Abstract
Oleic acid consumption is considered cardio-protective according to studies conducted examining effects of the Mediterranean diet. However, animal models have shown that oleic acid consumption increases LDL particle cholesteryl oleate content which is associated with increased LDL-proteoglycan binding and atherosclerosis. The objective was to examine effects of varying oleic, linoleic and docosahexaenoic acid consumption on human LDL-proteoglycan binding in a non-random subset of the Canola Oil Multi-center Intervention Trial (COMIT) participants. COMIT employed a randomized, double-blind, five-period, cross-over trial design. Three of the treatment oil diets; 1) a blend of corn/safflower oil (25:75); 2) high oleic canola oil; and 3) DHA-enriched high oleic canola oil were selected for analysis of LDL-proteoglycan binding in 50 participants exhibiting good compliance. LDL particles were isolated from frozen plasma by gel filtration chromatography and LDL cholesteryl esters quantified by mass-spectrometry. LDL-proteoglycan binding was assessed using surface plasmon resonance. LDL particle cholesterol ester fatty acid composition was sensitive to the treatment fatty acid compositions, with the main fatty acids in the treatments increasing in the LDL cholesterol esters. The corn/safflower oil and high-oleic canola oil diets lowered LDL-proteoglycan binding relative to their baseline values (p=0.0005 and p=0.0012, respectively). At endpoint, high-oleic canola oil feeding resulted in lower LDL-proteoglycan binding than corn/safflower oil (p=0.0243) and DHA-enriched high oleic canola oil (p=0.0249), although high-oleic canola oil had the lowest binding at baseline (p=0.0344). Our findings suggest that high-oleic canola oil consumption in humans increases cholesteryl oleate percentage in LDL, but in a manner not associated with a rise in LDL-proteoglycan binding.
Keywords: Nutrition, fatty acids, LDL, cholesterol, oleic acid, proteoglycan
Introduction
The Mediterranean diet supplemented with olive oil or nuts has been shown to reduce the incidence of major cardiovascular events in individuals with elevated cardiovascular disease (CVD) risk1. The Mediterranean diet has also been shown to be effective in secondary prevention of coronary heart disease (CHD)2. Many key components of this diet including olive oil and nuts are rich sources of monounsaturated fatty acids (MUFAs). Epidemiological evidence and meta-analyses suggest that, compared to diets high in saturated fatty acids (SFA), diets rich in MUFA are associated with lower LDL-C concentrations and reduced relative risk of CHD3-5. Similarly, metabolic ward trials which compare diets high in SFA, MUFA and polyunsaturated fatty acid (PUFA) or carbohydrate show that high MUFA diets reduce LDL-C, without decreasing HDL-C concentrations, compared to PUFAs 6, and do so without raising triglyceride levels as is typically observed with high carbohydrate diets 7.
Oleic acid accounts for over 90% of MUFA consumed in the USA 8. Recently, in response to the need to reduce trans-fats and increase product shelf life, a shift towards high oleic acid oilseed varieties has been implemented. This shift to MUFAs has come primarily at the cost of PUFAs 9. While it is often recommended to substitute SFA with MUFAs for cardiovascular disease prevention 10, some concerns persist as to whether MUFA consumption is cardioprotective, especially when replacing PUFAs 11. Notably, this concern is based on research in animal models which shows that elevated oleic acid consumption alters hepatic lipid metabolism, enriching LDL particles with cholesteryl oleate, and promoting the development of atherosclerosis 12, 13. Mouse knockout models as well as African green and cynomolgus monkey experiments show enrichment of LDL cholesteryl oleate and decreased cholesteryl linoleate content following consumption of oleic acid rich diets14-16. Importantly, the enrichment of LDL cholesteryl oleate is also associated with the extent of atherosclerosis in these animal models which is equivalent to the levels observed when saturated fat is fed. It has been demonstrated in mice and monkeys that increased LDL cholesteryl oleate enhances arterial proteoglycan binding, increasing arterial retention of LDL and promoting atherosclerosis11, 13, 17.
Human evidence regarding MUFA consumption, cholesteryl oleate content in LDL particles, and the promotion of atherosclerosis is less clear. MUFA content of cholesterol esters (CEs) was positively associated with average carotid intima-media thickness in the Atherosclerosis Risk in Communities (ARIC) Study 18. However, in that study, SFA content of CEs was also positively associated with MUFA content of CEs and average carotid intima-media thickness. In that trial, plasma MUFA content failed to correlate with MUFA intake, but did correlate with SFA intake, suggesting that the association between MUFA CE content may have served as a surrogate of SFA intake 18.
The objective of this investigation, therefore, was to examine using a controlled feeding human intervention trial the effect of increased dietary oleic acid consumption on LDL particle core CE composition and LDL particle interactions with arterial proteoglycans. To accomplish this goal, surface plasmon resonance (SPR) technology was used to quantify LDL particle proteoglycan binding in samples from a sub-group of the Canola Oil Multi-center Intervention Trial (COMIT) 19. COMIT was a randomized controlled cross-over trial designed to evaluate the effects of five diets that provided oils/oil blends differing in fatty acid content on CVD risk factors including plasma lipids in individuals with abdominal obesity20. Three of these diets were selected to investigate the impact of varying fatty acid intakes on CE composition and LDL proteoglycan binding.
Materials and methods
Trial design
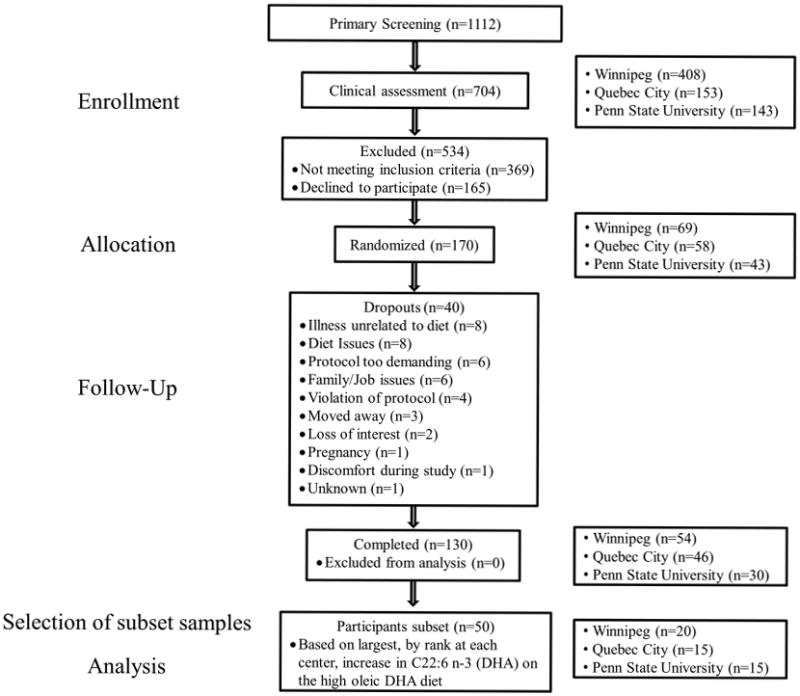
A multi center, double blind, randomized crossover controlled feeding trial was conducted at the Richardson Centre for Functional Foods and Nutraceuticals (RCFFN) (University of Manitoba), the Institute of Nutrition and Functional Foods (INAF) (Laval University), and the Department of Nutritional Sciences, Pennsylvania State University (PSU), as previously reported 19. Briefly the trial included 5 treatment periods of 4 weeks, separated by 2 to 4-week washout intervals (Figure 1). The date range for participant recruitment to last follow–up was September 20, 2010 to Dec 13, 2011 at the RCFFN, November 1st, 2010 to December 22, 2011 at INAF and November 16th, 2010 to April 12th, 2012 at PSU.
