Abstract
Central nervous system (CNS) relapse after allogeneic hematopoietic stem cell transplantation (HSCT) confers a poor prognosis in adult patients with acute lymphoblastic leukemia (ALL). Preventing CNS relapse after HSCT remains a therapeutic challenge, and criteria for post-HSCT CNS prophylaxis have not been addressed. In a three-center retrospective analysis, we reviewed the data for 457 adult patients with ALL who received a first allogeneic HSCT in first or second complete remission (CR). All patients received CNS prophylaxis as part of their upfront therapy for ALL, but post transplant CNS prophylaxis practice varied by institution, and was administered to 48% of the patients. Eighteen patients (4%) developed CNS relapse after HSCT (isolated CNS relapse, n=8; combined bone marrow and CNS relapse, n=10). Patients with a prior history of CNS involvement with leukemia had a significantly higher rate for CNS relapse (P=0.002), and pre transplant CNS involvement was the only risk factor for post transplant CNS relapse found in this study. We failed to find a significant effect of post-transplant CNS prophylaxis to prevent relapse after transplant. Furthermore, no benefit for post-transplant CNS prophylaxis could be detected when a sub-group analysis of patients with (p=0.10) and without prior CNS involvement (p=0.52) was performed. Finally, we couldn’t find any significant impact for intensity of the transplant conditioning regimen on CNS relapse after HSCT. In conclusion, CNS relapse is an uncommon event following HSCT for ALL in CR1 or CR2, but with higher risk among patients with CNS involvement pre transplant. Furthermore, neither the use of post-HSCT CNS prophylaxis nor the intensity of the HSCT conditioning regimen made a significant difference in the rate of post-HSCT CNS relapse.
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (HSCT) is an effective treatment for acute lymphoblastic leukemia (ALL) resulting in long-term remissions (1). Despite advances in therapy, disease progression remains the major cause of mortality following allogeneic HSCT accounting for 20–50% of all deaths (2–4). The central nervous system (CNS) is the most common extra-medullary site of disease progression after transplant in ALL (4).
Although the use of CNS prophylaxis as part of the upfront treatment for ALL has led to significant decreases in CNS relapse and improved outcomes overall (5, 6), the routine use of post-HSCT prophylactic CNS therapy as a strategy to prevent CNS relapse after transplant is still controversial. Studies that utilized post-HSCT CNS prophylaxis have reported disparate results and there is no generalized consensus regarding the role of post-transplant CNS prophylaxis to prevent CNS relapse (7–10). Furthermore, the increasing use of reduced intensity transplant conditioning therapies with possibly decreased CNS penetration makes it even more imperative that we try to understand the benefit, if any, of post HSCT CNS prophylaxis.
The practice of post-HSCT prophylactic CNS therapy varies from center to center. In order to determine the role of post-HSCT prophylactic CNS therapy in preventing CNS relapse in ALL patients, we designed a study in centers with different post-HSCT CNS therapy practice and reviewed the data of 457 patients with ALL who received first allogeneic HSCT in the first or second complete remission (CR).
PATIENTS AND METHODS
Population
In this multi-center retrospective study, we studied all adult (age≥18) ALL patients who underwent a first allogeneic HSCT at the MD Anderson Cancer Center (MDACC), Fred Hutchinson Cancer Research Center (FHCRC), or the Rabin Medical Center (RMC) in Israel between 2000 and 2011. We included all adult ALL patients who were transplanted in first or second CR. Before the transplantation procedure, all patients received intrathecal methotrexate (MTX) and/or cytarabine (Ara-C), and/or craniospinal irradiation (CSI) as part of their standard induction and consolidation treatment.
