Abstract
Background and Purpose
Atrial fibrillation (AF) is prevalent and there is a clinical need for biomarkers to identify individuals at higher risk for AF. Fixed throughout a life course and assayable early in life, genetic biomarkers may meet this need. Here, we investigate whether multiple single nucleotide polymorphisms (SNPs) together as an AF genetic risk score (AF-GRS) can improve prediction of one's risk for AF.
Methods
In 27,471 participants of the Malmö Diet and Cancer Study, a prospective, community-based cohort, we used Cox models that adjusted for established AF risk factors to assess the association of AF-GRS with incident AF and ischemic stroke. Median follow-up was 14.4 years for incident AF and 14.5 years for ischemic stroke. The AF-GRS comprised 12 SNPs that had been previously shown to be associated with AF at genome-wide significance.
Results
During follow-up, 2,160 participants experienced a first AF event and 1,495 had a first ischemic stroke event. Participants in the top AF-GRS quintile were at increased risk for incident AF (HR = 2.00; 95%CI = 1.73 to 2.31; P=2.7×10−21) and ischemic stroke (HR = 1.23; 95%CI = 1.04 to 1.46; P=0.02) when compared with the bottom quintile. Addition of the AF-GRS to established AF risk factors modestly improved both discrimination and reclassification (P<0.0001 for both).
Conclusions
An AF-GRS can identify 20% of individuals who are at approximately two-fold increased risk for incident AF and at 23% increased risk for ischemic stroke. Targeting diagnostic or therapeutic interventions to this subset may prove clinically useful.
Keywords: atrial fibrillation, stroke, genetic risk score, single nucleotide polymorphisms
Introduction
Stroke is the second leading cause of mortality worldwide, split almost evenly between ischemic and non-ischemic stroke.1 Atrial fibrillation (AF) and the commonly ensuing cardioembolic complications are an important cause of ischemic stroke. In the United States, AF has been estimated to affect nearly 3 million adults and this number has been projected to double by 2050.2-4 Approximately 20% to 25% of individuals will be affected by AF in their lifetime.5,6 In addition to cardioembolic stroke, AF is also associated with heart failure and other thromboembolic complications4, 7, 8 and results in substantial public health costs.9
Identifying individuals at the highest risk for AF and then aggressively addressing modifiable risk factors in these individuals may be beneficial with respect to health outcomes and cost. Risk factors for AF include hypertension, smoking, obesity, diabetes mellitus, age, male sex, and heart disease (myocardial infarction, heart failure, and valvular heart disease).10 The fraction of the risk for AF attributable to these factors is roughly 50%,11 with hypertension being the most prominent modifiable risk factor.12
Heredity also affects risk of AF, as evidenced by the increased risk of AF among those with documented familial AF.13-17 The heritability of AF has motivated genome-wide association studies (GWAS) that have screened for common single nucleotide polymorphisms (SNPs) associated with AF. To date, GWAS have identified 12 common SNPs that are associated with AF at genome-wide significance levels.18-23
Here, we build on these observations to test two hypotheses: (1) an AF genetic risk score (AF-GRS) that combines 12 SNPs is associated with risk for AF beyond established risk factors; and (2) the same AF-GRS is associated with ischemic stroke. We tested these hypotheses in a large, population-based prospective study of middle-aged men and women.
Methods
Study participants
The Malmö Diet and Cancer (MDC) study is a community-based, prospective observational study of 30,447 participants drawn from ~230,000 residents of Malmö, Sweden. Men aged 46 to 73 years and women aged 45 to 73 years were invited to participate and were enrolled between 1991 and 1996.24 Details of the MDC design have been previously reported.24,25 In the current study, we excluded participants who did not provide DNA with sufficient quantity or quality for genotyping (n=2693), participants with prevalent AF (n=282), and one participant who was lost to follow-up.
The primary end points of the study were time to first occurrence of AF and time to first occurrence of ischemic stroke. For incident ischemic stroke analyses, we also excluded participants with prevalent ischemic stroke (n=226).
Participants completed a baseline examination that included a medical history review, physical examination, and a blood draw. Blood pressure was measured using a mercury-column sphygmomanometer after 10 minutes of rest in the supine position. Hypertension was defined as systolic blood pressure of at least 140 mmHg, diastolic blood pressure of at least 90 mmHg, or use of antihypertensive medication. Prevalent diabetes was defined as previously described26 by participants’ report of a physician diagnosis, self-reported use of diabetes medication, fasting whole blood glucose ≥ 6.1 mmol/L (which corresponds to fasting plasma glucose ≥7.0 mm0ol/L),27 or having been registered in local or national diabetes registries. Body mass index (BMI) was defined as body weight in kilograms divided by the square of height measured in meters. Current cigarette use was defined as any self-reported use within the past year. Prevalence of AF and ischemic stroke was based on assessment of participants’ medical records at baseline examination. Prevalent coronary heart disease was defined as a diagnosis code of myocardial infarction on the basis of ICD9 and ICD10 codes 410 and I21, respectively. The MDC study protocols were approved by the ethics committee at Lund University. All participants provided written informed consent.