Figure 1. Canola Oil Multi-center Intervention Trial flow diagram.
Ethics Statement
Institutional ethics boards within the participating universities reviewed and approved the trial protocol. Written informed consent was obtained from participants as prescribed by institutional research ethics boards. The trial was registered with clinicaltrials.gov (NCT01351012). The registration of the clinical trial with clinicaltrials.gov (March 14, 2011) was delayed due to staff turnover and did not occur until after the enrolment of participants had begun at the RCFFN center (September 20th, 2010). The authors confirm that all ongoing and related trials for this intervention are registered.
Sample collection
Fasting blood samples were collected on days 1 and 2 (baseline) as well as 29 and 30 (endpoint) of all phases. Samples from all clinical sites were frozen then shipped to a central laboratory at St. Michaels Hospital, University of Toronto and analyzed for serum lipid parameters total cholesterol (TC), LDL-C, HDL -C, and TG. Baseline and endpoint blood lipid values were separately assayed and determined as mean sample values of day 1 and day 2, and day 29 and day 30, respectively20.
Selection of subset samples
Three of the COMIT diets, a corn and safflower oil with the highest n-6 PUFA content, high oleic canola oil, with the highest n-9 MUFA content, and a high oleic canola oil with DHA blend, with the highest long chain n-3 PUFA (Table 1), were selected to investigate the effect of these varying fatty acid intakes on CE composition and LDL proteoglycan binding. Of the total 130 participants who completed all 5 periods of the trial, a subset of 50 participants (20, 15 and 15 from RCFFN, INAF, and PSU, respectively), were assessed as good compliers to the dietary intervention and selected because they had the largest, by rank at each center, increase in C22:6 n-3 (DHA) as a percentage of plasma fatty acids at the end of DHA-enriched high oleic canola oil treatment period. These individuals' samples then underwent analysis of CE composition and LDL proteoglycan binding. Baseline characteristics of these 50 participants are shown in Table 2. A subset of 50 participants, as opposed to the full 130 participants, was selected for the LDL-biglycan binding assay as this was an exploratory analysis, the impact of dietary fatty acid modification on LDL proteoglycan binding in humans was unknown, and a limited budget was available.
Table 1. Fatty acid profile of treatments oils.
| Corn/safflower | High-oleic canola | DHA-enriched high-oleic canola | |
|---|---|---|---|
| C16:0 palmitic acid | 5.9 | 3.7 | 5.3 |
| C18:0 stearic acid | 1.9 | 1.8 | 1.7 |
| C18:1 oleic acid | 17.6 | 71.5 | 63.3 |
| C18:2 linoleic acid | 69.3 | 14.7 | 12.7 |
| C18:3 α-linolenic acid | 0.3 | 2.3 | 2.0 |
| C20:4 arachidonic acid | 0.0 | 0.0 | 0.1 |
| C20:5 EPA | 0.0 | 0.0 | 0.2 |
| C22:5 DPA | 0.0 | 0.0 | 2.4 |
| C22:6 DHA | 0.0 | 0.0 | 5.8 |
| Total saturated | 7.9 | 6.9 | 9.0 |
| Total monounsaturated | 17.7 | 73.2 | 64.7 |
| Total polyunsaturated | 69.6 | 17.0 | 23.1 |
The values are % of total fatty acids. The daily 3000 kcal diet block contained 60 g of treatment oil. The amount of treatment oil was adjusted to contribute 18% of energy for each calorie level.
Table 2. Baseline characteristics of COMIT participant subset at each center.
| RCFFN | INAF | PSU | Total | |
|---|---|---|---|---|
| Male, n | 6 | 12 | 8 | 26 |
| Female, n | 14 | 3 | 7 | 24 |
| BMI (Kg/m2) | 31.4 ± 5.9 | 29.8 ± 3.6 | 29.8 ± 3.9 | 30.4 ± 4.7 |
| Age (years) | 42.8 ± 14.6 | 49.0 ± 13.7 | 46.6 ± 9.2 | 45.8 ± 12.9 |
| TC (mmol/L) | 5.49 ± 0.98 | 5.61 ± 0.98 | 5.35 ± 0.73 | 5.49 ± 0.89 |
| HDL-C (mmol/L) | 1.23 ± 0.29 | 1.16 ± 0.36 | 1.21 ± 0.22 | 1.20 ± 0.29 |
| LDL-C (mmol/L) | 3.45 ± 0.75 | 3.48 ± 0.86 | 3.46 ± 0.65 | 3.46 ± 0.74 |
| TG (mmol/L) | 1.81 ± 1.25 | 2.14 ± 0.87 | 1.49 ± 0.57 | 1.81 ± 0.98 |
| Glucose (mmol/L) | 5.40 ± 1.07 | 5.28 ± 0.63 | 5.30 ± 0.37 | 5.33 ± 0.77 |
| Body weight (kg) | 84.3 ± 18.7 | 86.1 ± 15.8 | 91.8 ± 16.3 | 87.1 ± 17.1 |
| Waist | 98.3 ± 17.1 | 102.9 ± 8.9 | 102.0 ± 8.6 | 100.8 ± 12.7 |
| SBP (mmHg) | 125.2 ± 25.6 | 123.8 ± 14.3 | 123.3 ± 13.3 | 124.2 ± 19.1 |
| DBP (mmHg) | 82.9 ± 13.6 | 75.2 ± 11.9 | 84.0 ± 9.6 | 80.9 ± 12.4 |
TC: total cholesterol; LDL-C: low density lipoprotein cholesterol; HDL-C: high density lipoprotein cholesterol; TG: triglycerides; SBP: systolic blood pressure; DBP: diastolic blood pressure; RCFFN: Richardson Centre for Functional Foods and Nutraceuticals, Winnipeg, MB Canada; INAF: Institute of Nutrition and Functional Foods, Quebec City, QC, Canada; PSU: Pennsylvania State University, State College, PA, USA.
