronectin that can contribute to stellate cell activation. Upon their activation, stellate cells develop an enhanced contractile phenotype and produce increased extracellular matrix. A number of paracrine and autocrine interactions occur between sinusoidal endothelial cells and stellate cells with some of the putative molecules and their associated functions shown (gray box). HSC, hepatic stellate cells; LSEC, liver sinusoidal endothelial cell.
Current evidence indicates that during wound healing, stellate cells undergo a transformation process. This process, termed “activation”, involves a number of complex and integrated features. Characteristics of activation include morphologic and functional changes, such as loss of vitamin A, acquisition of stress (actin) bundles, development of a prominent rough endoplasmic reticulum, and production of increased quantities of extracellular matrix [31]. One of the most prominent features of stellate cell activation is an increase in the expression of smooth muscle α actin. Thus, these data point to stellate cells as liver specific myofibroblasts. Myofibroblasts appear to be key elements in the wounding response to injury in many tissues, producing abundant quantities of extracellular matrix, as well as smooth muscle proteins [32–36]. The functional role of myofibroblasts in the liver is most likely tied to fibrogenesis, and thus indirectly linked to portal hypertension, since it is believed that both perisinusoidal and bridging scar formation contributes to portal hypertension at the sinusoidal level, and perhaps at the whole liver level, respectively.
From a cell biological and molecular signaling pathway, the upregulation of smooth muscle α actin in the activation process is complex, and involves interplay between stellate and other cells, cytokines and other soluble elements, and the extracellular matrix itself. Having said that, there appear to be some parallels between the regulation of smooth muscle α actin and fibrogenesis, the latter being a major component of the activation process. For example, transforming growth factor beta-1 (TGF-β1) the most profibrogenic cytokine for stellate cells, may also regulate smooth muscle α actin gene expression [37]. Recent evidence indicates that smooth muscle α actin expression in stellate cells is controlled transcriptionally by canonical muscle specific transcription factors, including serum response factor [38]. Further, it appears that there may be interplay between the smooth muscle α actin cytoskeleton and various stellate cell functions, including cell motility and contractility [39]. These data indicate that smooth muscle α actin itself is not a static component of the cytoskeleton, but rather a dynamic regulator of stellate cell behaviour and function.
Sinusoidal vascular remodelling
Sinusoidal vascular remodelling involves a complex interplay of hepatic cells and appears to contribute to increased intrahepatic resistance. For example, when quiescent HSCs become activated and start to deposit extracellular matrix proteins this creates a basement membrane around the sinusoids. In turn vigorous “coverage” of the sinusoids with activated HSCs could contribute to increased intrahepatic resistance in cirrhotic livers [40]. Furthermore, with injury, reduced LSEC NO production appears to stimulate cell proliferation, upregulates various arterial surface markers and contributes to loss of LSEC fenestrations, resulting in de-differentiation and capillarisation of the hepatic microvascular bed [41]. These changes facilitate remodelling and constriction of the sinusoidal vasculature, which increases hepatic vascular resistance and is an early feature of intrahepatic portal hypertension.
Angiogenesis
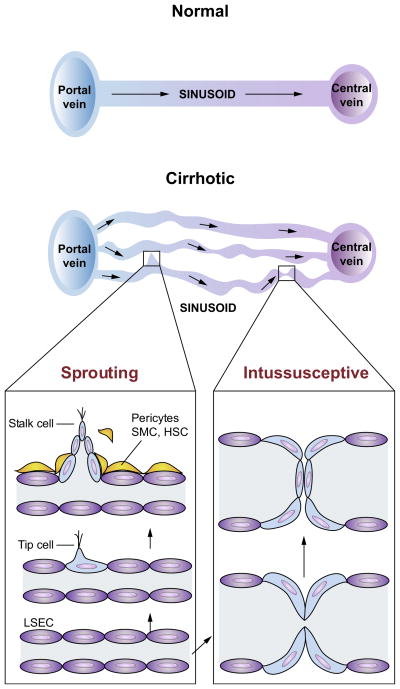
Angiogenesis, the process of new blood vessel formation from pre-existing vascular beds, takes place in two distinctive manners, namely through sprouting from the existing vasculature or splitting of the existing vasculature. In sprouting angiogenesis, angiogenic growth factors, through activation of endothelial cells, facilitate the degradation of the basement membrane in pre-existing blood vessels, which allows endothelial cells, pericytes and smooth muscle cells to detach and migrate towards angiogenic stimuli (Fig. 3). Endothelial cells then proliferate and form solid sprouts connecting neighbouring sprouts or blood vessels. Endothelial cells finally stop proliferating and bind to each other, to the pericytes and to the basement membrane, forming a new blood vessel [42,43]. Sprouting angiogenesis appears to involve a complex interplay between numerous signalling pathways such as Notch and Notch ligands, vascular endothelial growth factor (VEGF) and VEGF receptors (VEGFRs), semaphorins, and netrins [44], while signaling pathways regulating intussusceptive angiogenesis are less well studied but include Notch, Notch ligands, Tek/Tie-2, mTOR, ephrins and Eph receptors [45].
Fig. 3. Sprouting and intussusceptive angiogenesis in cirrhosis and portal hypertension.
Normal architecture of sinusoidal vasculature is shown in left panel with normal flow from portal venules, through sinusoidal microcirculation, to central veins. The sinusoidal vasculatures in the cirrhotic livers (right panel) are altered significantly, with increased number of sinusoidal vessels (angiogenesis) of various diameters and flow pattern. This is a simplified diagram of the sinusoidal microcirculation in liver cirrhosis. In cirrhosis, however, the relationship between the portal and central veins is not maintained because of fibrous septa and regenerative nodules, which disrupt the normal vascular architecture of portal venules, sinusoid and central vein. In sprouting angiogenesis, it is proposed that an endothelial ‘tip cell’ leads the vessel sprouts at the forefront and a trailing endothelial ‘stalk cell’ elongates behind the tip cell of a growing branch of vessels. Intussusceptive angiogenesis involves splitting vessels by the formation of translumenal pillars. Both forms of angiogenesis require the recruitment of pericytes or smooth muscle cells (SMCs). In cirrhotic livers, activated hepatic stellate cells (HSCs) are thought to serve as a role of pericytes and SMCs.
Intussusceptive angiogenesis, also known as splitting angiogenesis, was discovered relatively recent as an alternative process [46]. In intussusceptive angiogenesis, the two opposing walls of a capillary extend towards each other and form an intraluminal pillar. The cellular junctions of opposing endothelial cells are reorganised, which facilitates further growth of the pillar and finally results in splitting of the capillary into two new vessels [47]. Intussusceptive angiogenesis relies less on endothelial cell proliferation and generates blood vessels more rapidly [44,48]. Therefore, intussusceptive angiogenesis is particularly important in embryonic development where pre-exiting blood vessels are limited to create new vessels [49].
Both forms of angiogenesis, sprouting and intussusceptive, appear to be important in normal liver physiology and in pathophysiologic states, including liver organogenesis [50,51], liver regeneration [12,52], chronic liver diseases with fibrosis [53], nodular regenerative hyperplasia [45], hepatocarcinogenesis [54], and tumour angiogenesis [45].