Determination of AF and ischemic stroke outcomes
Incident AF and incident stroke events were assessed by ICD8, ICD9, and ICD10 codes from The Swedish Hospital Discharge Register and the Swedish Cause of Death Register which are administered by the Swedish National Board of Health and Welfare. Atrial fibrillation was defined as a diagnosis code of 427.92 (ICD-8), 427D (ICD-9), or I48 (ICD-10). This definition of atrial fibrillation was shown to have a 94% validation rate from ECG records.28 Ischemic stroke was defined on the basis of codes 434 (ICD-9) and code I63 (ICD-10). All ischemic stroke events that occurred within the catchment area of Malmö hospitals (92% of all events) were validated within the stroke registry in Malmö (STROMA),29 in which original medical records were examined including computed tomography and other imaging modalities, where available. Participants with transient ischemic attack were not included as a stroke event. Hemorrhagic strokes (ICD9 430, 431, or 432) were excluded from the analysis of incident ischemic strokes. The last assessment of incident events for MDC participants was performed in 2009.
Genotyping
Genotypes of the MDC participants were determined using a multiplex method that combines polymerase chain reaction (PCR), allele-specific oligonucleotide ligation assays, and hybridization to oligonucleotides coupled to Luminex® 100TM xMAP™ microspheres (Luminex, Austin, TX).30
Modeling of genetic risk score
The AF-GRS includes 12 SNPs (Table 1). These are all the SNPs that have been reported to be associated with AF at a genome-wide significance level (P<5×10−8), including 4 SNPs at PITX2 locus.18-23 The linkage disequilibrium between these SNPs is presented in Supplemental Figure and Supplemental Table. The AF-GRS for each individual in the current study was calculated as follows. For each SNP the natural log transformed risk estimate for the minor allele was multiplied by the number of minor alleles carried by that individual; these 12 products were then summed. The risk estimate for the minor allele at each SNP was obtained from the largest published study or from a published meta-analysis.
Table 1.
Association of single nucleotide polymorphisms with incident atrial fibrillation in the Malmo Diet and Cancer Study
| Locus | Gene | SNP | Modeled allele | Other allele | MAF‡ | Risk estimate from literature (weight) | Ref |
Risk estimate from MDC study |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||||||||
| HR (95%CI) | P value | HR (95%CI) | P value | ||||||||
| 1q21 | KCNN3 | rs13376333 | T | C | 0.34 | 1.13 (0.12) | [22] | 1.09 (1.02-1.16) | 6.7×10−3 | 1.10 (1.03-1.17) | 5.5×10−3 |
| 1q24 | PRRX1 | rs3903239 | G | A | 0.47 | 1.14 (0.13) | [23] | 1.11 (1.04-1.18) | 8.3×10−4 | 1.11 (1.05-1.19) | 6.4×10−4 |
| 4q25 | PITX2 | rs10033464* | T | G | 0.09 | 1.39 (0.33) | [18] | 1.16 (1.05-1.29) | 3.2×10−3 | 1.16 (1.04-1.29) | 5.7×10−3 |
| 4q25 | PITX2 | rs2200733† | T | C | 0.10 | 1.72 (0.54) | [18] | 1.46 (1.34-1.60) | 2.2×10−16 | 1.45 (1.32-1.59) | 8.1×10−15 |
| 4q25 | PITX2 | rs17570669* | T | A | 0.08 | 0.73 (−0.31) | [20] | 0.76 (0.67-0.85) | 8.7×10−6 | 0.76 (0.67-0.86) | 2.4×10−5 |
| 4q25 | PITX2 | rs3853445* | C | T | 0.27 | 0.86 (−0.15) | [20] | 0.85 (0.80-0.92) | 1.3×10−5 | 0.84 (0.78-0.90) | 1.8×10−6 |
| 7q31 | CAV1 | rs3807989 | A | G | 0.41 | 0.9 (−0.11) | [23] | 0.92 (0.86-0.98) | 5.9×10−3 | 0.90 (0.85-0.96) | 2.3×10−3 |
| 9q22 | C9orf3 | rs10821415 | A | C | 0.41 | 1.11 (0.10) | [23] | 1.02 (0.96-1.08) | 0.55 | 1.03 (0.96-1.09) | 0.4 |
| 10q22 | SYNPO2L | rs10824026 | G | A | 0.16 | 0.87 (−0.14) | [23] | 0.85 (0.78-0.92) | 1.4×10−4 | 0.83 (0.76-0.91) | 6.4×10−5 |
| 14q23 | SYNE2 | rs1152591 | A | G | 0.49 | 1.13 (0.12) | [23] | 1.02 (0.96-1.09) | 0.48 | 1.03 (0.97-1.10) | 0.35 |
| 15q24 | HCN4 | rs7164883 | G | A | 0.17 | 1.19 (0.17) | [23] | 1.09 (1.00-1.17) | 0.04 | 1.09 (1.01-1.18) | 0.032 |
| 16q22 | ZFHX3 | rs2106261 | T | C | 0.18 | 1.24 (0.22) | [23] | 1.10 (1.02-1.19) | 0.016 | 1.10 (1.01-1.19) | 0.025 |
Model 1: adjusted for age and sex
Model 2: adjusted for age, sex, BMI, systolic and diastolic blood pressure, use of antihypertensive medications, current smoking, prevalent diabetes, prevalent coronary heart disease, and prevalent heart failure.