Post transplant CNS prophylaxis
The practice of CNS disease prophylaxis varied between MDACC, FHCRC and the RMC. At MDACC, generally only patients with a prior history of CNS disease were offered post transplant CNS prophylaxis with intrathecal cytarabine alternating with methotrexate for 6–8 monthly infusions as tolerated; alternatively they could receive craniospinal XRT (dose 24 Gy in 12 daily fractions) or boost (12 Gy in 6 daily fractions) to the CNS as part of their scheduled TBI (12 Gy in 4 daily fractions) at the time of transplant conditioning. At the FHCRC, all patients, with and without history of CNS involvement pre transplant, were routinely administered post transplant CNS prophylaxis, most commonly with intrathecal or intraventricular methotrexate for 4–6 doses every two weeks as tolerated. Patients with CNS involvement incidentally found during the pre-transplant evaluation that failed to clear with 1–2 doses of MTX received cranial-spinal irradiation immediately before or during conditioning. Similarly, at RMC, all patients, irrespective of prior CNS disease, were given 4 injections of intrathecal methotrexate, with the first dose usually administered one month after transplantation. On-going transplant toxicities and/or thrombocytopenia would have been some reasons for the patients at FHCRC or RMC to not receive post HSCT CNS prophylaxis.
Definitions
Relapse into the CNS was defined as unequivocal morphologic evidence of leukemic blasts in the cerebrospinal fluid (CSF) or cranial nerve palsies, or a non-hemorrhagic mass seen in cranial computed tomography (CT) or magnetic resonance imaging (MRI) due to infiltration by leukemia cells. Cytogenetic abnormalities were classified based on previously published reports (11). The intensity of the conditioning regimens were defined according to the Center for International Blood and Marrow Transplantation Research criteria (12).
Statistics
We assessed the cumulative incidence of systemic and CNS relapses in a competing risks framework with a competing risk of non-relapse death. Since data from second and subsequent relapses were not available, patients whose first relapse did not include CNS involvement were censored at the time of relapse. To analyze the association between post-HSCT CNS prophylactic therapy (which was given within the first three months post HSCT) and CNS relapse, we used a landmark cumulative incidence analysis, including only patients who had not relapsed or died by 3 months post HSCT; landmark analysis was not used for total relapse or survival analyses. Fisher’s exact test and the Wilcoxon rank-sum test were used to compare categorical and continuous variables between patients with CNS prophylaxis and those without.
RESULTS
Patient and transplant characteristics