Angiogenesis in the intrahepatic circulation
In portal hypertension, angiogenesis plays a crucial role in both intra and extra hepatic circulations. In the intrahepatic circulation, for example, it is reported that conditional Notch 1 knockout mice develop intussusceptive angiogenesis, nodular regenerative hyperplasia and portal hypertension. LSECs from these mice show reduced endothelial fenestrae. These observations indicate that Notch1 in LSEC is required for fenestration of LSECs and the loss of Notch 1 results in pathological intussusceptive angiogenesis and the development of nodular regenerative hyperplasia and portal hypertension [45]. Irregular flow patterns generated as a result of intussusceptive angiogenesis might contribute to an increase in resistance within the intrahepatic circulation, leading to portal hypertension.
Furthermore, angiogenesis occurring after liver injury appears to increase the vascular volume in response to inflammation and hypoxia induced in the fibrogenic process [55]. Histological analyses of cirrhotic livers indicate an increased number of vessels in the fibrotic septa and surrounding regenerative nodules [56]. This observation has led to the hypothesis that activated HSCs and/or other myofibroblasts such as portal myofibroblasts promote angiogenesis in liver cirrhosis. In fact, activated HSCs are known to enhance activation of LSECs by releasing angiogenic factors, such as angiopoietins [10,40,57] and VEGF [58].
Mesenteric vascular pathophysiology
In portal hypertension, increased portal blood inflow from the splanchnic circulation augments portal pressure and thereby contributes to the maintenance and exacerbation of portal hypertension. Arterial vasodilation in the splanchnic circulation plays a critical role in increasing the blood flow to the portal vein. To ameliorate portal hypertension, therefore, blocking arterial vasodilation in the splanchnic circulation is necessary. Further, blocking the development of collaterals could be useful for decreasing the incidence of portosystemic encephalopathy and variceal bleeding.
Vasodilation in the mesenteric vasculature
Arterial vasodilation in the splanchnic and systemic circulations is an important feature of portal hypertension. Splanchnic arterial vasodilation increases the blood inflow to the portal system and exacerbates portal hypertension. Splanchnic arterial vasodilation is attributed to abnormal cell function in different layers of the vasculature, namely, endothelial cells, smooth muscle cells and the adventitial layer that contains neuronal termini. Because of the disparate regulation of the vascular tone in the intrahepatic and extrahepatic circulations (i.e., vasoconstriction in the intrahepatic circulation vs. vasodilation in the extrahepatic circulation), the organ/tissue specific modulation of the vasodilator molecules is of paramount importance.
Increased vasodilator molecules in endothelial cells
eNOS-derived NO is increased in the splanchnic and systemic circulation and plays a principal role in arterial vasodilation. Complex regulatory mechanisms of eNOS activation appear to be important in these pathological vasculature structures. For example, a recent study [59] described a new mechanism for the modulation of eNOS in cirrhosis, which involves the renin-angiotensin (Ang) system. The renin-Ang system plays a critical role in blood pressure control, body fluid and electrolyte homeostasis. Angiotensin II is a vasoconstrictor generated by the action of angiotensin-converting enzyme (ACE) and is further cleaved by ACE2 to generate a biologically active peptide, Ang-(1-7). Ang-(1-7) is however a vasodilator, which binds to the G-protein coupled receptor Mas (MasR) [60] and leads to eNOS activation and NO production in endothelial cells [61]. In an animal model of cirrhosis, expression of ACE2 and MasR in mesenteric arteries and Ang-(1-7) production in mesenteric arterial beds was increased in an ACE2 dependent manner [59]. Furthermore, Ang-(1-7)/MasR contributed to vasodilation in mesenteric arterial beds of cirrhotic rats, suggesting that NO might mediate this vasodilation.
Of note, NO may also contribute to the regulation of lymphatic flow by modulating smooth muscle cell contractility [62]. For example, mesenteric lymphatic vessels in cirrhotic rats were found to have increased endothelial cell eNOS expression and decreased smooth muscle cell coverage [63]; this diminished smooth muscle cell coverage was reversed by inhibition of eNOS. These and other data emphasise the importance of the lymphatic vascular system in liver diseases [64].
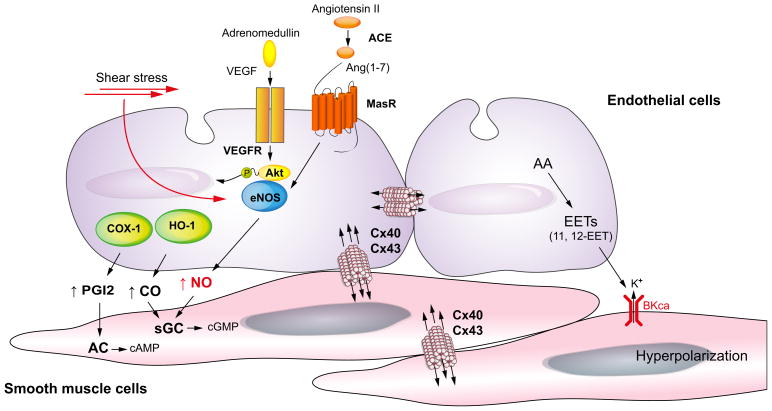
Besides NO, other vasodilator molecules, such as CO, prostacyclin (PGI2), adrenomedullin, endocannabinoids and endothelium-derived hyperpolarising factors (EDHF), also mediate arterial vasodilation. Some controversy surrounds the identity of EDHF in the hepatic system [65]. Candidate molecules include arachidonic acid metabolites (epoxyeicosatrienoic acid [EET]), the monovalent cation K+, components of gap junctions, and hydrogen peroxide. A recent study showed that in small resistance mesenteric arteries of cirrhotic rats, an arachidonic acid metabolite (11,12-EET) and gap junctions (in particular connexins 40 and 43) mediate increased vasodilation in the splanchnic circulation [66]. Collectively, the data suggest that multiple factors are involved in the excessive vasodilation, observed in the splanchnic and systemic circulations (Fig. 4).
Fig. 4. Mechanisms of increased vasodilation in arteries of the splanchnic circulation in cirrhosis with portal hypertension.
In the splanchnic arterial circulation, agonists such as vascular endothelial growth factor (VEGF) and Ang(1-7) or physical stimuli such as shear stress activate endothelial nitric oxide (NO) synthase (eNOS). In cirrhosis, Ang(1-7) levels are significantly increased by upregulation of angiotensin-converting enzyme (ACE)-2. In addition, MasR, a receptor of Ang(1-7) is up-regulated in cirrhosis, resulting in an increase in eNOS activity and NO production. Besides NO, carbon monoxide (CO) produced by hemeoxygenase-1 (HO-1) causes vasodilation by activating soluble guanylate cyclase (sGC) to generate cyclic guanosine monophosphate (cGMP) in vascular smooth muscle cells. Prostacyclin (PGI2) is synthesised by cyclooxygenase (COX) and relaxes smooth muscle cells by stimulating adenylate cyclase (AC) to generate cyclic adenosine monophosphate (cAMP). Arachidonic acid (AA) metabolites [epoxyeicosatrienoic acids (EETs)] cause hyperpolarisation/relaxation, acting through voltage-gated potassium channel (BKCa) and gap junction [in particular connexins (Cx) 40 and 43].
Smooth muscle cell hypocontractility
Concomitant with vasodilation, splanchnic and systemic arteries exhibit decreased contractile response to vasoconstrictors. This is caused not only by increases in vasodilator molecules mentioned above, but also by impaired contractile RhoA/Rho-kinase signaling in smooth muscle cells (see [67] for further review) and sympathetic nerve regression in these arteries [68]. A variety of vasoconstrictor molecules are also decreased in smooth muscle cells in the arteries of the splanchnic and systemic circulations; these include neuropeptide Y [68], urotensin II [69,70], angiotensin [71] and bradykinin [72,73]; this sets up impairment of contractility in the mesenteric vasculature in portal hypertension.