Adjusted for rs2200733
Adjusted for rs10033464
MAF is minor allele frequency in this study. This allele is also the minor allele among Utah residents with ancestry from northern and western Europe (CEU) reported by the International HapMap project. The minor allele is the modeled allele.
Statistical Analysis
All statistical analyses were performed in R.31 Participant characteristics at baseline were described by count and percent, or by mean and standard deviation (Table 2). Deviation from Hardy-Weinberg equilibrium assumptions was evaluated by an exact test for biallelic markers. The association of each SNP with incident AF was assessed from Cox proportional hazards regression models that were adjusted for age, sex, BMI, systolic and diastolic blood pressure, use of antihypertensive medications, current smoking, prevalent diabetes, coronary events, and heart failure. The association of the AF-GRS with incident AF or incident ischemic stroke was also assessed using Cox proportional hazards models. The cumulative fraction of events was estimated as 1 minus the Kaplan-Meier estimate of survival free of first AF event. The differences of the cumulative fraction of events between subgroups were assessed by the log-rank test. C-statistics were calculated by the method of Gönen and Heller.32 To obtain unbiased estimates of the c-statistics, the data were randomly split into equal sized training and test sets. The training set was used to estimate the Cox model coefficients and the c-statistic was determined in the test set. To provide a P value for the difference between the c-statistics of two models, we randomly permuted the AF-GRS of the participants 10000 times, and calculated the difference between the c-statistics of the two models for each iteration. The P value was calculated as the proportion of iterations in which the difference in c-statistics was as or more extreme than the observed difference. Continuous net reclassification improvement (NRI) was calculated according to Pencina et al.33 The c-statistics and continuous NRI are reported for 10-year risk for incident AF events, and for 15-year risk for incident ischemic stroke events that occurred after or coincident with a diagnosis of AF and are therefore based on events occurring in the first 10-year and 15-year of follow-up, respectively.
Table 2.
Characteristics of participants in the Malmo Diet and Cancer Study
| Baseline Characteristic | n = 27,471 |
|---|---|
| Age, years | 58.0±7.7 |
| Male, n (%) | 10,829 (39.4) |
| Body mass index, kg/m2 | 25.8±4.0 |
| Current smoker, n (%) | 7,244 (28.1) |
| Systolic blood pressure, mmHg | 141.1±20.1 |
| Diastolic blood pressure, mmHg | 85.5±10.0 |
| Use of anti-hypertensives, n (%) | 4,678 (17.0) |
| Prevalent diabetes, n (%) | 1,186 (4.3) |
| Prevalent coronary heart disease, n (%) | 531 (1.9) |
| Prevalent heart failure, n (%) | 54 (0.2) |
| Prevalent ischemic stroke, n (%) | 226 (0.8) |
Data are means ± standard deviation unless stated otherwise
Results
Study population
The baseline characteristics of the 27,471 MDC participants in this study are provided in Table 2. Median time at risk for incident AF and ischemic stroke was 14.4 years and 14.5 years, respectively. During follow-up, 2,160 participants experienced a first AF event and 1,495 participants experienced a first ischemic stroke event. Of those participants with a first ischemic stroke event, 208 had a diagnosis of AF that preceded or coincided with the diagnosis of stroke.
Individual genetic variants and risk for incident AF
We genotyped 12 SNPs reported in the literature to be associated with AF at a genome-wide significance level (P<5×10−8). None of the 12 SNPs deviated from Hardy-Weinberg equilibrium assumptions (P>0.1). We tested the association of each SNP with incident AF and for all 12 SNPs, the effect size estimates were in the same direction as those reported in the literature. For 10 of these SNPs, the association exceeded nominal significance in the MDC study (P<0.05, Table 1). In this study population, a model that includes these 10 SNPs provide as much information about genetic risk for AF as a model that includes all 12 SNPs according to both forward and backward model selection algorithms. The magnitude of effect conferred by individual SNPs in MDC was consistent with the original discovery reports (correlation coefficient = 0.98, P=1.2×10−8, Supplemental Figure).
A multi-locus AF-GRS and risk for incident AF
We calculated a weighted 12-SNPs AF-GRS for each participant using the literature risk estimates as weights for the minor allele of each of the 12 SNPs. The AF-GRS in the study population ranged from −1.028 to +2.198. The median AF-GRS was 0.529 (IQR 0.262 to 0.795) among those with incident AF event and 0.431 (IQR 0.206 to 0.684) among those who did not experience an incident AF event. The prevalence of each of the 12 SNPs and the contribution of each weighted SNP to the AF-GRS in each quintile is provided in Supplemental Table.