Plasma fatty acid composition
Plasma fatty acid concentrations were analyzed as previously reported 19. Briefly, plasma fatty acids were extracted by the Folch method 21 using chloroform-methanol with added butylated hydroxytoluene, followed by methylation with methanolic hydrochloric acid with minor modifications 22. Fatty acid methyl esters were then analyzed using gas chromatography with flame ionization detection. Heptadecanoic acid (C17:0) was used as an internal standard. Authenticated fatty acid standards were used to identify the individual fatty acids of interest. Fatty acids were reported as percentage values of total identified fatty acids measured.
LDL particle cholesteryl ester fatty acid composition
LDL particles used in the binding experiments were isolated by gel filtration chromatography using a Superose-6 10/30 column (GE Healthcare) run at a flow rate of 0.4 ml/min as previously described 16. LDL CEs were detected and quantified by mass-spectrometry as previously described16, 23. Isolated LDL was diluted in methanol with a C17:0 CE internal standard. CEs were measured using a Quattro II mass spectrometer equipped with a Z-spray interface (capillary voltage = 3.2 kV; cone voltage = 50 V; source temperature= -80°C; desolvation temperature = 200°C) in positive-ion mode, monitoring the common neutral loss of 368.5 Da. CE molar concentrations were calculated from individual profiles using the internal standard (C17:0 CE) and reported as percentages of total CE mass.
LDL proteoglycan binding
LDL-biglycan binding was quantified at Wake Forest University School of Medicine using a Biacore T100 SPR platform as previously described16. Briefly, human recombinant biglycan, a dermatan sulfate proteoglycan found in human atherosclerotic plaques24, was produced using 293-EBNA cells transfected to produce biglycan. The biglycan was immuno-bound to CM5 biosensor chips (Biacore, Inc.; Piscataway, NJ) in the sample channels of the Biacore T100, then isolated LDL samples, normalized to a cholesterol content of 10 μg/ml then added to both the sample and the reference channels. The 10 μg/ml normalization concentration was selected based on dose response data from previously run human LDL samples (Supplementary data) and the low sample volumes/concentrations and the large amount of samples. LDL-biglycan binding was corrected for nonspecific binding in the biglycan-free reference channel and is reported as the peak value for LDL binding in resonance units (RU). A representative kinetic profile of the LDL-biglycan binding assay shows rapid binding and slow dissociation (Supplementary Data). Though less than ideal based on previous studies16, 25, 26 the plasma samples used for assay of binding had necessarily been frozen, and this has the potential to modify the physical state of the cholesterol ester core or the apolipoprotein content 25, 26 of the LDL particles so that the binding may not fully reflect that of native LDL particles.
Statistical analysis
Mixed-effects repeated-measures analysis of variance were utilized for data with treatment, age and sex used as fixed effects, participant was used as a repeated factor, whereas multi-center site was added as a random effect and an autocorrelation structure of order 1 (AR1). Normality and homogeneity was checked by visual inspections of plots of residuals against fitted values. Statistical comparisons were conducted between treatment baselines and treatment endpoints. Tukey-Kramer post hoc tests were used to examine differences between treatments. Significance of changes within each treatment (baseline to endpoint) was assessed using paired t-tests. Least squares means with standard errors are presented in the text and tables. SAS version 9.2 (SAS Institute Inc., Cary, NC) and Graphpad Prism 5 (Graphpad Software Inc, San Diego, CA) were used for data analysis.
Results
Serum lipids
Changes in serum lipids in the 130 participants of COMIT for all 5 treatments have been reported20. Serum lipid profiles for the subset of participants used for LDL-biglycan binding are summarized in Table 3. Total cholesterol decreased baseline to endpoint for each treatment (p<0.05 for all). No differences were observed among treatments at baseline (p=0.1452) or endpoint (p=0.3643) for total cholesterol. LDL-C concentrations decreased from baseline to endpoint for each treatment (p<0.05 for all). LDL-C concentrations were lower at endpoint after high-oleic canola oil and corn/safflower oil treatments compared to DHA-enriched high oleic canola oil (p=0.0340 and p=0.0004, respectively). These findings were similar to those reported for the full COMIT population, except at endpoint corn/safflower oil had lower total cholesterol than DHA-enriched high oleic canola oil20.
Table 3. Baseline and endpoint serum lipid values for a subset of 50 COMIT participants across the high oleic canola, high oleic canola and DHA blend, and corn and safflower blend treatments.
| High-oleic canola oil | DHA-high-oleic canola oil | Corn/safflower oil | Diet effect | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Start | End | Start | End | Start | End | Start | End | |
| TC | 5.44 ± 0.01 | 4.91± 0.01* | 5.40 ± 0.01 | 4.95 ± 0.01* | 5.57 ± 0.01 | 4.87 ± 0.01* | p=0.1452 | p=0.3643 |
| LDL-C | 3.40 ± 0.10 | 2.92 ± 0.09a* | 3.32 ± 0.10 | 3.10 ± 0.09b* | 3.51 ± 0.10 | 2.90 ± 0.09a* | p=0.0775 | p=0.0007 |
| HDL-C | 1.22 ± 0.04 | 1.16 ± 0.04a* | 1.21 ± 0.04 | 1.25 ± 0.04b* | 1.26 ± 0.04 | 1.20 ± 0.04a* | p=0.0209 | p=0.0010 |
| TG | 1.81 ± 0.18 | 1.80 ± 0.17a | 1.92 ± 0.18 | 1.31 ± 0.17b* | 1.76 ± 0.18 | 1.68 ± 0.17a | p=0.0674 | p<0.0001 |
TC= total cholesterol, LDL-C =low density lipoprotein cholesterol, HDL-C = high density lipoprotein cholesterol, TG= triglycerides. All values in mmol/L. The values are given as least squares mean ± SE for 50 individuals. Mixed-effects repeated-measures analysis of variance with treatment, age, sex as fixed effect, center as a random effect, and the measures for each participant repeated by period were used for the data analysis with Tukey-Kramer adjustment for multiple comparisons.
Mean values with different superscript letters across diets denote significant differences at p<0.05.
denotes significant change from start value of same diet.
Plasma fatty acid composition
Total plasma fatty acid profiles are summarized in Table 4. Changes in plasma fatty acids reflected their dietary intake after 28-d of treatment. Participants on the high-oleic canola oil diet showed the highest level of MUFA (p=0.05), consistent with the higher C18:1 n-9 content compared with other treatments. Corn/safflower oil elevated the n-6 PUFA content compared to high-oleic canola oil and DHA-enriched high oleic canola oil (p<0.05), due to higher levels of C18:2 n-6 in the test diet. DHA-enriched high oleic canola oil produced higher levels of C20:5 n-3 compared to the other treatments (p<0.05).