Arterial thinning
Vascular remodelling of the mesenteric vascular bed is another major event in portal hypertension. In a murine model of liver cirrhosis with portal hypertension, the thinning of arterial walls is observed in the splanchnic and systemic circulations [74,75]. Arterial walls consist of endothelial cells, smooth muscle cells and adventitia. The cellular and molecular mechanisms responsible for arterial thinning remain to be fully elucidated. One hypothesis is that increased apoptosis of smooth muscle cells in the mesenteric artery leads to thinning [76]. Through these structural changes as well as possible changes in the levels of proteins important for arterial integrity and function, arterial thinning may help to impair contractile responses of the arteries. Further, arterial thinning may contribute to increased permeability through structural and compositional changes in vessel junctions and thereby facilitate the development of ascites and oedema. Thus, arterial thinning that results from hemodynamic changes caused by portal hypertension may further help to sustain arterial vasodilation and worsen portal hypertension [65,77].
Extrahepatic collateral vessel formation
Porto-systemic collaterals (or shunts) develop through the opening of pre-existing vessels in response to an increase in portal pressure [78,79]. Changes in portal pressure are sensed first by the intestinal microcirculation and then by arteries of the splanchnic circulation [80]. It also appears that these vascular beds in turn produce various angiogenic factors, such as VEGF [81–83] and placental growth factor (PlGF) [84], which stimulate angiogenesis and promote the formation of portosystemic collaterals. Using a vascular corrosion technique, it was demonstrated that collaterals are formed in the splanchnic circulation in portal hypertensive mice by both sprouting and intussusceptive angiogenesis [85].
Collaterals develop to decompress the portal system. However, portal pressure remains elevated because of increased splanchnic blood flow resulting from splanchnic vasodilation. Additionally, these collaterals cause serious complications such as variceal bleeding and hepatic encephalopathy [65]. To what extent collaterals develop through the opening of pre-existing vessels, sprouting and intussusceptive angiogenesis remains an active area of investigation.
Studies in experimental models of portal hypertension and cirrhosis have shown that portal systemic collaterals can be reduced by a variety of approaches, including inhibiting VEGF (with anti-VEGFR2) or a combination of anti-VEGF (rapamycin)/anti-platelet derived growth factor (PDGF) (gleevec), PlGF [84], apelin [86], sorafenib [87,88] and cannabinoid signaling [89]. However, the reduction of collateral vessels does not always result in a decrease in portal pressure to the normal level, since this reduction does not significantly change the portal blood flow [84,90].
Translational implications and future directions
Much has been learned in the area of hepatic vascular biology in the last decade, and this new information holds great promise for the development of novel therapies for patients with portal hypertension. Based on an understanding of vascular pathology in chronic liver disease and portal hypertension, many potential therapies are evolving (Table 1). Here, as examples, we review three specific potential biological systems and associated therapies.
Table 1. Target molecules and experimental approaches/drugs that are reported to decrease portal pressure.
(See below-mentioned references for further information.)
| Target molecules | Approaches/drugs |
|---|---|
|
Intrahepatic circulation
| |
| Nitric oxide (NO)/endothelial NO synthase (eNOS) | Statins [111], eNOS gene transfer [112], neuronal NOS gene transfer [113], Akt gene transfer [114], tetrahydrobiopterin (BH4) [115,116] |
| TXA2 | COX-1 inhibitor (SC-560) [117], PGH2/TXA2 receptor blocker (SQ-29548) [118] |
| Superoxide radicals | Antioxidants: vitamin C [119], vitamin E [120], superoxide dismutase (SOD) gene transfer [121], SOD mimetic [121,122], N-acetylcysteine [123] |
| Hydrogen sulfide (H2S) | Sodium hydrogen sulfide [124] |
| Notch 1 | Notch 1 KO mice [79] |
| Nogo-B | Nogo-B KO mice [125] |
| Toll like receptor 4 | TLR4 mutant mice [126] |
| The farnesoid-X-receptor (FXR) | A selective FXR agonist, obeticholic acid (INT-747) [102] |
|
| |
|
Splanchnic and systemic circulation
| |
| Cannabinoid receptor type 2(CB2) receptor | CB2 receptor agonist (JWH-015) [89] |
| RhoA/Rho-kinase | Neuropeptide Y [68] |
| Peroxisome proliferator-activated receptor gamma (PPARγ) | PPARγ agonist (pioglitazone) [127] |
| Vascular endothelial growth factor (VEGF) | Anti-VEGFR2 monoclonal antibody (SU5416) [82,128], receptor tyrosine kinase inhibitors (sorafenib [87,88], sunitinib [129]) |
| Placental growth factor (PIGF) | PlGF KO mice and anti-PIGF monoclonal antibody [84] |
| Apelin | Apelin antagonist (F13A) [86] |
Statins and intrahepatic resistance
The statin class of compounds has gained considerable interest in vascular biology and in portal hypertension because they appear to stimulate eNOS and activate endothelial NO production [91]. It is also possible that statins may stimulate downstream signaling molecules that have beneficial effects in the liver. One mechanism of statins appears to be via selective effects on LSECs through stimulation of the expression of the KLF2 transcription factor [92,93]. KLF2 is highly expressed in vascular endothelial cells and protects the endothelium by upregulating the expression of a wide variety of vasoprotector genes [94,95], including eNOS [96]. Interestingly, LSECs overexpressing KLF-2 lead to HSC quiescence when these cells are co-cultured, suggesting that the statins’ anti-fibrogenic effect through up-regulation of KLF-2 is LSEC mediated [93]. However, statins may also exert their anti-fibrotic effect via direct activity on HSCs. Early atorvastatin exposure in a rat model of hepatic fibrosis attenuated HSC activation and fibrosis [97]; additionally, atorvastatin induced senescence in hepatic myofibroblasts in vitro and in vivo [98].
It has also been documented that atorvastatin decreases portal pressure in cirrhotic rats by inhibiting Rho-kinase and by activating eNOS [91]. Rho-kinase contributes to increased intra-hepatic resistance in cirrhosis, by mediating contraction of activated HSCs. Further, HSC-specific inhibition of Rho-kinase decreased intrahepatic resistance and lowered portal pressure in an experimental model [99].
Initial studies have indicated that statins can reduce portal pressure in cirrhotic patients and clinical trials are ongoing in patients with cirrhosis that are aimed at identifying a clinical niche for statins [100].
Obeticholic acid
Obeticholic acid is a semisynthetic bile acid analogue and a potent selective farnesoid-X receptor agonist [101]. A recent study demonstrated that obeticholic acid decreased intrahepatic resistance and ameliorated portal hypertension in both thioacetamide (TAA) treated – and bile duct ligated rats, by increasing intrahepatic eNOS activity through down-regulation of Rho-kinase and through up-regulation of dimethylarginine dimethyl-aminohydrolase 2(DDAH-2), respectively [102].