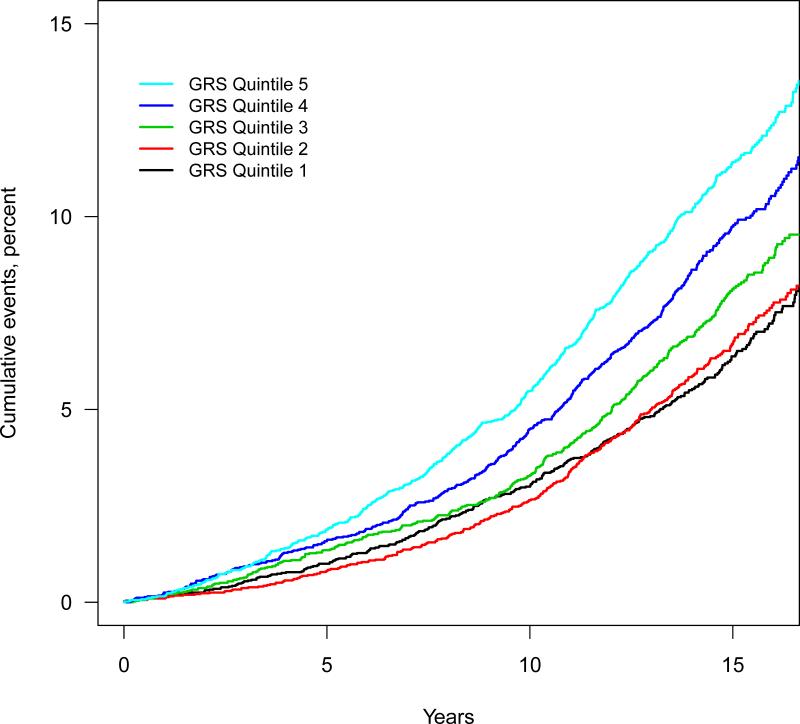
The probability of a first AF event, assessed from the cumulative event rate after 15 year follow-up increased smoothly with increased AF-GRS (Supplemental Figure). The AF-GRS was associated with incident AF (Table 3, Ptrend =1.8×10−28) after adjusting for age, sex, BMI, systolic and diastolic blood pressure, use of antihypertensive medication, smoking, diabetes, coronary events, and heart failure; and those in the top quintile of AF-GRS had a two-fold greater risk of incident AF compared with those at the bottom quintile (HR = 2.00; 95%CI 1.73 to 2.31, P=2.7×10−21). Kaplan-Meier estimates of the cumulative AF rate increased according to quintile of the AF-GRS (Figure 1).
Table 3.
Atrial fibrillation and ischemic stroke event rates and risk estimates according to AF-GRS quintiles
| AF-GRS Quintile |
Ptrend | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | |||
| Atrial fibrillation | ||||||
| N (Event) | 4,839 (292) | 4,868 (307) | 4,845 (367) | 4,890 (442) | 4,885 (527) | |
| Event rate (95%CI) | 4.33 (3.84 - 4.86) | 4.52 (4.02 - 5.05) | 5.44 (4.89 - 6.03) | 6.59 (5.98 - 7.23) | 7.90 (7.23 - 8.61) | |
| HR (95%CI) | Reference | 1.09 (0.93 - 1.28) | 1.30 (1.12 - 1.52) | 1.60 (1.38 - 1.86) | 2.00 (1.73 - 2.31) | 1.8×10−28 |
| P value (vs. Q1) | 0.3 | 7.7×10−4 | 4.7×10−10 | 2.7×10−21 | ||
| Ischemic stroke | ||||||
| N (Event) | 4,804 (254) | 4,831 (251) | 4,800 (237) | 4,857 (260) | 4,851 (296) | |
| Event rate (95%CI) | 3.80 (3.34 - 4.29) | 3.74 (3.29 - 4.24) | 3.53 (3.09 - 4.01) | 3.87 (3.41 - 4.37) | 4.41 (3.92 - 4.94) | |
| HR (95%CI) | Reference | 1.01 (0.85 - 1.21) | 0.95 (0.80 - 1.14) | 1.03 (0.87 - 1.23) | 1.23 (1.04 - 1.46) | 0.02 |
| P value (vs. Q1) | 0.9 | 0.6 | 0.7 | 0.015 | ||
Quintile boundaries: 1 GRS≤0.1453; 2 GRS>0.1453 and ≤0.3469; 3 GRS >0.3469 and ≤0.5284; 4 GRS>0.5284 and ≤0.7643; 5 GRS >0.7643.
Event rates are per 1000 person-years.
Hazard ratios from Cox proportional hazards models adjusted for age, sex, BMI, systolic and diastolic blood pressure, use of antihypertensive medications, current smoking, prevalent diabetes, prevalent coronary heart disease, and prevalent heart failure.
Figure 1. Cumulative incident atrial fibrillation events according to AF-GRS quintile.
Black: GRS Quintile 1. Red: GRS Quintile 2. Green: GRS Quintile 3. Blue: GRS Quintile 4. Light blue: GRS Quintile 5.