We included 457 adult patients with ALL with median age 38 years (range 18 – 76 years). Characteristics of patients are presented in Table 1. Two hundred seventy three patients were transplanted in first CR and 184 in second CR. Sixty seven patients (15%) had a history of pre-HSCT CNS involvement either at diagnosis (n= 38) or after first relapse (n=29). The median follow-up for the 213 surviving patients was 3.0 years. Due to differing practice patterns, only 19% of patients at MDACC received post-transplant CNS prophylaxis in contrast to 79% of patients from FHCRC and RMC (P<0.0001). Overall 217 patients (47%) received post-transplant intrathecal CNS prophylaxis with intrathecal MTX, Ara-C, or both agents for a median of 4 treatment cycles (range, 1–10). Two patients received prophylactic CNS radiotherapy after transplant.
Table 1.
Patient and Transplant Characteristics
| No post-HSCT CNS prophylaxis (n=238) | Received post-HSCT CNS prophylaxis (n=219) | P-value | |
|---|---|---|---|
|
| |||
| Center | <0.0001 | ||
| MDACC | 193 | 45 | |
| FHCRC | 38 | 161 | |
| RMC | 7 | 13 | |
|
| |||
| Median age at HSCT (range, years) | 38 (18 – 70) | 38 (19 – 76) | 0.78 |
|
| |||
| Sex | 0.34 | ||
| Female | 92 | 95 | |
| Male | 146 | 124 | |
|
| |||
| Lineage | 1.00 | ||
| B-cell | 201 | 188 | |
| T-cell | 33 | 30 | |
| Unknown | 4 | 1 | |
|
| |||
| Cytogenetic Risk Group | 0.65 | ||
| Good | 13 | 8 | |
| Intermediate | 86 | 87 | |
| Poor | 110 | 102 | |
| Unknown | 29 | 22 | |
|
| |||
| Status at HSCT | 0.002 | ||
| CR1 | 126 | 147 | |
| CR2 | 112 | 72 | |
|
| |||
| Pre-HSCT CNS Disease | 0.06 | ||
| None | 209 | 174 | |
| At Diagnosis | 15 | 23 | |
| At First Relapse | 11 | 18 | |
| Missing | 3 | 4 | |
|
| |||
| Preparative regimen | 0.89 | ||
| Myeloablative | 204 | 189 | |
| Non-myeloablative or RIC | 34 | 30 | |
|
| |||
| Allotype | 0.95 | ||
| HLA Matched-Related | 98 | 91 | |
| HLA Matched Unrelated | 86 | 80 | |
| HLA Mismatched-Related | 9 | 10 | |
| HLA Mismatched Unrelated | 45 | 38 | |
|
| |||
| Graft Source | 0.001 | ||
| Bone Marrow | 65 | 48 | |
| Peripheral Blood | 136 | 157 | |
| Cord Blood | 37 | 14 | |
|
| |||
| Relapse | 0.08 | ||
| Total | 60 | 74 | |
| CNS | 6 | 12 | |
|
| |||
| Graft-versus-Host Disease | |||
| Acute, grades II–IV | 100 | 156 | |
| Chronic, limited and/or extensive | 55 | 125 | |
CNS Relapse after transplant
The incidence of CNS relapse after transplant in the entire cohort was 4%, with incidence of 13% in patients with history of CNS involvement pre transplant. Characteristics of the 18 patients who developed CNS relapse after transplants are described in Table 2. Among the 18 relapses, eight were isolated CNS relapse and 10 were combined bone marrow and CNS relapse. The CNS relapses occurred at a median of 231 days (range 38–1414) after HSCT. Nine of these patients (50%) had pre-HSCT CNS involvement. Among the nine patients with CNS involvement before HSCT, four had CNS involvement at diagnosis and five patients had CNS involvement during first relapse and were transplanted in second CR.