VEGF
A number of preclinical studies support the concept that inhibition of VEGF may have beneficial therapeutic effects in portal hypertension. Mechanisms by which VEGF inhibition may be beneficial include attenuation of mesenteric angiogenesis and portosystemic collaterals as well as reduction in intrahepatic vascular remodelling and fibrogenesis. Additional effects of VEGF inhibition on reduction in vascular permeability and ascites are also documented [103]. However, further studies are needed in humans and this is being pursued in an indirect manner through analysis of small molecule inhibitors of receptor tyrosine kinases such as sorafenib (with the understanding that these inhibitors target a multitude of receptor tyrosine kinases on different cell types) [10,104]. It should be pointed out that based on data with VEGF inhibition in the cancer arena, unanticipated effects of VEGF inhibition may be possible. Furthermore, some data indicate that VEGF itself may be important in hepatic tissue healing, sinusoidal normalisation, and regeneration. For example, VEGF may induce fibrosis regression through effects on macrophage infiltration and ensuing matrix degradation [105]. Further, in one study, reestablishment of LSEC fenestrae via restoration of VEGF function fully reversed portal hypertension and its secondary manifestations [8]. Finally, VEGF facilitates the recruitment of bone marrow-derived LSEC progenitor cells during liver regeneration [106]. Thus, the role of VEGF in liver injury, fibrosis, and portal hypertension, as well as its role in the recovery from these processes will require further exploration.
Future
Here, we have reviewed current concepts in the area of intra- and extra-vascular pathophysiology in portal hypertension. A number of novel areas are on the horizon. For example, an attractive future area will likely include inter-organ relationships in the pathogenesis of portal hypertension in the context of vascular biology. An excellent example in portal hypertension will be the gut-liver axis. The importance of bacterial translocation from the gut to the portal circulation has been long recognised in the study of portal hypertension, but the molecular basis of this relationship has been little investigated. The vascular biology associated with the gut-liver axis, including an understanding of how TLR signaling to key cellular players is initiated, or how vascular permeability may be changed facilitating bacterial translocation, will be important.
An understanding of VEGF function in the liver and mesenteric circulation is just now coming into focus. VEGF has different isoforms and distinct VEGF receptors mediate a variety of signaling cascades in cell specific manners [107]. Therefore, a detailed characterisation of VEGF induced pathways is needed to understand the intra- and extra-hepatic neovascularisation in portal hypertension. In addition, angiocrine growth factors in addition to VEGF are likely to play a role in the neovascularisation typical of portal hypertension and will be important.
An accumulating body of evidence suggests that neovascularisation is a key pathological feature of patients with non-alcoholic steatohepatitis (NASH) [108]. In mouse models of NASH, treatment with anti-mouse VEGFR2 antibody improved disrupted liver vasculature, decreased inflammatory gene expressions, and reduced steatosis to some extent, suggesting that VEGF is involved in the pathogenesis of NASH [109]. However, the mechanisms underlining hepatic neovascularisation in NASH are largely unknown and are important for investigation.
Other unexplored, but yet important areas include the role of the extracellular matrix components and their signaling in the vascular cells such as integrins as well as that of non-coding RNAs in portal hypertension. In particular, certain extracellular matrix components may have an important role in vascular remodelling associated with portal hypertension, as suggested by a recent study emphasising the role of integrins in fibrosis in several organs, including the liver [110].
Finally, available data suggest that the pathogenesis of portal hypertension is linked to specific and targetable molecular pathways. This suggests that approaches exist for manipulating these pathways. For example, correcting the defect in eNOS signaling in intrahepatic LSECs is attractive. Alternatively, abrogating the effect of the abundant vasoconstrictors in the intrahepatic circulation, or approaching neoangiogenesis in the mesenteric circulation would be therapeutically attractive. Indeed, the prospects for new therapy in the area of vascular biology of the liver are bright.
Key Points.
Significant alterations in the intra- and extrahepatic vasculature are prominent in chronic liver diseases
Hepatic vascular cells, such as sinusoidal endothelial cells and pericyte-like hepatic stellate cells, have paracrine and autocrine effects on each other and themselves, and play important roles in the pathogenesis of liver fibrosis/cirrhosis and portal hypertension
An important feature of liver injury is development of an increase in intra-hepatic resistance, which is associated with alterations in hepatic vascular cells and their signaling pathway that causes portal hypertension
Portal hypertension leads to the collateral vessel formation and arterial vasodilation in the splanchnic and systemic circulation, which worsens portal hypertension
This review summarizes the current state and future directions in hepatic vascular biology and portal hypertension - with a focus on the physiology, pathophysiology of cells and molecules within the liver, as well as those in the mesenteric vascular circulation. Translational implications of current research are highlighted
Acknowledgments
Financial support
This work was supported by grants R01DK082600 (Iwakiri), R01DK59615 (Shah), and R01DK57830 (Rockey) from the National Institutes of Health.
Abbreviations
- No
nitric oxide
- eNOS
endothelial nitric oxide synthase
- VEGF
vascular endothelial growth factor
- LSECs
liver sinusoidal endothelial cells
- HSCs
hepatic stellate cells
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- PIGF
placental growth factor
- KLF2
kruppel-like factor-2 (KLF2)
- VEGFR
VEGF receptor
- PDGF
platelet derived growth factor
- SMC
smooth muscle cells
Footnotes
Conflict of interest
D. Rockey has disclosed a research agreement with Actelion Pharmaceuticals Ltd. and consulting within the past year with Ono Pharmaceuticals Co. The other authors declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- 1.Iwakiri Y, Grisham M, Shah V. Vascular biology and pathobiology of the liver: report of a single-topic symposium. Hepatology. 2008;47:1754–1763. doi: 10.1002/hep.22203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wisse E, De Zanger RB, Jacobs R, McCuskey RS. Scanning electron microscope observations on the structure of portal veins, sinusoids and central veins in rat liver. Scanning Microsc. 1983:1441–1452. [PubMed] [Google Scholar]
- 3.Bhunchet E, Fujieda K. Capillarization and venularization of hepatic sinusoids in porcine serum-induced rat liver fibrosis: a mechanism to maintain liver blood flow. Hepatology. 1993;18:1450–1458. [PubMed] [Google Scholar]