Table 4. Plasma fatty acid profile of participants at the end of each dietary phase in 50 COMIT participants.
| High-oleic canola | DHA-high-oleic canola | Corn/safflower | Diet effect | |
|---|---|---|---|---|
| C14:0 | 0.77±0.12 | 0.68±0.12 | 0.66±0.12 | p=0.2644 |
| C14:1 n-5 | 0.08 ±0.03 | 0.08±0.03 | 0.07±0.03 | p=0.9758 |
| C16:0 | 27.21±0.20a | 27.88±0.20b | 27.35±0.20a | p=0.0012 |
| C16:1 n-7 | 1.08±0. 13a | 0.86±0.13b | 0.97±0.11c | p<0.0001 |
| C18:0 | 12.02±0.17a | 12.61±0.17 | 12.63±0.17b | p<0.0001 |
| C18:1 n-9 | 15.63 ±0.9a | 13.44±0.9b | 11.48±0.9c | p<0.0001 |
| C18:2 n-6 | 2l.60±0.34a | 18.27±0.34b | 26.41±0.34c | p<0.0001 |
| c18:3 n-6 | 0.18±0.01a | 0.10±0.01b | 0.19±0.01a | p<0.0001 |
| c18:3 n-3 | 0.62±0.04a | 0.54±0.04b | 0.50±0.04b | p<0.0001 |
| c20:0 | 0.44±0.05ab | 0.46±0.05a | 0.41±0.05b | p<0.0001 |
| c20:1 n-9 | 0.31±0.02a | 0.24±0.02b | 0.21±0.02b | p<0.0001 |
| c20:2 n-6 | 0.35±0.05a | 0.19±0.05b | 0. 33±0.05ab | p=0.0290 |
| c20:3 n-6 | 2.72 ±0. 22a | 1.83±0.22b | 2.43±0.22c | p<0.0001 |
| c20:4 n-6 | 9.38 ±0.42 | 9.50±0.42 | 9.38±0.42 | p=0.7846 |
| c20:5 n-3 | 0.86±0.08a | 1.61±0.08b | 0.47±0.08c | p<0.0001 |
| c22:0 | 0.88±0.13a | 1.00±0.13b | 0.91±0.13a | p=0.0040 |
| c22:4 n-6 | 0.32±0.04 | 0.21±0.04 | 0.33±0.04 | p=0.0283 |
| c22:5 n-3 | 0.±0.03a | 0.36±0.03b | 0.63±0.03c | p<0.0001 |
| c22:6 n-3 | 2.75±0.15a | 7.89±0.15b | 2.67±0.15a | p<0.0001 |
| c24:0 | 0.62±0.07a | 0.71±0.07b | 0.67±0.07ab | p=0.0198 |
| c24:1 n-9 | 1.39±0.09a | 1.48±0.09b | 1.24±0.09a | p<0.0001 |
|
| ||||
| Total SFA | 41.91±0.23a | 43.32±0.23b | 42.61±0.23c | p<0.0001 |
| Total MUFA | 18.48±0.99a | 16.09±0.99 | 13.97±0.99c | p<0.0001 |
| Total PUFA | 39.59±0.90a | 40.57±0.90b | 43.40±0.90c | p<0.0001 |
The values are % abundance of each fatty acid to total fatty acids given as least squares mean ± SE for 50 individuals. Mixed-effects repeated-measures analysis of variance with treatment, age, sex as fixed effect, center as a random effect, and the measures for each participant repeated by period were used for the data analysis with Tukey-Kramer adjustment for multiple comparisons.
Mean values with different superscript letters across rows denote significant differences at p<0.05
LDL cholesteryl ester fatty acid composition
Individual CE compositions of LDL expressed as percentages based on FA components are shown in Table 5. Dietary treatment altered the LDL particle core CE FA profiles in a manner consistent with the dietary FA profile. Baseline LDL CE compositions differed across treatments. At baseline the high-oleic canola oil diet had higher LDL CE C16:0 than corn/safflower oil (p=0.0014) and DHA-enriched high oleic canola oil (p<0.0001); LDL CE C16:1 (p=0.0084) and C18:2 (p=0.047) was higher for high-oleic canola oil than corn/safflower oil; LDL CE C20:4 was higher in high-oleic canola with DHA oil, than high-oleic canola oil (p=0.0006) or corn/safflower oil (p=0.018); and LDL CE C18:3 was higher (p=0.0126) in DHA-enriched high oleic canola oil than corn/safflower oil. Consumption of the corn/safflower oil diet increased the C18:2 CE (p<0.0001) content, primarily at the expense of C16:1, C18:1 CE,(p<0.0001 for both), but also C18:3 (p<0.0001); C20:5 (p<0.0001); C22:6 (p=0.0001) and saturated FA (C16:0,p<0.0001; C18:0, p=0.0001) CE percentage. The high-oleic canola oil diet increased C18:1 in CE (p<0.0001) percentage, while decreasing the C16:1 (p<0.0001), C18:0 (p=0.0049) and C16:0 (p<0.0001) CE percentage. The DHA-enriched high oleic canola oil increased C18:1 (p=0.0015), C20:4 (p=0.0007), C20:5 (p<0.0001) and C22:6 (p<0.0001) CE percentage, primarily at the expense of C18:3 (p<0.0001) in CE.
Table 5. Individual cholesteryl esters of LDL for a subset of 50 COMIT participants.
| Baseline Cholesteryl Ester Fatty Acid by weight % | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| C16:0 | C18:0 | C16:1 | C18:1 | C18:2 | C20:4 | C18:3 | C20:5 | C22:6 | |
| Corn/safflower | 11.8 (0.7)a | 1.2 (0.1) | 2.8 (0.2)a | 20.2 (1.2) | 52.5 (1.7)a | 8.1 (0.3)a | 2.0 (0.1)a | 1.2 (0.2) | 0.5 (0.1) |
| High-oleic canola | 12.8 (0.7)b | 1.2 (0.1) | 3.1 (0.2)b | 20.4 (1.2) | 51.0 (1.7)b | 7.8 (0.3)a | 2.2 (0.1)ab | 1.1 (0.2) | 0.4 (0.1) |
| DHA-high-oleic canola | 11.8 (0.7)a | 1.2 (0.1) | 2.7 (0.2)ab | 20.3 (1.2) | 51.5 (1.7)ab | 8.6 (0.3)b | 2.2 (0.1)b | 1.3 (0.2) | 0.5 (0.1) |
|
| |||||||||
| Baseline | p=0.0001 | p=0.5527 | p=0.0233 | p=0.7592 | p=0.0048 | p=0.0014 | p=0.0149 | p=0.1324 | p=0.2704 |
|
| |||||||||
| Endpoint Cholesteryl Ester Fatty Acid by weight % | |||||||||
|
|
|||||||||
| C16:0 | C18:0 | C16:1 | C18:1 | C18:2 | C20:4 | C18:3 | C20:5 | C22:6 | |
|
| |||||||||
| Corn/safflower | 10.3 (0.6)a* | 0.9 (0.1)* | 1.6 (0.3)a* | 14.6 (1.0)a* | 62.1 (1.2)a* | 8.0 (0.2)a | 1.6 (0.1)a* | 0.5 (0.1)a* | 0.4 (0.1)a* |
| High-oleic canola | 11.8 (0.6)b* | 0.9 (0.1)* | 2.0 (0.3)b* | 22.2(1.0)b* | 51.7 (1.2)b | 8.0 (0.2)a | 2.0 (0.1)b | 1.0 (0.1)b | 0.4 (0.1)a |
| DHA-high-oleic canola | 11.8 (0.6)b | 1.0 (0.1)* | 1.8 (0.3)ab* | 21.5(1.0)b* | 50.1 (1.2)c* | 9.4 (0.2)b* | 1.5 (0.1)a* | 1.9 (0.1)c* | 1.2 (0.1)b* |
|
| |||||||||
| Endpoint | p<0.0001 | p=0.6096 | p=0.0011 | p<0.0001 | p<0.0001 | p<0.0001 | p<0.0001 | p<0.0001 | p<0.0001 |
Cholesteryl esters are expressed as a percentage of the total CE of the LDL as determined by mass spectrometry. All values represent least squares means (±SEM) of n=50 participants for each diet. Different superscripts indicate differences across diets calculated by mixed-effects repeated-measures analysis of variance with treatment, age, sex as fixed effect, center as a random effect, and the measures for each participant repeated by period were used for the data analysis with Tukey-Kramer adjustment for multiple comparisons.