Table 2.
Characteristics of Patients with Post-Transplant CNS Relapse
| Pts | Center | S E X |
Age | Histology | Previous CNS Involvement |
WBC (k/uL) |
Cytogenetic Risk Group |
Pre-HSCT CNS Therapy |
Disease Status at HSCT |
Prep. Regimen |
Post-HSCT CNS Therapy |
Relapse Sites |
Relapse Post HSCT (Day) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MDACC | F | 24 | B | Relapse 1 | 28.9 | Poor | 8 MTX+10 Ara-C | CR2 | MA+TBI | 4 MTX | BM/CNS | 687 |
| 2 | MDACC | M | 31 | T | Diagnosis | 170 | Intermediate | 8 MTX+11 Ara-C | CR2 | MA+TBI | 5 MTX + 3 Ara-C | BM/CNS | 136 |
| 3 | MDACC | F | 26 | B | Relapse 1 | 2.6 | Poor | 2 MTX + 3 Ara-C | CR2 | MA+TBI | 1 MTX | CNS | 231 |
| 4 | MDACC | M | 29 | B | Relapse 1 | 5.1 | Poor | 1 Ara-C | CR2 | MA+TBI | 2 Ara-C | CNS | 399 |
| 5 | FHCRC | F | 38 | B | Relapse 1 | 200 | Poor | 8 MTX | CR2 | RIC | No prophylaxis | CNS | 38 |
| 6 | FHCRC | F | 42 | B | Relapse 1 | 22 | Intermediate | 7 Ara-C | CR2 | MA+TBI | 4 MTX | BM/CNS | 244 |
| 7 | FHCRC | F | 20 | B | None | 150 | Poor | 8 MTX | CR1 | MA+TBI | 5 MTX | BM/CNS | 96 |
| 8 | FHCRC | F | 50 | B | None | 14 | Poor | 2 MTX | CR1 | MA+TBI | 6 MTX | BM/CNS | 1414 |
| 9 | FHCRC | F | 27 | B | Diagnosis | 1.2 | Unknown | 8 MTX | CR2 | MA+TBI | 7 MTX | CNS | 1397 |
| 10 | FHCRC | M | 48 | B | None | Unk | Intermediate | 4 MTX | CR1 | MA+TBI | 8 MTX | BM/CNS | 231 |
| 11 | MDACC | M | 23 | B | None | 3.2 | Intermediate | 6 MTX + 6 Ara-C | CR2 | MA | 9 MTX | CNS | 860 |
| 12 | MDACC | M | 35 | B | None | 2.6 | Poor | unknown # of IT | CR1 | MA+TBI | No prophylaxis | CNS | 52 |
| 13 | MDACC | M | 27 | T | None | 3900 | Intermediate | 1 MTX | CR1 | MA | No prophylaxis | BM/CNS | 63 |
| 14 | RMC | M | 53 | B | Diagnosis | 200 | Poor | 3 MTX | CR1 | MA | 4 MTX | BM/CNS | 86 |
| 15 | MDACC | F | 24 | B | None | 42 | Poor | 2 MTX + 1 Ara-C | CR1 | MA | No prophylaxis | CNS | 638 |
| 16 | MDACC | M | 51 | B | None | .5 | Poor | 4 MTX + 4 Ara-C | CR1 | MA | 5 Ara-C | CNS | 350 |
| 17 | MDACC | M | 24 | B | Relapse 1 | 215 | Poor | 4 MTX + 3 Ara-C | CR2 | MA | No prophylaxis | CNS | 82 |
| 18 | MDACC | F | 41 | B | None | 3 | Intermediate | 6 Ara-C | CR1 | MA | No prophylaxis | BM/CNS | 140 |
Predictors of CNS relapse
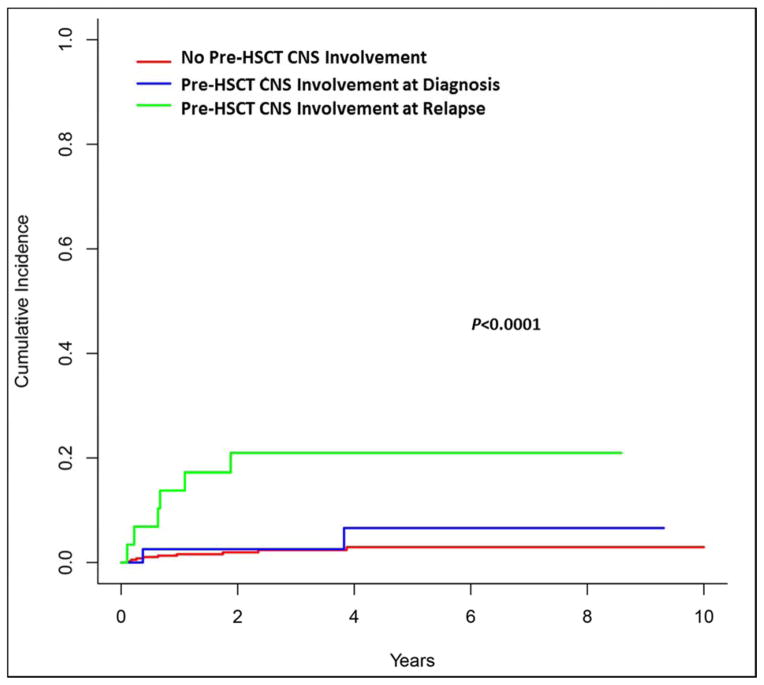
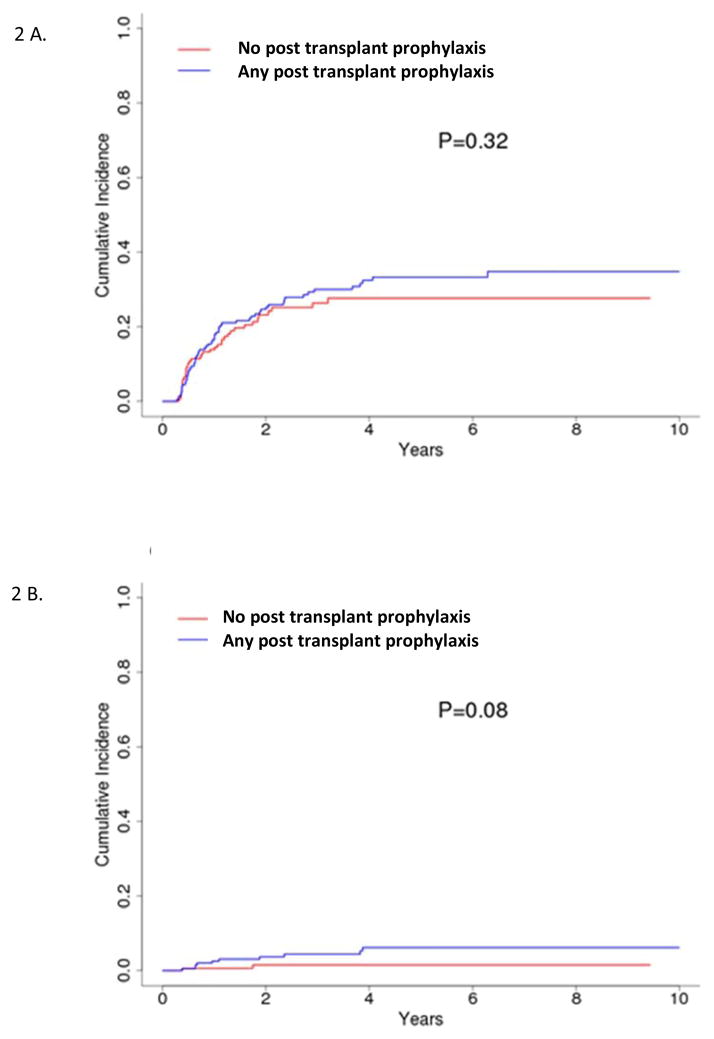