- 4.Svistounov D, Warren A, McNerney GP, Owen DM, Zencak D, Zykova SN, et al. The relationship between fenestrations, sieve plates and rafts in liver sinusoidal endothelial cells. PLoS One. 2012;7:e46134. doi: 10.1371/journal.pone.0046134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah V, Toruner M, Haddad F, Cadelina G, Papapetropoulos A, Choo K, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999;117:1222–1228. doi: 10.1016/s0016-5085(99)70408-7. [DOI] [PubMed] [Google Scholar]
- 6.Funyu J, Mochida S, Inao M, Matsui A, Fujiwara K. VEGF can act as vascular permeability factor in the hepatic sinusoids through upregulation of porosity of endothelial cells. Biochem Biophys Res Commun. 2001;280:481–485. doi: 10.1006/bbrc.2000.4148. [DOI] [PubMed] [Google Scholar]
- 7.Yokomori H, Oda M, Yoshimura K, Nagai T, Ogi M, Nomura M, et al. Vascular endothelial growth factor increases fenestral permeability in hepatic sinusoidal endothelial cells. Liver Int. 2003;23:467–475. doi: 10.1111/j.1478-3231.2003.00880.x. [DOI] [PubMed] [Google Scholar]
- 8.May D, Djonov V, Zamir G, Bala M, Safadi R, Sklair-Levy M, et al. A transgenic model for conditional induction and rescue of portal hypertension reveals a role of VEGF-mediated regulation of sinusoidal fenestrations. PLoS One. 2011;6:e21478. doi: 10.1371/journal.pone.0021478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou L, Cao S, Kang N, Huebert RC, Shah VH. Fibronectin induces endothelial cell migration through beta1 integrin and Src-dependent phosphorylation of fibroblast growth factor receptor-1 at tyrosines 653/654 and 766. J Biol Chem. 2012;287:7190–7202. doi: 10.1074/jbc.M111.304972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thabut D, Routray C, Lomberk G, Shergill U, Glaser K, Huebert R, et al. Complementary vascular and matrix regulatory pathways underlie the beneficial mechanism of action of sorafenib in liver fibrosis. Hepatology. 2011;54:573–585. doi: 10.1002/hep.24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah V, Haddad FG, Garcia-Cardena G, Frangos JA, Mennone A, Groszmann RJ, et al. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J Clin Invest. 1997;100:2923–2930. doi: 10.1172/JCI119842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Wang X, Xie G, Wang L, Hill CK, DeLeve LD. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J Clin Invest. 2012;122:1567–1573. doi: 10.1172/JCI58789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505(7841):97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rautou PE, Bresson J, Sainte-Marie Y, Vion AC, Paradis V, Renard JM, et al. Abnormal plasma microparticles impair vasoconstrictor responses in patients with cirrhosis. Gastroenterology. 2012;143:e166. doi: 10.1053/j.gastro.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 16.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhan S, Chan CC, Serdar B, Rockey DC. Fibronectin stimulates endothelin-1 synthesis in rat hepatic myofibroblasts via a Src/ERK-regulated signaling pathway. Gastroenterology. 2009;136:e2341–e2344. doi: 10.1053/j.gastro.2009.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344–351. doi: 10.1016/s0016-5085(98)70487-1. [DOI] [PubMed] [Google Scholar]
- 19.Rockey DC. Vascular mediators in the injured liver. Hepatology. 2003;37:4–12. doi: 10.1053/jhep.2003.50044. [DOI] [PubMed] [Google Scholar]
- 20.Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 21.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S, Premont RT, Rockey DC. G-protein-coupled receptor kinase interactor-1 (GIT1) is a new endothelial nitric-oxide synthase (eNOS) interactor with functional effects on vascular homeostasis. J Biol Chem. 2012;287:12309–12320. doi: 10.1074/jbc.M111.320465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Premont RT, Rockey DC. eNOS is activated through GIT1 tyrosine phosphorylation and Src. J Biol Chem. 2014;289(26):18163–18174. doi: 10.1074/jbc.M113.521203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langer DA, Das A, Semela D, Kang-Decker N, Hendrickson H, Bronk SF, et al. Nitric oxide promotes caspase-independent hepatic stellate cell apoptosis through the generation of reactive oxygen species. Hepatology. 2008;47:1983–1993. doi: 10.1002/hep.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deleve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 2008;48:920–930. doi: 10.1002/hep.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie G, Wang X, Wang L, Wang L, Atkinson RD, Kanel GC, et al. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology. 2012;142:e916. doi: 10.1053/j.gastro.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Povero D, Eguchi A, Niesman IR, Andronikou N, de Mollerat du Jeu X, Mulya A, et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Science signaling. 2013;6:ra88. doi: 10.1126/scisignal.2004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 29.Hutchison N, Fligny C, Duffield JS. Resident mesenchymal cells and fibrosis. Biochim Biophys Acta. 2013;1832:962–971. doi: 10.1016/j.bbadis.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80:1170–1181. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 31.Kent G, Gay S, Inouye T, Bahu R, Minick OT, Popper H. Vitamin A-containing lipocytes and formation of type III collagen in liver injury. Proc Natl Acad Sci U S A. 1976;73:3719–3722. doi: 10.1073/pnas.73.10.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 33.Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1990;3:507–511. doi: 10.1165/ajrcmb/3.5.507. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, et al. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991;87:847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leslie KO, Taatjes DJ, Schwarz J, vonTurkovich M, Low RB. Cardiac myofibroblasts express alpha smooth muscle actin during right ventricular pressure overload in the rabbit. Am J Pathol. 1991;139:207–216. [PMC free article] [PubMed] [Google Scholar]
- 36.Rockey DC, Housset CN, Friedman SL. Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest. 1993;92:1795–1804. doi: 10.1172/JCI116769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lakner AM, Steuerwald NM, Walling TL, Ghosh S, Li T, McKillop IH, et al. Inhibitory effects of microRNA 19b in hepatic stellate cell-mediated fibrogenesis. Hepatology. 2012;56:300–310. doi: 10.1002/hep.25613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Z, Rockey DC. Interferon-gamma-mediated inhibition of serum response factor-dependent smooth muscle-specific gene expression. J Biol Chem. 2010;285:32415–32424. doi: 10.1074/jbc.M110.164863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rockey DC, Weymouth N, Shi Z. Smooth Muscle alpha Actin (Acta 2) and Myofibroblast Function during Hepatic Wound Healing. PLoS One. 2013;8:e77166. doi: 10.1371/journal.pone.0077166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53:976–980. doi: 10.1016/j.jhep.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 41.De Spiegelaere W, Cornillie P, Van den Broeck W, Plendl J, Bahramsoltani M. Angiopoietins differentially influence in vitro angiogenesis by endothelial cells of different origin. Clin Hemorheol Microcirc. 2011;48:15–27. doi: 10.3233/CH-2011-1393. [DOI] [PubMed] [Google Scholar]