indicates endpoint value is significantly different from baseline, tested by paired t-test (p<0.05).
LDL binding to biglycan as a function of diet
Mean LDL-biglycan binding values for baseline and endpoint of each diet are presented in Figure 1. At baseline, LDL-biglycan binding was lower (p=0.0344) before the high-oleic canola oil compared to the corn/safflower oil diet. Both the corn/safflower oil and high-oleic canola oil diets lowered LDL-biglycan binding relative to their baseline values (p=0.0005 and p=0.0012, respectively), whereas the DHA-enriched high oleic canola oil diet failed to produce a change in binding (p=0.5619). At endpoint, high-oleic canola oil exhibited lower LDL-biglycan binding than both corn/safflower oil (p=0.0243) and DHA-enriched high oleic canola oil (p=0.0249) diets. Linear regression enable investigation of the relationship between LDL-biglycan binding and LDL CE fatty acid composition (Figure 2). Results revealed an inverse relationship between percentage C18:1 CE in LDL and LDL-biglycan binding at baseline and endpoint of the high-oleic canola oil diet (r2=0.1093, p=0.0190 and r2=0.1027, p=0.0232. for baseline and endpoint, respectively, n=50 for both). This relationship was not seen in DHA-enriched high oleic canola oil or corn/safflower oil diets.
Figure 2. Peak biglycan binding responses of LDL particles.
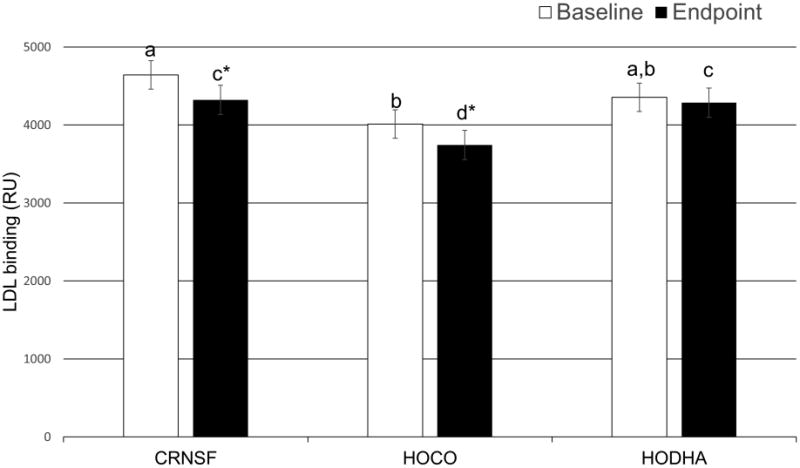
n=50 for each bar. Bars with different letters designate differences across diets at that time (p<0.05) calculated by mixed-effects repeated-measures analysis of variance with treatment, age, sex as fixed effect, center as a random effect, and the measures for each participant repeated by period were used for the data analysis with Tukey-Kramer adjustment for multiple comparisons. * indicates a differences from baseline of same diet using paired t-test (p<0.05). CRNSF = corn and safflower oil blend, = high oleic canola oil, HODHA= high oleic canola oil with DHA.
Discussion
The principal finding of this trial is that diet modification, through the consumption of high oleic canola oil, led to the highest percentage of C18:1 CE in LDL particles, but did not increase LDL binding affinity to biglycan, measured using established methods. In contrast consumption of high oleic canola oil slightly decreased LDL-biglycan binding compared to baseline, as did corn/safflower oil consumption. These results indicate that enrichment of C18:1 in LDL CEs may result in a reduced residence time of LDL particles in the arterial wall, which could potentially decrease the development of atherosclerosis.
The present results contrast those of Melchior et al.16 who investigated the effects of dietary fatty acid modifications on LDL-biglycan binding in apoB-100-only Ldlr -/- mice. In the work by Melchior et al., increasing levels of C18:1 CE in LDL particles was consistently associated with increased LDL-biglycan binding affinity. The amount of biglycan binding was also shown to be proportional to the development of atherosclerosis in the apoB-100-only Ldlr -/- mice. Similar results to those of Melchior et al. have also been seen in other rodent and primate models, which have repeatedly demonstrated that consumption of MUFAs and SFA, as opposed to n-3 or n-6 PUFA, results in the production of LDL particles with an increased affinity for proteoglycans 17, 27, 28. These animal trials showed associations between the content of cholesteryl oleate in the CE of the LDL particle core with the amount of LDL proteoglycan binding, both of which were also associated with the amount of atherosclerosis present. Unlike the COMIT, the diets used in these animal trials were supplemented with dietary cholesterol to model hypercholesterolemia, but similar to the COMIT diets the oleic acid sources in the animal trials were from vegetable oils.
The binding data obtained in the COMIT samples may not be the entirely representative of LDL particles in their native state because the samples were frozen prior to analysis, and the changes in binding seen were small and may not reflect the maximal effects possible. In addition, the rationale for using LDL isolated by gel filtration chromatography has been to avoid disruption of the minor apolipoproteins associated with LDL particles 16. These components may differ between the current trial participants and the animal models studied to date, where the apoE/apoB ratio was found to be a major determinant of binding17, 28.