Patients with a prior history of CNS involvement with leukemia had a significantly higher rate for CNS relapse compared to patients with no history of CSN involvement pre transplant, 13.4% vs. 2.6% (P=0.002), and furthermore, patients who had CNS involvement in first relapse, compared with those with CNS involvement at diagnosis, had the highest rate for CNS progression post transplant (Figure 1). We failed to find a significant effect of post-transplant intrathecal chemotherapy or radiation therapy to prevent CNS relapse, or any relapse, after transplant (Figure 2A and 2B). The 4-year cumulative incidence of CNS relapse was 6% and 1.5% for patients with and without CNS prophylaxis after HSCT, respectively (P=0.08, Figure 2A). No benefit for post-transplant CNS prophylaxis could be detected when a sub-group analysis of patients with (p=0.10) and without prior CNS involvement (p=0.52) was performed. The 4-year rate of CNS relapse was also not impacted by the intensity of the transplant conditioning regimen, p=0.33, as shown in univariate analysis in Table 3.
Figure 1.
Cumulative incidence of CNS relapse by pre-HSCT CNS involvement.
Figure 2.
Cumulative incidence of (A) any relapse or (B) CNS relapse by CNS prophylaxis using landmark analysis starting at day 100.
Table 3.
Transplant Outcomes, Univariate Analyses
| Variable | CNS relapse | Total Relapse | Overall Mortality | |||
|---|---|---|---|---|---|---|
|
| ||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
|
| ||||||
| Age, continuous variable | 0.97 (0.94, 1.004) | 0.08 | 0.99 (0.98, 1.002) | 0.09 | 1.01 (1.001, 1.02) | 0.03 |
|
| ||||||
| Sex | ||||||
| Female | -------- | -------- | -------- | -------- | -------- | -------- |
| Male | 0.69 (0.27, 1.73) | 0.42 | 1.02 (0.72, 1.43) | 0.92 | 1.12 (0.86, 1.44) | 0.40 |
|
| ||||||
| Lineage | ||||||
| B-cell | -------- | -------- | -------- | -------- | -------- | -------- |
| T-cell | 0.83 (0.19, 3.59) | 0.80 | 1.71 (1.10, 2.67) | 0.02 | 1.09 (0.76, 1.59) | 0.61 |
|
| ||||||
| Cytogenetic risk group | * | * | ||||
| Good | -------- | -------- | -------- | -------- | ||
| Intermediate | 1.21 (0.55, 2.67) | 0.64 | 0.83 (0.45, 1.53) | 0.55 | ||
| Poor | 1.13 (0.78, 1.62) | 0.52 | 0.87 (0.48, 1.58) | 0.65 | ||
|
| ||||||
| Pre CNS involvement | 5.84 (2.33, 14.64) | 0.0002 | 1.17 (0.73, 1.86) | 0.52 | 1.33 (0.96, 1.85) | 0.09 |
|
| ||||||
| Disease status | ||||||
| CR1 | -------- | -------- | -------- | -------- | -------- | -------- |
| CR2 | 1.47 (0.59, 3.69) | 0.41 | 1.87 (1.34, 2.63) | 0.0003 | 1.95 (1.52, 2.51) | <0.0001 |
|
| ||||||
| Conditioning regimen | ||||||
| MA | -------- | -------- | -------- | -------- | -------- | -------- |
| Others | 0.36 (0.05, 2.77) | 0.33 | 1.20 (0.75, 1.91) | 0.45 | 1.19 (0.83, 1.68) | 0.34 |
|
| ||||||
| Graft Source | * | * | ||||
| Bone Marrow | -------- | -------- | -------- | -------- | ||
| Cord Blood | 0.66 (0.31, 1.39) | 0.27 | 2.33 (1.52, 3.57) | 0.0001 | ||
| Peripheral Blood | 1.25 (0.84, 1.87) | 0.28 | 1.22 (0.90, 1.66) | 0.20 | ||
|
| ||||||
| Allotype | ||||||