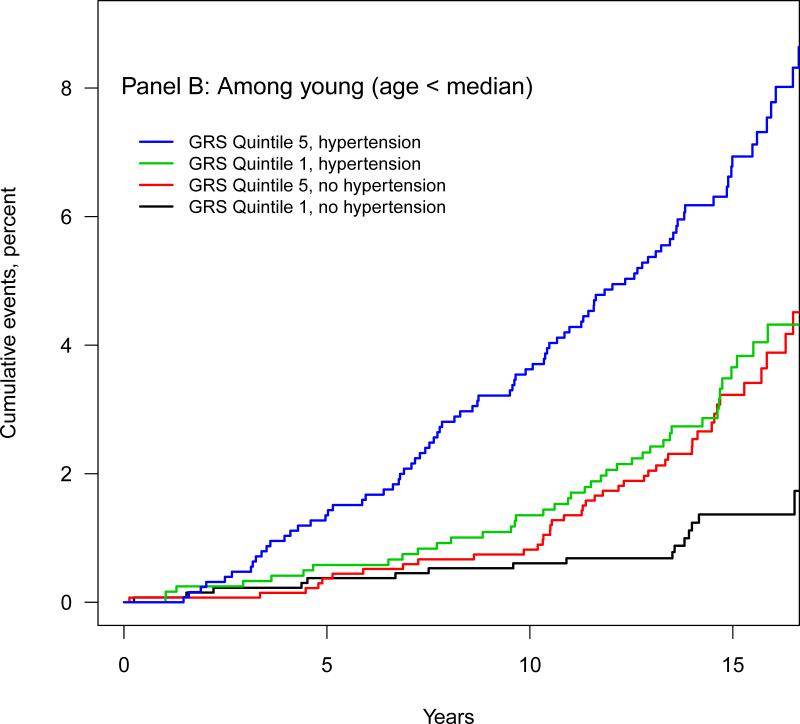
Since hypertension is a clinically important modifiable risk factor for AF we compared the AF event rate in those with a high and a low AF-GRS and in those with and without hypertension (defined as systolic blood pressure ≥140mmHg, diastolic blood pressure ≥90mmHg, or use of antihypertensive medications). Among young participants (those below the median age of 57.6) the rate of AF among those with a low AF-GRS (Q1) and hypertension was 2.34 per 1000 person-years (95%CI, 1.68 to 3.17) (Table 4). This rate of AF was similar to rate among those with a high AF-GRS (Q5) but without hypertension (2.37 per 1000 person-years, 95%CI, 1.74 to 3.15). This equivalent magnitude of the AF-GRS and hypertension as AF risk factors was also observed among individuals above the median age. Similarly, Kaplan-Meier estimates showed that the cumulative AF rate among those with a low AF-GRS and hypertension was similar to that of participants with a high AF-GRS but without hypertension among both the old (above median age, Figure 2A) and the young (below median age, Figure 2B).
Table 4.
Atrial fibrillation event rates according to median age, AF-GRS quintile, and hypertension
| Hypertension | AF-GRS Quintile | Event Rate (95%CI) |
|
|---|---|---|---|
| Age < 57.6 years | Age ≥57.6 years | ||
| Yes | 5th | 4.87 (3.91 - 5.99) | 14.52 (13.01 - 16.15) |
| Yes | 1st | 2.34 (1.68 - 3.17) | 8.52 (7.44 - 9.72) |
| No | 5th | 2.37 (1.74 - 3.15) | 9.73 (7.78 - 12.01) |
| No | 1st | 0.82 (0.47 - 1.34) | 3.88 (2.68 - 5.42) |
Event rate is per 1,000 person-years.
Hypertension defined as systolic blood pressure ≥140mmHg, diastolic blood pressure ≥90mmHg, or use of antihypertensive medications.
Figure 2. Cumulative incident atrial fibrillation events according to median age, AFGRS quintile, and hypertension status.
Panel A: Subgroup with age greater than median. Blue: The highest GRS (Quintile 5) with hypertension. Green: The lowest GRS (Quintile 1) with hypertension. Red: The highest GRS (Quintile 5) without hypertension. Black: The lowest GRS (Quintile 1) without hypertension. Median age for this older group is 64.2, IQR 61.1 to 67.6.
Panel B: Subgroup with age below median. Blue: The highest GRS (Quintile 5) with hypertension. Green: The lowest GRS (Quintile 1) with hypertension. Red: The highest GRS (Quintile 5) without hypertension. Black: The lowest GRS (Quintile 1) without hypertension. Median age for this group is 51.1 IQR 48.8 to 54.2.
The risk per quintile increase in AF-GRS was 1.20 (95 %CI 1.16 to 1.24), a magnitude of risk that is comparable to the dichotomized smoking status (HR = 1.26; 95%CI, 1.13 to 1.40) or dichotomized diabetes (HR = 1.32; 95%CI, 1.11 to 1.58) in this study (Supplemental Table).
We investigated whether the discrimination of a model based on established risk factors differed from that of a model that also included the AF-GRS. The established risk factor model was similar to those described by Schnabel et al.10 and Smith et al.34 (which included age, sex, BMI, systolic and diastolic blood pressure, use of antihypertensive medication, history of coronary events, and LDL-C); however, the model we used replaced LDL-C with ApoB because LDL-C was not available for the entire MDC study. The c-statistic for the established risk factors model was 0.735, and increased to 0.738 (P<0.0001) after the addition of the AF-GRS to the model. To assess the discriminatory potential of AF-GRS, we also assessed continuous NRI and found that adding the AFGRS to the established risk factors model improved reclassification (continuous NRI =0.225; (95%CI 0.187 to 0.323, P<0.0001). Among those with incident events, 54% were reclassified to higher risk and 46% to lower risk, while among those without events, 59% were reclassified to lower risk and 41% were reclassified to higher risk.