The evidence linking oleic acid intake to CVD in humans is often seen in instances where animal fat was the source of oleic acid. For instance 11 cohort studies that contained dietary intake and coronary event reporting were analyzed by Jakobsen et al.29 to investigate the effects of different types of dietary fatty acid on coronary heart disease (CHD) risk. The combined cohort contained 344,696 participants, 5249 coronary events and 2155 coronary deaths occurred during a 4-10 year follow up. In this combined cohort a theoretical shift of 5% of energy from SFA was to MUFA and an increase in coronary event (hazard ratio (95% CI) = 1.19 (1.00, 1.42)), but not coronary death (hazard ratio (95% CI) = 1.01 (0.73, 1.41)) risk was reported. However, the main source of MUFA in this combined cohort was animal fat, which contrasts with the use of vegetable oils used in the present trial. In addition, the authors noted that some vegetable sources of MUFA likely contained trans fatty acids29.
Furthermore, associations between MUFA and CVD risk are complicated by the fact that MUFAs can be synthesized de novo from SFA, so differences in concentrations between individuals may also reflect stearoyl-CoA desaturase (SCD) activity, and not just dietary intake. In this regard, elevated SCD activity, measured by desaturase index using a product-to-precursor fatty acid ratios, was shown to predict cardiovascular mortality in the Uppsala Longitudinal Study of Adult Men population-based cohort study30. In that study, elevated LDL cholesteryl oleate was associated with increased cardiovascular disease and total mortality.
Serum and LDL-CE fatty acid composition data at the end of the treatment phases were responsive to the dietary interventions, with the main component fatty acids of each treatment increasing in response to consumption, suggesting that participants had good compliance to the diets. C18:2 was the major fatty acid in LDL CE at baseline and endpoint at each treatment. This was expected as the majority of the CE in human plasma is derived from the enzyme lecithin:cholesterol acyltransferase (LCAT) and linoleic acid is the predominant fatty acid in the sn2 position of the phosphatidylcholine that serves as the LCAT substrate. Accordingly C18:2 is typically the main fatty acid seen in CE in humans 31 and is very responsive to dietary linoleic acid showing a large increase during the linoleic acid rich corn/safflower oil treatment. C16:0 was a minor fatty acid, and C20:4 was absent from all the dietary treatments, however, these were higher in plasma and LDL CE due to lipogenesis of C16:0 and elongation from C18:2 to C20:4. While very little C20:5 n-3 was present in the DHA-enriched high oleic canola oil diet, C20:5 n-3 was elevated in plasma and in LDL CE at endpoint of the DHA-enriched high oleic canola oil diet possibly due to retro-conversion of C22:6 n-3 32, 33.
All three diets reduced total and LDL-C from baseline, however, high-oleic canola oil and corn/safflower oil feeding produced lower LDL-C compared with high-oleic canola with DHA oil, while DHA-enriched high oleic canola oil produced lower TG levels. These results are consistent with previous clinical trials which consistently show hypocholesterolemic effects of both PUFA or MUFA consumption 34, and TG lowering by C22:6 n-3 consumption 35. The effect of the test diets on LDL-binding differed compared to their effects on the Framingham 10-year CHD scores20. The DHA-enriched high oleic canola oil diet showed a greater reduction in CHD score than both the corn/safflower oil and high-oleic canola oil diets. This discrepancy may be due to the mechanisms underlying the cardio-protective effects of DHA consumption being independent of LDL-proteoglycan interactions36.
The SPR method used to assess LDL-biglycan binding provides a measurements of the extent of formation and dissociation of LDL-biglycan complexes, which is comparable to solid-phase LDL-biglycan binding measurements37. Gel filtration chromatography was used to isolate LDL for the SPR binding assays in an attempt to prevent modifications that might occur with ultracentrifugation. Only a small center portion of the LDL peak was selected for the binding assays. The entire LDL peak has been used for the compositional analysis of CE fatty acid content. This approach introduces the possibility that there were differences in composition in the LDL particles used in the binding assay and those used to measure composition 16.
The higher level of oleic acid present in the LDL CE at baseline of all treatments suggests that participants in this trial were likely already consuming high amounts of oleic acid both at baseline and during the washout periods 18, 31. However, this is not surprising as most saturated fat sources contain comparable amounts of MUFA, primarily oleic acid, and many vegetable oils, such as safflower, sunflower, and canola have been modified to high oleic varieties 9. This high baseline oleic acid content could have reduced the impact of the high-oleic canola oil and DHA-enriched high oleic canola oil diets on the LDL CE composition, while increasing the impact of the corn/safflower oil diet. The effect of this high baseline cholesteryl oleate percentage on the changes in LDL-biglycan binding induced by the treatment diets is unknown. However, a weak negative association between cholesteryl oleate percentage in LDL and LDL-biglycan binding was seen when all the baseline samples were pooled, which also was seen when all endpoint samples were pooled. These results suggest that, at least in our trial population, the presence of cholesteryl oleate in LDL particles appeared to be associated with slightly reduced LDL interactions with proteoglycans, which would be assumed to be cardio-protective.
Strengths of this trial include the controlled feeding, randomized crossover design which minimizes the potential impact of confounding factors when each participant acts as their own control. Participants were provided all meals, with one meal a day eaten under supervision for the majority of days. Good compliance was achieved as assessed from plasma fatty acid and LDL CE fatty acid composition responses which reflected each dietary treatment. A limitation of this trial is that the binding assays were necessarily conducted on LDL particles isolated from previously frozen plasma. Accordingly, we cannot be certain that the freezing and subsequent thawing may not have altered LDL particle structure, which in turn may have influenced the LDL proteoglycan interactions. Freezing and subsequent thawing of isolated LDL particles has been shown to alter LDL structural and cell-binding characteristics26. However, whatever the effect of freezing may have had, it would presumably impact all the dietary treatments in a similar manner. It is also relevant to underscore that binding curves generated by the samples were of high quality. Another limitation of this trial is the selection of a subset of 50 participants, rather than the complete 130 participants. The selection criteria, based on the increase in DHA in plasma fatty acids, was used as a measure of trial compliance, however, this selection criteria may also have selected for some unknown factors which may have impacted the results.
Conclusions
These findings suggest that high oleic acid canola oil consumption for 4 weeks led to a small enrichment of LDL CE with oleic acid, but this was not associated with increased LDL-biglycan binding, as has been reported with cholesterol oleate enrichment of LDL within rodent and primate models. In fact, both corn/safflower oil and high-oleic canola oil diets appeared to lead to small reductions in LDL-biglycan binding. Our trial provides evidence that diets high in oleic acid from canola may have comparable effects to diets rich in linoleic acid, from corn and safflower, on LDL-biglycan binding over a 4 week period in humans. The data support evidence from prospective clinical trials such as the PREDIMED trial1, that diets rich in oleic acid from vegetable oils have benefits for cardiovascular health. It is unknown if longer feeding of the diets as in this trial would show similar effects to the longer feeding studies of the experimental animals.
Supplementary Material
Figure 3. LDL cholesteryl oleate and its relationship to LDL-biglycan binding.