- 42.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 43.Luttun A, Carmeliet G, Carmeliet P. Vascular progenitors: from biology to treatment. Trends Cardiovasc Med. 2002;12:88–96. doi: 10.1016/s1050-1738(01)00152-9. [DOI] [PubMed] [Google Scholar]
- 44.Ribatti D, Crivellato E. “Sprouting angiogenesis”, a reappraisal. Dev Biol. 2012;372:157–165. doi: 10.1016/j.ydbio.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Dill MT, Rothweiler S, Djonov V, Hlushchuk R, Tornillo L, Terracciano L, et al. Disruption of Notch1 induces vascular remodeling, intussusceptive angiogenesis, and angiosarcomas in livers of mice. Gastroenterology. 2012;142:e962. doi: 10.1053/j.gastro.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 46.Caduff JH, Fischer LC, Burri PH. Scanning electron microscope study of the developing microvasculature in the postnatal rat lung. Anat Rec. 1986;216:154–164. doi: 10.1002/ar.1092160207. [DOI] [PubMed] [Google Scholar]
- 47.Nagy JA, Morgan ES, Herzberg KT, Manseau EJ, Dvorak AM, Dvorak HF. Pathogenesis of ascites tumor growth: angiogenesis, vascular remodeling, and stroma formation in the peritoneal lining. Cancer Res. 1995;55:376–385. [PubMed] [Google Scholar]
- 48.De Spiegelaere W, Casteleyn C, Van den Broeck W, Plendl J, Bahramsoltani M, Simoens P, et al. Intussusceptive angiogenesis: a biologically relevant form of angiogenesis. J Vasc Res. 2012;49:390–404. doi: 10.1159/000338278. [DOI] [PubMed] [Google Scholar]
- 49.Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev Dyn. 2004;231:474–488. doi: 10.1002/dvdy.20184. [DOI] [PubMed] [Google Scholar]
- 50.Gouysse G, Couvelard A, Frachon S, Bouvier R, Nejjari M, Dauge MC, et al. Relationship between vascular development and vascular differentiation during liver organogenesis in humans. J Hepatol. 2002;37:730–740. doi: 10.1016/s0168-8278(02)00282-9. [DOI] [PubMed] [Google Scholar]
- 51.Sugiyama Y, Takabe Y, Nakakura T, Tanaka S, Koike T, Shiojiri N. Sinusoid development and morphogenesis may be stimulated by VEGF-Flk-1 signaling during fetal mouse liver development. Dev Dyn. 2010;239:386–397. doi: 10.1002/dvdy.22162. [DOI] [PubMed] [Google Scholar]
- 52.Wack KE, Ross MA, Zegarra V, Sysko LR, Watkins SC, Stolz DB. Sinusoidal ultrastructure evaluated during the revascularization of regenerating rat liver. Hepatology. 2001;33:363–378. doi: 10.1053/jhep.2001.21998. [DOI] [PubMed] [Google Scholar]
- 53.Coulon S, Heindryckx F, Geerts A, Van Steenkiste C, Colle I, Van Vlierberghe H. Angiogenesis in chronic liver disease and its complications. Liver Int. 2011;31:146–162. doi: 10.1111/j.1478-3231.2010.02369.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8:292–301. doi: 10.1038/nrclinonc.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, et al. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010–1021. doi: 10.1053/jhep.2002.32524. [DOI] [PubMed] [Google Scholar]
- 56.Rappaport AM, MacPhee PJ, Fisher MM, Phillips MJ. The scarring of the liver acini (Cirrhosis). Tridimensional and microcirculatory considerations. Virchows Arch. 1983;402:107–137. doi: 10.1007/BF00695054. [DOI] [PubMed] [Google Scholar]
- 57.Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729–1738. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 58.Novo E, Cannito S, Zamara E, Valfre di Bonzo L, Caligiuri A, Cravanzola C, et al. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am J Pathol. 2007;170:1942–1953. doi: 10.2353/ajpath.2007.060887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grace JA, Klein S, Herath CB, Granzow M, Schierwagen R, Masing N, et al. Activation of the MAS receptor by angiotensin-(1-7) in the renin-angiotensin system mediates mesenteric vasodilatation in cirrhosis. Gastroenterology. 2013;145:e875. doi: 10.1053/j.gastro.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 60.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heitsch H, Brovkovych S, Malinski T, Wiemer G. Angiotensin-(1-7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension. 2001;37:72–76. doi: 10.1161/01.hyp.37.1.72. [DOI] [PubMed] [Google Scholar]
- 62.Hagendoorn J, Padera TP, Kashiwagi S, Isaka N, Noda F, Lin MI, et al. Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics. Circ Res. 2004;95:204–209. doi: 10.1161/01.RES.0000135549.72828.24. [DOI] [PubMed] [Google Scholar]
- 63.Ribera J, Pauta M, Melgar-Lesmes P, Tugues S, Fernandez-Varo G, Held KF, et al. Increased nitric oxide production in lymphatic endothelial cells causes impairment of lymphatic drainage in cirrhotic rats. Gut. 2013;62:138–145. doi: 10.1136/gutjnl-2011-300703. [DOI] [PubMed] [Google Scholar]
- 64.Chung C, Iwakiri Y. The lymphatic vascular system in liver diseases: its role in ascites formation. Clin Mol Hepatol. 2013;19:99–104. doi: 10.3350/cmh.2013.19.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121–S131. doi: 10.1002/hep.20993. [DOI] [PubMed] [Google Scholar]
- 66.Bolognesi M, Zampieri F, Di Pascoli M, Verardo A, Turato C, Calabrese F, et al. Increased myoendothelial gap junctions mediate the enhanced response to epoxyeicosatrienoic acid and acetylcholine in mesenteric arterial vessels of cirrhotic rats. Liver Int. 2011;31:881–890. doi: 10.1111/j.1478-3231.2011.02509.x. [DOI] [PubMed] [Google Scholar]
- 67.Hennenberg M, Trebicka J, Sauerbruch T, Heller J. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut. 2008;57:1300–1314. doi: 10.1136/gut.2007.144584. [DOI] [PubMed] [Google Scholar]
- 68.Moleda L, Trebicka J, Dietrich P, Gabele E, Hellerbrand C, Straub RH, et al. Amelioration of portal hypertension and the hyperdynamic circulatory syndrome in cirrhotic rats by neuropeptide Y via pronounced splanchnic vasoaction. Gut. 2011;60(8):1122–1132. doi: 10.1136/gut.2010.226407. [DOI] [PubMed] [Google Scholar]
- 69.Trebicka J, Leifeld L, Hennenberg M, Biecker E, Eckhardt A, Fischer N, et al. Hemodynamic effects of urotensin II and its specific receptor antagonist palosuran in cirrhotic rats. Hepatology. 2008;47:1264–1276. doi: 10.1002/hep.22170. [DOI] [PubMed] [Google Scholar]
- 70.Kemp W, Krum H, Colman J, Bailey M, Yandle T, Richards M, et al. Urotensin II: a novel vasoactive mediator linked to chronic liver disease and portal hypertension. Liver Int. 2007;27:1232–1239. doi: 10.1111/j.1478-3231.2007.01539.x. [DOI] [PubMed] [Google Scholar]
- 71.Hennenberg M, Trebicka J, Kohistani AZ, Heller J, Sauerbruch T. Vascular hyporesponsiveness to angiotensin II in rats with CCl(4)-induced liver cirrhosis. Eur J Clin Invest. 2009;39:906–913. doi: 10.1111/j.1365-2362.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 72.Chu CJ, Wu SL, Lee FY, Wang SS, Chang FY, Lin HC, et al. Splanchnic hyposensitivity to glypressin in a haemorrhage/transfused rat model of portal hypertension: role of nitric oxide and bradykinin. Clin Sci (Lond) 2000;99:475–482. [PubMed] [Google Scholar]
- 73.Chen CT, Chu CJ, Lee FY, Chang FY, Wang SS, Lin HC, et al. Splanchnic hyposensitivity to glypressin in a hemorrhage-transfused common bile duct-ligated rat model of portal hypertension: role of nitric oxide and bradykinin. Hepatogastroenterology. 2009;56:1261–1267. [PubMed] [Google Scholar]