| HLA Matched-Related | -------- | -------- | -------- | -------- | -------- | -------- |
| HLA Matched Unrelated | 0.50 (0.17, 1.43) | 0.20 | 0.70 (0.48, 1.03) | 0.07 | 0.88 (0.66, 1.18) | 0.40 |
| HLA Mismatched-Related | 0.85 (0.10, 7.00) | 0.89 | 0.67 (0.27, 1.64) | 0.38 | 1.40 (0.78, 2.50) | 0.25 |
| HLA Mismatched Unrelated | 0.20 (0.03, 1.54) | 0.12 | 0.57 (0.34, 0.97) | 0.04 | 1.53 (1.10, 2.14) | 0.01 |
|
| ||||||
| Post-HSCT CNS prophylaxis | 3.88 (0.85, 17.61) | 0.08 | 1.22 (0.82, 1.83) | 0.32 | 0.47 (0.36, 0.61) | <0.0001 |
|
| ||||||
| Post-HSCT IT prophylactic cycles | ||||||
| <4 | -------- | -------- | -------- | -------- | -------- | -------- |
| ≥4 | 2.54 (0.77, 8.41) | 0.13 | 1.01 (0.68, 1.49) | 0.98 | 0.45 (0.34, 0.60) | <0.0001 |
Predictors of Total Relapse and OS
The 4-year cumulative incidence of relapse after HSCT among all patients was 32.3% and 27.9% for patients with and without CNS prophylaxis after HSCT, respectively (P=0.32) (Figure 2B). There was no difference in survival between patients with and without pre-HSCT CNS disease (P=0.09) while the disease status (CR1 vs CR2 at time of transplant) was a significant predictor of survival (P<0.0001) (Table 3).
DISCUSSION
Effective strategies to control the primary disease and to reduce the incidence of CNS relapse, including early and frequent combination therapy with systemic CNS active agents, CNS irradiation, and intrathecal therapy, have reduced the incidence of CNS progression prior to, and after, transplant (13, 14). Indeed, the rate for CNS relapse following transplant was only 4% in our study, and is consistent with previous studies that have demonstrated that CNS relapse is an uncommon event following HSCT for ALL in first or second remission (4, 7–10).
We failed to show a significant benefit for post-transplant CNS prophylaxis in preventing CNS relapse. Previous studies have reported disparate effects for post-HSCT CNS prophylaxis. While some studies showed beneficial effects for post-HSCT CNS prophylaxis (10), others have not shown benefit, (7, 8) and still others showed even adverse effects for prophylaxis as a result of clinical toxicity (9). In our study, one could note that the rate of CNS relapse was higher in patients who received post-HSCT CNS prophylaxis. This may be partially explained by the fact that the rate of pre HSCT CNS involvement was higher among patients who received CNS prophylaxis (19% versus 11%). In addition, at MDACC generally only patients with a predicted higher rate for CNS relapse post-transplant (those with prior CNS involvement) routinely received post HSCT prophylaxis (51% vs. 13%, respectively, P <0.001). Patients from the FHCRC and RMC received post-HSCT CNS prophylaxis routinely, but the numbers of patients were too small to perform sub-group analyses by center.