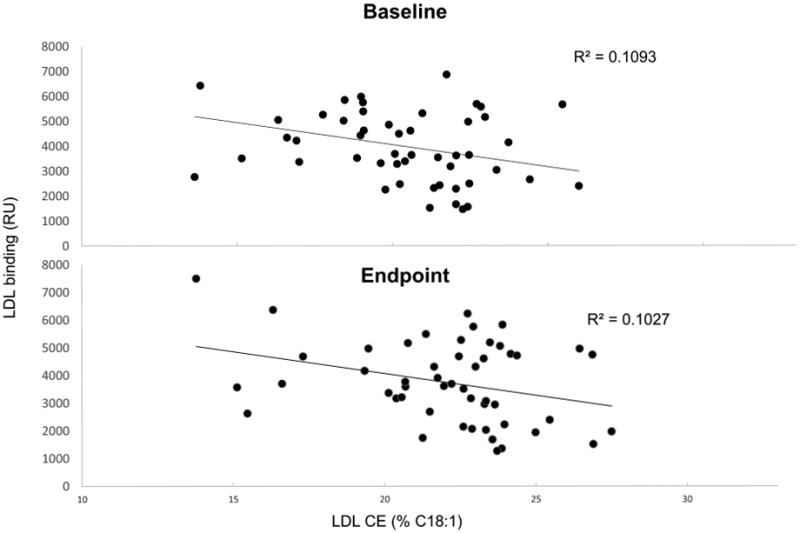
Regression analysis comparing LDL binding to immobilized BGN with the percentage of cholesteryl oleate in the LDL particles in the high oleic canola oil diet (n=50).
Highlights.
This trial found that, in subset of participants from a randomized controlled trial, consumption of high oleic acid canola oil for 4 weeks led to a small enrichment of oleic acid in LDL cholesteryl esters. This enrichment was not associated with increased LDL-biglycan binding. In fact, both the high oleic acid canola oil diet, and a diet high in linoleic acid containing a blend of corn/safflower oil (25:75), led to small reductions in LDL-biglycan binding following 4 weeks of consumption. These results do not agree with results from animal trials in which increases in LDL particle cholesteryl oleate content were associated with increased LDL-biglycan binding and the development of atherosclerosis. The trial data supports other clinical evidence that diets rich in oleic acid from vegetable oils have benefits for cardiovascular health.
Acknowledgments
Sources of funding: Supported by grants from Canola Council of Canada, Flax Council of Canada, Agriculture and Agri Food Canada, Dow Agrosciences and Western Grains Research Foundation and Canada Research Chairs Program collectively provided funding for this trial through the Growing Forward program of Agriculture and Agri Food Canada. Also supported by NIH grant HL 49373 and National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000127. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Peter Jones reported receiving grants from Advanced Foods and Materials Network (AFM Net), Danone, Enzymotec, Unilever, the Canadian Institutes of Health Research (CIHR) and Canada Research Chair Endowment (CRCE) of the Federal Government of Canada. Dr. Jones also serves as President of Nutritional Fundamentals for Health Inc, which markets plant sterols among other nutraceuticals.
Drs. Lamarche and Couture have received research grants from the Dairy Farmers of Canada, Dairy Australia. Dr. Lamarche has received research funding from the Danone Institute and Atrium Innovations, and honoraria from Unilever, Danone, and the Dairy Farmers of Canada. Dr. Lamarche is Chair in Nutrition and Cardiovascular Health, supported in part by Provigo/Loblaws.
Dr. Jenkins reported serving on the Scientific Advisory Board of Unilever, Sanitarium Company, California Strawberry Commission, Loblaw Supermarket, Herbal Life International, Nutritional Fundamental for Health, Pacific Health Laboratories, Metagenics, Bayer Consumer Care, Orafti, Dean Foods, Kellogg's, Quaker Oats, Procter & Gamble, Coca-Cola, NuVal Griffin Hospital, Abbott, Pulse Canada, Saskatchewan Pulse Growers, and Canola Council of Canada; receiving honoraria for scientific advice from the Almond Board of California, International Tree Nut Council Nutrition Research and Education Foundation, Barilla, Unilever Canada, Solae, Oldways, Kellogg's, Quaker Oats, Procter & Gamble, Coca-Cola, NuVal Griffin Hospital, Abbott, Canola Council of Canada, Dean Foods, California Strawberry Commission, Haine Celestial, and Alpro Foundation; being on the speakers panel for the Almond Board of California; receiving research grants from Loblaw Brands Ltd, Unilever, Barilla, Almond Board of California, Solae, Haine Celestial, Sanitarium Company, Orafti, International Tree Nut Council, and Peanut Institute; and receiving travel support to meetings from the Almond Board of California, Unilever, Alpro Foundation, and International Tree Nut Council, Canadian Institutes for Health Research, Canada Foundation for Innovation, Ontario Research Fund. Dr. Jenkins receives salary support as a Canada Research Chair from the federal government of Canada. Dr. Jenkins' wife is a director of Glycemic Index Laboratories, Toronto, Ontario, Canada.
Dr. West has received research funding and consulting fees from the Canola Council of Canada and Flax Canada 2013.
Dr. Kris-Etherton serves on the Unilever Scientific Advisory Board.