- 74.Fernandez-Varo G, Morales-Ruiz M, Ros J, Tugues S, Munoz-Luque J, Casals G, et al. Impaired extracellular matrix degradation in aortic vessels of cirrhotic rats. J Hepatol. 2007;46:440–446. doi: 10.1016/j.jhep.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 75.Fernandez-Varo G, Ros J, Morales-Ruiz M, Cejudo-Martin P, Arroyo V, Sole M, et al. Nitric oxide synthase 3-dependent vascular remodeling and circulatory dysfunction in cirrhosis. Am J Pathol. 2003;162:1985–1993. doi: 10.1016/S0002-9440(10)64331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tashiro K, Utsumi T, Chen T, Iwakiri Y. EMMPRIN/CD147 induction in the adventitia of superior mesenteric arteries facilitates thinning of arteries in cirrhotic rats with portal hypertension. Hepatology. 2012;56:305A. [Google Scholar]
- 77.Iwakiri Y. Endothelial dysfunction in the regulation of cirrhosis and portal hypertension. Liver Int. 2012;32:199–213. doi: 10.1111/j.1478-3231.2011.02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sieber CC, Sumanovski LT, Stumm M, van der Kooij M, Battegay E. In vivo angiogenesis in normal and portal hypertensive rats: role of basic fibroblast growth factor and nitric oxide. J Hepatol. 2001;34:644–650. doi: 10.1016/s0168-8278(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 79.Sumanovski LT, Battegay E, Stumm M, van der Kooij M, Sieber CC. Increased angiogenesis in portal hypertensive rats: role of nitric oxide. Hepatology. 1999;29:1044–1049. doi: 10.1002/hep.510290436. [DOI] [PubMed] [Google Scholar]
- 80.Abraldes JG, Iwakiri Y, Loureiro-Silva M, Haq O, Sessa WC, Groszmann RJ. Mild increases in portal pressure upregulate vascular endothelial growth factor and endothelial nitric oxide synthase in the intestinal microcirculatory bed, leading to a hyperdynamic state. Am J Physiol Gastrointest Liver Physiol. 2006;290:G980–G987. doi: 10.1152/ajpgi.00336.2005. [DOI] [PubMed] [Google Scholar]
- 81.Huang HC, Haq O, Utsumi T, Sethasine S, Abraldes JG, Groszmann RJ, et al. Intestinal and plasma VEGF levels in cirrhosis: the role of portal pressure. J Cell Mol Med. 2012;16:1125–1133. doi: 10.1111/j.1582-4934.2011.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fernandez M, Vizzutti F, Garcia-Pagan JC, Rodes J, Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126:886–894. doi: 10.1053/j.gastro.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 83.Geerts AM, De Vriese AS, Vanheule E, Van Vlierberghe H, Mortier S, Cheung KJ, et al. Increased angiogenesis and permeability in the mesenteric microvasculature of rats with cirrhosis and portal hypertension: an in vivo study. Liver Int. 2006;26:889–898. doi: 10.1111/j.1478-3231.2006.01308.x. [DOI] [PubMed] [Google Scholar]
- 84.Van Steenkiste C, Geerts A, Vanheule E, Van Vlierberghe H, De Vos F, Olievier K, et al. Role of placental growth factor in mesenteric neoangiogenesis in a mouse model of portal hypertension. Gastroenterology. 2009;137:e2111–e2116. doi: 10.1053/j.gastro.2009.08.068. [DOI] [PubMed] [Google Scholar]
- 85.Van Steenkiste C, Trachet B, Casteleyn C, van Loo D, Van Hoorebeke L, Segers P, et al. Vascular corrosion casting: analyzing wall shear stress in the portal vein and vascular abnormalities in portal hypertensive and cirrhotic rodents. Lab Invest. 2010;90:1558–1572. doi: 10.1038/labinvest.2010.138. [DOI] [PubMed] [Google Scholar]
- 86.Tiani C, Garcia-Pras E, Mejias M, de Gottardi A, Berzigotti A, Bosch J, et al. Apelin signaling modulates splanchnic angiogenesis and portosystemic collateral vessel formation in rats with portal hypertension. J Hepatol. 2009;50:296–305. doi: 10.1016/j.jhep.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 87.Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245–1256. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]
- 88.Reiberger T, Angermayr B, Schwabl P, Rohr-Udilova N, Mitterhauser M, Gangl A, et al. Sorafenib attenuates the portal hypertensive syndrome in partial portal vein ligated rats. J Hepatol. 2009;51:865–873. doi: 10.1016/j.jhep.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 89.Huang HC, Wang SS, Hsin IF, Chang CC, Lee FY, Lin HC, et al. Cannabinoid receptor 2 agonist ameliorates mesenteric angiogenesis and portosystemic collaterals in cirrhotic rats. Hepatology. 2012;56:248–258. doi: 10.1002/hep.25625. [DOI] [PubMed] [Google Scholar]
- 90.Van Steenkiste C, Ribera J, Geerts A, Pauta M, Tugues S, Casteleyn C, et al. Inhibition of placental growth factor activity reduces the severity of fibrosis, inflammation, and portal hypertension in cirrhotic mice. Hepatology. 2011;53:1629–1640. doi: 10.1002/hep.24238. [DOI] [PubMed] [Google Scholar]
- 91.Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242–253. doi: 10.1002/hep.21673. [DOI] [PubMed] [Google Scholar]
- 92.Gracia-Sancho J, Russo L, Garcia-Caldero H, Garcia-Pagan JC, Garcia-Cardena G, Bosch J. Endothelial expression of transcription factor Kruppel-like factor 2 and its vasoprotective target genes in the normal and cirrhotic rat liver. Gut. 2011;60:517–524. doi: 10.1136/gut.2010.220913. [DOI] [PubMed] [Google Scholar]
- 93.Marrone G, Russo L, Rosado E, Hide D, Garcia-Cardena G, Garcia-Pagan JC, et al. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. J Hepatol. 2013;58:98–103. doi: 10.1016/j.jhep.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 94.Anderson KP, Kern CB, Crable SC, Lingrel JB. Isolation of a gene encoding a functional zinc finger protein homologous to erythroid Kruppel-like factor: identification of a new multigene family. Mol Cell Biol. 1995;15:5957–5965. doi: 10.1128/mcb.15.11.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuo CT, Veselits ML, Barton KP, Lu MM, Clendenin C, Leiden JM. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 1997;11:2996–3006. doi: 10.1101/gad.11.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trebicka J, Hennenberg M, Odenthal M, Shir K, Klein S, Granzow M, et al. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol. 2010;53:702–712. doi: 10.1016/j.jhep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 98.Klein S, Klosel J, Schierwagen R, Korner C, Granzow M, Huss S, et al. Atorvastatin inhibits proliferation and apoptosis, but induces senescence in hepatic myofibroblasts and thereby attenuates hepatic fibrosis in rats. Lab Invest. 2012;92:1440–1450. doi: 10.1038/labinvest.2012.106. [DOI] [PubMed] [Google Scholar]
- 99.Klein S, Van Beuge MM, Granzow M, Beljaars L, Schierwagen R, Kilic S, et al. HSC-specific inhibition of Rho-kinase reduces portal pressure in cirrhotic rats without major systemic effects. J Hepatol. 2012;57:1220–1227. doi: 10.1016/j.jhep.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 100.Abraldes JG, Albillos A, Banares R, Turnes J, Gonzalez R, Garcia-Pagan JC, et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651–1658. doi: 10.1053/j.gastro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 101.Rizzo G, Passeri D, De Franco F, Ciaccioli G, Donadio L, Rizzo G, et al. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol. 2010;78:617–630. doi: 10.1124/mol.110.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, et al. Obeticholic acid, a farnesoid-X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59(6):2286–2298. doi: 10.1002/hep.26939. [DOI] [PubMed] [Google Scholar]