A prior history of CNS involvement was the only identified predictor for post-transplant CNS relapse in our study, similar to other reports (7, 9, 10). In our study, the incidence of CNS relapse after transplant in patients with pre-HSCT CNS involvement was 13%, which is consistent with previously published rates of 0 – 27% (7–10). Interestingly, our results showed that patients with CNS involvement at diagnosis had a lower rate of CNS relapse compared to patients who developed CNS relapse in first relapse (Figure 1). This may in part be explained by differences in biology between the two groups, and may also be impacted by differences in treatment approaches (15–18). Importantly, the use of post-transplant CNS prophylaxis did not lower the rate.
Our study is limited by the retrospective nature of the analyses, and by the small number of post-transplant CNS relapses that precluded extensive analyses of predictors for relapse and subpopulation analyses. Additionally, complete toxicity data for patients who received CNS-directed therapy was not available, as most patients had left the transplant center prior to completion of intrathecal therapy, and toxicity, if developed, was not reported to the transplant center. However, within this incomplete reporting of CNS toxicity, 30 patients among the 217 who received post HSCT prophylaxis (14%) had reporting of toxicity ranging from post lumbar puncture headache (n=16) to white matter changes noted on MRI and symptoms of encephalopathy (n=9). There was no correlation with type of treatment or number of intrathecal treatments. It is well established that intrathecal CNS therapy can be associated with acute, subacute, and chronic neurotoxicities (19). In addition, although CNS irradiation side effects are more pronounced in children, adults with older age are susceptible to cerebral atrophy and neurocognitive deficits following CNS irradiation (20, 21). Thus, the potential for toxicity needs to be taken into account when making the decision to administer post transplant prophylaxis.
Identifying the patient population who may benefit the most from CNS prophylactic therapy may help to increase the clinical benefit and avoid unnecessary treatment and toxicity. Our results suggest that only patients with a prior history of CNS involvement may benefit from post-HSCT prophylactic CNS therapy, with a trend for less relapse. However, our findings need to be confirmed in a larger, prospective study.
Highlights.
CNS relapse after allogeneic HSCT confers a poor prognosis in ALL patients.
We studied the impact of post HSCT CNS prophylaxis in this retrospective analysis.
48% of patients received post HSCT CNS prophylaxis.
4% of patients developed CNS relapse after HSCT.
No benefit was noted for post-HSCT CNS prophylaxis to prevent CNS relapse after HSCT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008 Feb 15;111(4):1827–33. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 2.Gratwohl A, Brand R, Frassoni F, Rocha V, Niederwieser D, Reusser P, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: An EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplantation. 2005;36(9):757–69. doi: 10.1038/sj.bmt.1705140. [DOI] [PubMed] [Google Scholar]
- 3.Spyridonidis A, Labopin M, Schmid C, Volin L, Yakoub-Agha I, Stadler M, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. 2012 Jun;26(6):1211–7. doi: 10.1038/leu.2011.351. print. [DOI] [PubMed] [Google Scholar]
- 4.Poon LM, Hamdi A, Saliba R, Rondon G, Ledesma C, Kendrick M, et al. Outcomes of Adults with Acute Lymphoblastic Leukemia Relapsing after Allogeneic Hematopoietic Stem Cell Transplantation. Biology of Blood and Marrow Transplantation. 2013 Jul;19(7):1059–64. doi: 10.1016/j.bbmt.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortes J, O’Brien SM, Pierce S, Keating MJ, Freireich EJ, Kantarjian HM. The value of high-dose systemic chemotherapy and intrathecal therapy for central nervous system prophylaxis in different risk groups of adult acute lymphoblastic leukemia. Blood. 1995 Sep 15;86(6):2091–7. [PubMed] [Google Scholar]