A multi-SNP AF-GRS and risk for incident ischemic stroke
The AF-GRS was associated with incident ischemic stroke (Table 3, Ptrend=0.02) after adjusting for age, sex, BMI, systolic and diastolic blood pressure, use of antihypertensive medication, smoking, diabetes, coronary events, and heart failure; an association with overall ischemic stroke that was not as strong as that of hypertension (Supplemental Table IV) Those in the top quintile of the AF-GRS, had about 23% greater risk of ischemic stroke compared with those in the bottom quintile (HR = 1.23; 95%CI 1.04 to 1.46, P=0.015).
A multi-SNP AF-GRS and risk for “likely cardioembolic” stroke
Since a high AF-GRS could be associated with a more deleterious type of AF, we also investigated the association between the AF-GRS and ischemic stroke among patients with a diagnosis of AF (n=2139; 282 prevalent and 2160 incident AF patients, excluding 303 patients with prevalent ischemic stroke, or without sufficient information). To further enrich the events for cardioembolic stroke this end point included only ischemic stroke events that were preceded by or coincided with a diagnosis of AF (n=208). We found that the AF-GRS was associated with these likely cardioembolic stroke events after adjustment for established risk factors (age, sex, BMI, systolic and diastolic blood pressure, use of antihypertensive medication, smoking, diabetes, coronary events, and heart failure). The hazard ratio for those in the top compared with the bottom quintile was 1.81 (95%CI, 1.20 to 2.73, P=0.005, Ptrend=0.009, Supplemental Table V). This association remained similar when we adjusted the association of the AF-GRS with likely cardioembolic stroke events for the CHADS2 score instead of established risk factors: for the top vs. bottom quintile, the HR was 1.74 (95%CI 1.15 to 2.62), P=0.009, Ptrend=0.014.
To assess whether the AF-GRS would add to the discrimination by the CHADS2 score among patients with AF, we compared the CHADS2 model with a model that also included the AF-GRS for prediction of likely cardioembolic stroke. The continuous NRI indicated that adding the AF-GRS to CHADS2 score improved reclassification (continuous NRI = 0.166 (95%CI 0.0175 to 0.315, P=0.03)). Among those with incident events, 51.1 % were reclassified to higher risk and 48.9% to lower risk, while among those without events, 57.3% were reclassified to lower risk and 42.7% were reclassified to higher risk.
In contrast to the results with overall ischemic stroke, the rate of likely cardioembolic stroke among those with a low AF-GRS (1st quartile) and hypertension was similar to that among those with a high AF-GRS (4th quartile) but without hypertension: 0.19 and 0.20 per 1000 person years for young individuals and 1.04 and 1.22 per 1000 person-years for old individuals (Supplemental Table IV).
Discussion
We sought to validate recently discovered genetic risk factors for AF and to estimate the magnitude of risk conferred by these genetic risk factors in 27,471 participants from a community-based prospective cohort. We found that an AF-GRS comprising 12 SNPs was associated with incident AF and ischemic stroke, even after accounting for non-genetic risk factors including age and hypertension. Individuals of European ancestry who were in the top quintile of genetic risk had an approximately two-fold greater risk for incident AF than those in the lowest quintile.
These findings suggest several conclusions. First, the associations of SNPs with AF in the discovery GWAS seem to generalize to the community-based, prospective setting. Second, the magnitudes of effects conferred by individual SNPs in the AF-GRS are consistent with the original discovery reports. Third, when comparing risk factors for AF, having a high AF-GRS seems to confer risk equivalent to having hypertension: those with a high AF-GRS but no hypertension had a rate of future AF events similar to the rate of those with a low AF-GRS but with hypertension. And this equivalence of the AF-GRS and hypertension as AF risk factors was observed in both younger and older individuals.
Everett and colleagues reported that a similar AF-GRS was associated with incident AF in the Women's Genome Health Study (WGHS),35 a study that comprised a subset of the female health professionals who participated in a randomized clinical trial of aspirin and vitamin E therapy.36 The AF-GRS in the WGHS comprised 12 SNPs, 11 of which were the same as the SNPs in the GRS of the current study, and the 12th SNP in both AF-GRS studies was in the same gene (ZFHX3). In the WGHS analysis, those in the top quintile of AF-GRS had a greater than two-fold increased risk for incident AF in a model that adjusted for non-genetic factors. The current study, extends the prediction of AF by a GRS to a community-based cohort that includes both men and women, and also reports the association of an AF-GRS with ischemic stroke—a severe clinical complication of AF.
Recently, Lubitz et al. reported on an AF-GRS comprising 12 SNPs in the same 9 genetic loci we report here.37 They found that this AF-GRS was associated with AF in populations of European descent as well as in a case-control study of a Japanese population, thus providing support for the notion that an AF-GRS could be used in diverse ethnic settings. However, these investigators did not report whether adjusting for established risk factors for AF modified the magnitude of the association. And a GRS for ischemic stroke has been reported by Malik et al.38 This GRS comprises 113 SNPs that had been previously reported to be associated with risk factors for ischemic stroke: AF, coronary artery disease, hypertension, and systolic blood pressure. This GRS was associated with ischemic stroke in a population-based validation study (P=0.016), but the association of the AF related SNPs in the GRS with ischemic stroke or AF was not reported in that validation study.