- 103.Becker G, Blum HE. VEGF Trap for the treatment of malignant ascites. Lancet Oncol. 2012;13:115–116. doi: 10.1016/S1470-2045(11)70394-1. [DOI] [PubMed] [Google Scholar]
- 104.Pinter M, Sieghart W, Reiberger T, Rohr-Udilova N, Ferlitsch A, Peck-Radosavljevic M. The effects of sorafenib on the portal hypertensive syndrome in patients with liver cirrhosis and hepatocellular carcinoma – A pilot study. Aliment Pharmacol Ther. 2012;35:83–91. doi: 10.1111/j.1365-2036.2011.04896.x. [DOI] [PubMed] [Google Scholar]
- 105.Yang L, Kwon J, Popov Y, Gajdos GB, Ordog T, Brekken RA, et al. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology. 2014;146(5):1339–1350. doi: 10.1053/j.gastro.2014.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang L, Wang X, Wang L, Chiu JD, van de Ven G, Gaarde WA, et al. Hepatic vascular endothelial growth factor regulates recruitment of rat liver sinusoidal endothelial cell progenitor cells. Gastroenterology. 2012;143:e1552. doi: 10.1053/j.gastro.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kitade M, Yoshiji H, Kojima H, Ikenaka Y, Noguchi R, Kaji K, et al. Leptin-mediated neovascularization is a prerequisite for progression of nonalcoholic steatohepatitis in rats. Hepatology. 2006;44:983–991. doi: 10.1002/hep.21338. [DOI] [PubMed] [Google Scholar]
- 109.Coulon S, Legry V, Heindryckx F, Van Steenkiste C, Casteleyn C, Olievier K, et al. Role of vascular endothelial growth factor in the pathophysiology of nonalcoholic steatohepatitis in two rodent models. Hepatology. 2013;57:1793–1805. doi: 10.1002/hep.26219. [DOI] [PubMed] [Google Scholar]
- 110.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abraldes JG, Rodriguez-Vilarrupla A, Graupera M, Zafra C, Garcia-Caldero H, Garcia-Pagan JC, et al. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J Hepatol. 2007;46:1040–1046. doi: 10.1016/j.jhep.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 112.Van de Casteele M, Omasta A, Janssens S, Roskams T, Desmet V, Nevens F, et al. In vivo gene transfer of endothelial nitric oxide synthase decreases portal pressure in anaesthetised carbon tetrachloride cirrhotic rats. Gut. 2002;51:440–445. doi: 10.1136/gut.51.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu Q, Shao R, Qian HS, George SE, Rockey DC. Gene transfer of the neuronal NO synthase isoform to cirrhotic rat liver ameliorates portal hypertension. J Clin Invest. 2000;105:741–748. doi: 10.1172/JCI7997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morales-Ruiz M, Cejudo-Martin P, Fernandez-Varo G, Tugues S, Ros J, Angeli P, et al. Transduction of the liver with activated Akt normalizes portal pressure in cirrhotic rats. Gastroenterology. 2003;125:522–531. doi: 10.1016/s0016-5085(03)00909-0. [DOI] [PubMed] [Google Scholar]
- 115.Matei V, Rodriguez-Vilarrupla A, Deulofeu R, Colomer D, Fernandez M, Bosch J, et al. The eNOS cofactor tetrahydrobiopterin improves endothelial dysfunction in livers of rats with CCl4 cirrhosis. Hepatology. 2006;44:44–52. doi: 10.1002/hep.21228. [DOI] [PubMed] [Google Scholar]
- 116.Matei V, Rodriguez-Vilarrupla A, Deulofeu R, Garcia-Caldero H, Fernandez M, Bosch J, et al. Three-day tetrahydrobiopterin therapy increases in vivo hepatic NOS activity and reduces portal pressure in CCl4 cirrhotic rats. J Hepatol. 2008;49:192–197. doi: 10.1016/j.jhep.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 117.Graupera M, March S, Engel P, Rodes J, Bosch J, Garcia-Pagan JC. Sinusoidal endothelial COX-1-derived prostanoids modulate the hepatic vascular tone of cirrhotic rat livers. Am J Physiol Gastrointest Liver Physiol. 2005;288:G763–G770. doi: 10.1152/ajpgi.00300.2004. [DOI] [PubMed] [Google Scholar]
- 118.Gracia-Sancho J, Lavina B, Rodriguez-Vilarrupla A, Garcia-Caldero H, Bosch J, Garcia-Pagan JC. Enhanced vasoconstrictor prostanoid production by sinusoidal endothelial cells increases portal perfusion pressure in cirrhotic rat livers. J Hepatol. 2007;47:220–227. doi: 10.1016/j.jhep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 119.Hernandez-Guerra M, Garcia-Pagan JC, Turnes J, Bellot P, Deulofeu R, Abraldes JG, et al. Ascorbic acid improves the intrahepatic endothelial dysfunction of patients with cirrhosis and portal hypertension. Hepatology. 2006;43:485–491. doi: 10.1002/hep.21080. [DOI] [PubMed] [Google Scholar]
- 120.Yang YY, Lee TY, Huang YT, Chan CC, Yeh YC, Lee FY, et al. Asymmetric dimethylarginine (ADMA) determines the improvement of hepatic endothelial dysfunction by vitamin E in cirrhotic rats. Liver Int. 2012;32:48–57. doi: 10.1111/j.1478-3231.2011.02651.x. [DOI] [PubMed] [Google Scholar]
- 121.Lavina B, Gracia-Sancho J, Rodriguez-Vilarrupla A, Chu Y, Heistad DD, Bosch J, et al. Superoxide dismutase gene transfer reduces portal pressure in CCl4 cirrhotic rats with portal hypertension. Gut. 2009;58:118–125. doi: 10.1136/gut.2008.149880. [DOI] [PubMed] [Google Scholar]
- 122.Garcia-Caldero H, Rodriguez-Vilarrupla A, Gracia-Sancho J, Divi M, Lavina B, Bosch J, et al. Tempol administration, a superoxide dismutase mimetic, reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J Hepatol. 2011;54:660–665. doi: 10.1016/j.jhep.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 123.Yang YY, Lee KC, Huang YT, Wang YW, Hou MC, Lee FY, et al. Effects of N-acetylcysteine administration in hepatic microcirculation of rats with biliary cirrhosis. J Hepatol. 2008;49:25–33. doi: 10.1016/j.jhep.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 124.Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, et al. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539–548. doi: 10.1002/hep.20817. [DOI] [PubMed] [Google Scholar]
- 125.Zhang D, Utsumi T, Huang HC, Gao L, Sangwung P, Chung C, et al. Reticulon 4B (Nogo-B) is a novel regulator of hepatic fibrosis. Hepatology. 2011;53:1306–1315. doi: 10.1002/hep.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu Q, Zou L, Jagavelu K, Simonetto DA, Huebert RC, Jiang ZD, et al. Intestinal decontamination inhibits TLR4 dependent fibronectin-mediated cross-talk between stellate cells and endothelial cells in liver fibrosis in mice. J Hepatol. 2012;56:893–899. doi: 10.1016/j.jhep.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schwabl P, Payer BA, Grahovac J, Klein S, Horvatits T, Mitterhauser M, et al. Pioglitazone decreases portosystemic shunting by modulating inflammation and angiogenesis in cirrhotic and non-cirrhotic portal hypertensive rats. J Hepatol. 2014;60(6):1135–1142. doi: 10.1016/j.jhep.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 128.Fernandez M, Mejias M, Angermayr B, Garcia-Pagan JC, Rodes J, Bosch J. Inhibition of VEGF receptor-2 decreases the development of hyperdynamic splanchnic circulation and portal-systemic collateral vessels in portal hypertensive rats. J Hepatol. 2005;43:98–103. doi: 10.1016/j.jhep.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 129.Tugues S, Fernandez-Varo G, Munoz-Luque J, Ros J, Arroyo V, Rodes J, et al. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46:1919–1926. doi: 10.1002/hep.21921. [DOI] [PubMed] [Google Scholar]
- 130.Vorobioff J, Bredfeldt JE, Groszmann RJ. Increased blood flow through the portal system in cirrhotic rats. Gastroenterology. 1984;87:1120–1126. [PubMed] [Google Scholar]