- 6.Kantarjian HM, O’Brien S, Smith TL, Cortes J, Giles FJ, Beran M, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2000 Feb;18(3):547–61. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 7.Ganem G, Kuentz M, Bernaudin F, Gharbi A, Cordonnier C, Lemerle S, et al. Central nervous system relapses after bone marrow transplantation for acute lymphoblastic leukemia in remission. Cancer. 1989;64(9):1796–804. doi: 10.1002/1097-0142(19891101)64:9<1796::aid-cncr2820640907>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Singhal S, Powles R, Treleaven J, Horton C, Tait D, Meller S, et al. Central nervous system relapse after bone marrow transplantation for acute leukemia in first remission. Bone Marrow Transplantation. 1996;17(4):637–41. [PubMed] [Google Scholar]
- 9.Oshima K, Kanda Y, Yamashita T, Takahashi S, Mori T, Nakaseko C, et al. Central Nervous System Relapse of Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation. Biology of Blood and Marrow Transplantation. 2008 Oct;14(10):1100–7. doi: 10.1016/j.bbmt.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Thompson CB, Sanders JE, Flournoy N, Buckner CD, Thomas ED. The risks of central nervous system relapse and leukoencephalopathy in patients receiving marrow transplants for acute leukemia. Blood. 1986 Jan;67(1):195–9. [PubMed] [Google Scholar]
- 11.Moorman AV, Harrison CJ, Buck GA, Richards SM, Secker-Walker LM, Martineau M, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007 Apr 15;109(8):3189–97. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 12.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the Intensity of Conditioning Regimens: Working Definitions. Biology of Blood and Marrow Transplantation. 2009;15(12):1628–33. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabbour E, Thomas D, Cortes J, Kantarjian HM, O’Brien S. Central nervous system prophylaxis in adults with acute lymphoblastic leukemia. Cancer. 2010;116(10):2290–300. doi: 10.1002/cncr.25008. [DOI] [PubMed] [Google Scholar]
- 14.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011 Feb 10;29(5):532–43. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]
- 15.Bleyer WA, Poplack DG. Prophylaxis and treatment of leukemia in the central nervous system and other sanctuaries. Seminars in oncology. 1985 Jun;12(2):131–48. [PubMed] [Google Scholar]
- 16.Kantarjian HM, Walters RS, Smith TL, Keating MJ, Barlogie B, McCredie KB, et al. Identification of risk groups for development of central nervous system leukemia in adults with acute lymphocytic leukemia. Blood. 1988 Nov;72(5):1784–9. [PubMed] [Google Scholar]
- 17.Lazarus HM, Richards SM, Chopra R, Litzow MR, Burnett AK, Wiernik PH, et al. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis: results from the international ALL trial MRC UKALL XII/ECOG E2993. Blood. 2006 Jul 15;108(2):465–72. doi: 10.1182/blood-2005-11-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharathkumar A, DeCamillo D, Bhambhani K, Cushing B, Thomas R, Mohamed AN, et al. Children with hyperdiploid but not triple trisomy (+4,+10,+17) acute lymphoblastic leukemia have an increased incidence of extramedullary relapse on current therapies: A single institution experience. American Journal of Hematology. 2008;83(1):34–40. doi: 10.1002/ajh.21011. [DOI] [PubMed] [Google Scholar]
- 19.Vezmar S, Becker A, Bode U, Jaehde U. Biochemical and clinical aspects of methotrexate neurotoxicity. Chemotherapy. 2003 May;49(1–2):92–104. doi: 10.1159/000069773. [DOI] [PubMed] [Google Scholar]
- 20.Tucker J, Prior PF, Green CR, Ede GM, Stevenson JF, Gawler J, et al. Minimal neuropsychological sequelae following prophylactic treatment of the central nervous system in adult leukaemia and lymphoma. British journal of cancer. 1989 Nov;60(5):775–80. doi: 10.1038/bjc.1989.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laack NN, Brown PD. Cognitive sequelae of brain radiation in adults. Seminars in oncology. 2004 Oct;31(5):702–13. doi: 10.1053/j.seminoncol.2004.07.013. [DOI] [PubMed] [Google Scholar]