The clinical value of using genetic markers to identify a subset of asymptomatic individuals at high risk for AF will depend on the availability of an effective intervention (diagnostic or therapeutic) that, when implemented in those at a high genetic risk, can alter health outcomes. Examples of interventions that might benefit those at high genetic risk for AF include aggressive blood pressure management and more intensive monitoring for AF in the ambulatory or postoperative settings. Further clinical studies could assess the potential benefit of additional diagnostic or therapeutic interventions among those at high genetic risk for AF.
While risk assessment tools for cardioembolic stroke in patients with AF, such as the CHADS2 score are currently used to assess the potential benefit of anticoagulant therapy for stroke prevention,39 we found that using the AF-GRS in addition to the CHADS2 score improved risk assessment for ischemic stroke among patients with AF in this population.
Our study has several limitations. The study population is Swedish middle-aged to elderly individuals; hence, the generalizability to other ethnicities or age groups is uncertain. However, the association of an AF-GRS in a Japanese population could indicate that an AF-GRS would be useful in other populations.37 In addition, although we could provide an estimate of the effect of the AF-GRS on risk for overall ischemic stroke, we did not have sufficient information about stroke subtypes to provide a risk estimate for validated cardioembolic stroke, a stroke subtype that is most strongly associated with AF. Also our SNP panel focused on genetic loci thought to be directly associated with AF and did not include SNPs associated with AF risk factors such as blood pressure or BMI. And information about family history of AF was not routinely available for this cohort.40 Although incident AF have been well validated in this study,28 the incidence rate might have been underestimated because ICD code information was derived from hospitalized participants and did not include AF diagnosis by general practitioners. Asymptomatic AF is often undiagnosed41 and is likely underdiagnosed in this study as well. This under-diagnosis underscores the clinical need for biomarkers associated with risk of AF.
In conclusion, an AF-GRS based on 12 SNPs can identify a subset of the population at more than two-fold increased risk for AF and ~25% increased risk for ischemic stroke. Diagnostic or therapeutic interventions targeted to this subset may lower the burden of AF and stroke.
Supplementary Material
Acknowledgments
Funding Sources: The Malmö Diet and Cancer study was made possible by grants from the Swedish Cancer Society, the Swedish Medical Research Council, the Swedish Dairy Association, the Albert Påhlsson and Gunnar Nilsson Foundations and the Malmö city council. Dr. Tada is supported by a grant from the Japanese Circulation Society to study in the United States. Dr. Smith is supported by governmental funding of clinical research within the Swedish National Health Service and grants from the Swedish Heart and Lung Foundation and Skåne University Hospital. Dr. Lubitz is supported by a research grant from NIH (1K23HL114724). Dr. Ellinor is supported by research grants from NIH and American Heart Association. Dr. Kathiresan is supported by a Research Scholar award from the Massachusetts General Hospital (MGH), the Howard Goodman Fellowship from MGH, and the Donovan Family Foundation. Dr. Melander is supported by the European Research Council (StG-282255), the Swedish Heart and Lung Foundation, Swedish Research Council; the Novo Nordisk Foundation, the Skåne University Hospital donation funds; the Medical Faculty, Lund University; the Governmental funding of clinical research within the national health services, the Albert Påhlsson Research Foundation, Region Skåne, the King Gustav V and Queen Victoria Foundation and the Marianne and Marcus Wallenberg Foundation. Genotyping of the AF-GRS SNPs in the MDC study was performed at the Quest Diagnostics Science and Innovation genotyping facility in Alameda, California. Drs. Melander and Kathiresan are the recipients of an investigator-initiated grant from Celera to study the genetics of AF in MDC.
Footnotes
Conflict of Interest Disclosures:
Dr. Tada is supported by a research grant from Japanese Circulation Society. Dr. Shiffman, Ms. Louie, Mr. Catanese, and Dr. Devlin are employees of Celera. Dr. Smith is supported by governmental funding of clinical research within the Swedish National Health Service and grants from the Swedish Heart and Lung Foundation and Skåne University Hospital. Dr. Lubitz is supported by a research grant from NIH. Dr. Ellinor is supported by research grants from NIH and American Heart Association. Dr. Catanese has stocks of Quest Diagnostics. Dr. Delvin participates in the employee stock purchase plan, and have received incentive stock options. Dr. Kathiresan is supported by a research grant from Celera, a Research Scholar award from the Massachusetts General Hospital (MGH), the Howard Goodman Fellowship from MGH, and the Donovan Family Foundation. Dr. Melander is supported by the European Research Council, the Swedish Heart and Lung Foundation, Swedish Research Council, the Novo Nordisk Foundation, the Skåne University Hospital donation funds, the Medical Faculty, Lund University, the Governmental funding of clinical research within the national health services, the Albert Påhlsson Research Foundation, Region Skåne, the King Gustav V and Queen Victoria Foundation and the Marianne and Marcus Wallenberg Foundation.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in AF (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Benjamin EJ. Status of the epidemiology of AF. Med Clin North Am. 2008;92:17–40, ix. doi: 10.1016/j.mcna.