Abbreviations
- DHA
docosahexaenoic acid
- CE
cholesteryl ester
- MUFA
monounsaturated fatty acid
- PUFA
polyunsaturated fatty acid
Footnotes
Disclosures: Other authors did not report anything to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. The New England journal of medicine. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 2.de Lorgeril M, Renaud S, Mamelle N, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343:1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 3.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arteriosclerosis and thrombosis: a journal of vascular biology/American Heart Association. 1992;12:911–919. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- 4.Gardner CD, Kraemer HC. Monounsaturated versus polyunsaturated dietary fat and serum lipids. A meta-analysis. Arterioscler Thromb Vasc Biol. 1995;15:1917–1927. doi: 10.1161/01.atv.15.11.1917. [DOI] [PubMed] [Google Scholar]
- 5.Artaud-Wild SM, Connor SL, Sexton G, et al. Differences in coronary mortality can be explained by differences in cholesterol and saturated fat intakes in 40 countries but not in France and Finland. A paradox. Circulation. 1993;88:2771–2779. doi: 10.1161/01.cir.88.6.2771. [DOI] [PubMed] [Google Scholar]
- 6.Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J Lipid Res. 1985;26:194–202. [PubMed] [Google Scholar]
- 7.Grundy SM. Comparison of monounsaturated fatty acids and carbohydrates for lowering plasma cholesterol. N Engl J Med. 1986;314:745–748. doi: 10.1056/NEJM198603203141204. [DOI] [PubMed] [Google Scholar]
- 8.US Department of Agriculture. Nutrient Intakes from Food: Mean Amounts Consumed per Individual, by Gender and Age. What We Eat in America. 2010:2007–2008. NHANES. [Google Scholar]
- 9.Rudel LL. Letter to the editor. Journal of clinical lipidology. 2011;5:500. doi: 10.1016/j.jacl.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 11.Degirolamo C, Rudel LL. Dietary monounsaturated fatty acids appear not to provide cardioprotection. Curr Atheroscler Rep. 2010;12:391–396. doi: 10.1007/s11883-010-0133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lada AT, Rudel LL. Associations of low density lipoprotein particle composition with atherogenicity. Current Opinion in Lipidology. 2004;15:19–24. doi: 10.1097/00041433-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Degirolamo C, Shelness GS, Rudel LL. LDL cholesteryl oleate as a predictor for atherosclerosis: evidence from human and animal studies on dietary fat. Journal of Lipid Research. 2009;50:S434–S439. doi: 10.1194/jlr.R800076-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudel LL, Johnson FL, Sawyer JK, et al. Dietary polyunsaturated fat modifies low-density lipoproteins and reduces atherosclerosis of nonhuman primates with high and low diet responsiveness. Am J Clin Nutr. 1995;62:463S–470S. doi: 10.1093/ajcn/62.2.463S. [DOI] [PubMed] [Google Scholar]
- 15.Rudel LL, Haines J, Sawyer JK, et al. Hepatic origin of cholesteryl oleate in coronary artery atherosclerosis in African green monkeys. Enrichment by dietary monounsaturated fat. J Clin Invest. 1997;100:74–83. doi: 10.1172/JCI119524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melchior JT, Sawyer JK, Kelley KL, et al. LDL particle core enrichment in cholesteryl oleate increases proteoglycan binding and promotes atherosclerosis. J Lipid Res. 2013;54:2495–2503. doi: 10.1194/jlr.M039644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manning JM, Gebre AK, Edwards IJ, et al. Dietary polyunsaturated fat decreases interaction between low density lipoproteins and arterial proteoglycans, Lipids. 1994;29:635–641. doi: 10.1007/BF02536098. [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Folsom AR, Lewis L, et al. Relation of plasma phospholipid and cholesterol ester fatty acid composition to carotid artery intima-media thickness: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 1997;65:551–559. doi: 10.1093/ajcn/65.2.551. [DOI] [PubMed] [Google Scholar]
- 19.Senanayake VK, Pu S, Jenkins DA, et al. Plasma fatty acid changes following consumption of dietary oils containing n-3, n-6, and n-9 fatty acids at different proportions: preliminary findings of the Canola Oil Multicenter Intervention Trial (COMIT), Trials. 2014;15:136. doi: 10.1186/1745-6215-15-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones PJ, Senanayake VK, Pu S, et al. DHA-enriched high-oleic acid canola oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am J Clin Nutr. 2014 doi: 10.3945/ajcn.113.081133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 22.Gillingham LG, Gustafson JA, Han SY, et al. High-oleic rapeseed (canola) and flaxseed oils modulate serum lipids and inflammatory biomarkers in hypercholesterolaemic subjects. British Journal of Nutrition. 2011;105:417–427. doi: 10.1017/S0007114510003697. [DOI] [PubMed] [Google Scholar]
- 23.Miller CD, Thomas MJ, Hiestand B, et al. Cholesteryl esters associated with acyl-CoA:cholesterol acyltransferase predict coronary artery disease in patients with symptoms of acute coronary syndrome. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 2012;19:673–682. doi: 10.1111/j.1553-2712.2012.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riessen R, Isner JM, Blessing E, et al. Regional differences in the distribution of the proteoglycans biglycan and decorin in the extracellular matrix of atherosclerotic and restenotic human coronary arteries. The American journal of pathology. 1994;144:962–974. [PMC free article] [PubMed] [Google Scholar]
- 25.Cohn JS, Rodriguez C, Jacques H, et al. Storage of human plasma samples leads to alterations in the lipoprotein distribution of apoC-III and apoE. J Lipid Res. 2004;45:1572–1579. doi: 10.1194/jlr.D300041-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Rumsey SC, Galeano NF, Arad Y, et al. Cryopreservation with sucrose maintains normal physical and biological properties of human plasma low density lipoproteins. Journal of lipid research. 1992;33:1551–1561. [PubMed] [Google Scholar]
- 27.Edwards IJ, Gebre AK, Wagner WD, et al. Reduced proteoglycan binding of low density lipoproteins from monkeys (Macaca fascicularis) fed a fish oil versus lard diet. Arteriosclerosis and thrombosis: a journal of vascular biology/American Heart Association. 1991;11:1778–1785. doi: 10.1161/01.atv.11.6.1778. [DOI] [PubMed] [Google Scholar]
- 28.Parks JS, Gebre AK, Edwards IJ, et al. Role of LDL subfraction heterogeneity in the reduced binding of low density lipoproteins to arterial proteoglycans in cynomolgus monkeys fed a fish oil diet. J Lipid Res. 1991;32:2001–2008. [PubMed] [Google Scholar]
- 29.Jakobsen MU, O'Reilly EJ, Heitmann BL, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. The American journal of clinical nutrition. 2009;89:1425–1432. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warensjo E, Sundstrom J, Vessby B, et al. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr. 2008;88:203–209. doi: 10.1093/ajcn/88.1.203. [DOI] [PubMed] [Google Scholar]
- 31.Zock PL, Mensink RP, Harryvan J, et al. Fatty acids in serum cholesteryl esters as quantitative biomarkers of dietary intake in humans. American journal of epidemiology. 1997;145:1114–1122. doi: 10.1093/oxfordjournals.aje.a009074. [DOI] [PubMed] [Google Scholar]
- 32.Grimsgaard S, Bonaa KH, Hansen JB, et al. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. The American journal of clinical nutrition. 1997;66:649–659. doi: 10.1093/ajcn/66.3.649. [DOI] [PubMed] [Google Scholar]
- 33.Conquer JA, Holub BJ. Dietary docosahexaenoic acid as a source of eicosapentaenoic acid in vegetarians and omnivores. Lipids. 1997;32:341–345. doi: 10.1007/s11745-997-0043-y. [DOI] [PubMed] [Google Scholar]
- 34.Mensink RP, Katan MB. Effect of a diet enriched with monounsaturated or polyunsaturated fatty acids on levels of low-density and high-density lipoprotein cholesterol in healthy women and men. N Engl J Med. 1989;321:436–441. doi: 10.1056/NEJM198908173210705. [DOI] [PubMed] [Google Scholar]
- 35.Geppert J, Kraft V, Demmelmair H, et al. Microalgal docosahexaenoic acid decreases plasma triacylglycerol in normolipidaemic vegetarians: a randomised trial. Br J Nutr. 2006;95:779–786. doi: 10.1079/bjn20051720. [DOI] [PubMed] [Google Scholar]
- 36.Mozaffarian D, Wu JH. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? The Journal of nutrition. 2012;142:614S–625S. doi: 10.3945/jn.111.149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skalen K, Gustafsson M, Rydberg EK, et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–754. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