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 7.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham heart study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 9.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 10.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Sr, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, McManus DD, et al. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011;124:1982–1993. doi: 10.1161/CIRCULATIONAHA.111.039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox CS, Parise H, D'Agostino RB, Sr, Lloyd-Jones DM, Vasan RS, Wang TJ, et al. Parental AF as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 14.Arnar DO, Thorvaldsson S, Manolio TA, Thorgeirsson G, Kristjansson K, Hakonarson H, et al. Familial aggregation of atrial fibrillation in Iceland. Eur Heart J. 2006;27:708–712. doi: 10.1093/eurheartj/ehi727. [DOI] [PubMed] [Google Scholar]
- 15.Christophersen IE, Ravn LS, Budtz-Joergensen E, Skytthe A, Haunsoe S, Svendsen JH, et al. Familial aggregation of atrial fibrillation: a study in Danish twins. Circ Arrhythm Electrophysiol. 2009;2:378–383. doi: 10.1161/CIRCEP.108.786665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellinor PT, Yoerger DM, Ruskin JN, MacRae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118:179–184. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 17.Marcus GM, Smith LM, Vittinghoff E, Tseng ZH, Badhwar N, Lee BK, et al. A first-degree family history in lone atrial fibrillation patients. Heart Rhythm. 2008;5:826–830. doi: 10.1016/j.hrthm.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 19.Lubitz SA, Sinner MF, Lunetta KL, Makino S, Pfeufer A, Rahman R, et al. Independent Susceptibility Markers for atrial fibrillation on Chromosome 4q25. Circulation. 2010;122:976–984. doi: 10.1161/CIRCULATIONAHA.109.886440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamin EJ, Rice KM, Arking DE, Pfeufer A, van Noord C, Smith AV, et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41:879–881. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gudbjartsson DF, Holm H, Gretarsdottir S, Thorleifsson G, Walters GB, Thorgeirsson G, et al. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet. 2009;41:876–878. doi: 10.1038/ng.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellinor PT, Lunetta KL, Glazer NL, Pfeufer A, Alonso A, Chung MK, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berglund G, Elmstähl S, Janzon L, Larsson SA. The Malmo Diet and Cancer Study. Design and feasibility. J Intern Med. 1993;233:45–51. doi: 10.1111/j.1365-2796.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith JG, Platonov PG, Hedblad B, Engström G, Melander O. Atrial fibrillation in the Malmö Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol. 2010;25:95–102. doi: 10.1007/s10654-009-9404-1. [DOI] [PubMed] [Google Scholar]
- 26.Enhörning S, Wang TJ, Nilsson PM, Almgren P, Hedblad B, Berglund G, et al. Plasma copeptin and the risk of diabetes mellitus. Circulation. 2010;121:2102–2108. doi: 10.1161/CIRCULATIONAHA.109.909663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl1):S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith JG, Platonov PG, Hedblad B, Engström G, Melander O. Atrial fibrillation in the Malmö Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol. 2010;25:95–102. doi: 10.1007/s10654-009-9404-1. [DOI] [PubMed] [Google Scholar]
- 29.Jerntorp P, Berglund G. Stroke registry in Malmö, Sweden. Stroke. 1992;23:357–361. doi: 10.1161/01.str.23.3.357. [DOI] [PubMed] [Google Scholar]
- 30.Shiffman D, O'Meara ES, Bare LA, Rowland CM, Louie JZ, Arellano AR, et al. Association of gene variants with incident myocardial infarction in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2008;28:173–179. doi: 10.1161/ATVBAHA.107.153981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
- 32.Gönen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92:965–970. [Google Scholar]
- 33.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JG, Newton-Cheh C, Almgren P, Struck J, Morgenthaler NG, Bergmann A, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;56:1712–1219. doi: 10.1016/j.jacc.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Everett BM, Cook NR, Conen D, Chasman DI, Ridker PM, Albert CM. Novel genetic markers improve measures of atrial fibrillation risk prediction. Eur Heart J. 2013;34:2243–2251. doi: 10.1093/eurheartj/eht033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glynn RJ, Ridker PM, Goldhaber SZ, Zee RY, Buring JE. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: report from the Women's Health Study. Circulation. 2007;116:1497–1503. doi: 10.1161/CIRCULATIONAHA.107.716407. [DOI] [PubMed] [Google Scholar]
- 37.Lubitz SA, Lunetta KL, Lin H, Arking DE, Trompet S, Li G, et al. Novel genetic markers associate with atrial fibrillation risk in europeans and Japanese. J Am Coll Cardiol. 2014;63:1200–1210. doi: 10.1016/j.jacc.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malik R, Bevan S, Nalls MA, Holliday EG, Devan WJ, Cheng YC, et al. Multilocus genetic risk score associates with ischemic stroke in case-control and prospective cohort studies. Stroke. 2014;45:394–402. doi: 10.1161/STROKEAHA.113.002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 40.Lubitz SA, Yin X, Fontes JD, Magnani JW, Rienstra M, Pai M, et al. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA. 2010;304:2263–2269. doi: 10.1001/jama.2010.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charitos EI, Pürerfellner H, Glotzer TV, Ziegler PD. Clinical Classifications of Atrial Fibrillation Poorly Reflect its Temporal Persistence: Insights From 1195 Patients Continuously Monitored with Implantable Devices. J Am Coll Cardiol. 2014;63:2840–2848. doi: 10.1016/j.jacc.2014.04.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.