Abstract
Lipid rafts are putative complexes of lipids and proteins in cellular membranes that are proposed to function in trafficking and signalling events. CTxB (cholera toxin B-subunit) has emerged as one of the most studied examples of a raft-associated protein. Consisting of the membrane-binding domain of cholera toxin, CTxB binds up to five copies of its lipid receptor on the plasma membrane of the host cell. This multivalency of binding gives the toxin the ability to reorganize underlying membrane structure by cross-linking otherwise small and transient lipid rafts. CTxB thus serves as a useful model for understanding the properties and functions of protein-stabilized domains. In the present chapter, we summarize current evidence that CTxB associates with and cross-links lipid rafts, discuss how CTxB binding modulates the architecture and dynamics of membrane domains, and describe the functional consequences of this cross-linking behaviour on toxin uptake into cells via endocytosis.
Keywords: cholera toxin, diffusion, endocytosis, giant plasma membrane vesicle, glycosphingolipid, lipid raft, membrane curvature, membrane domain
Introduction
The basic lipid raft model defines rafts as nanoscale, fluctuating lateral assemblies of proteins and lipids in cellular membranes generated in part through lipid–lipid interactions that lead to the formation of Lo (liquid ordered) domains [1]. Although rafts have long been proposed to regulate a range of biological processes including endocytosis and cell signalling, an ongoing challenge in the field has been to develop a fundamental understanding of the properties of these domains and how they might function at the mechanistic level.
Multiple lines of evidence have identified CTxB (cholera toxin B-subunit), the membrane-binding subunit of cholera toxin, as a bona fide marker of lipid rafts and subsequently it has become a commonly used tool for raft research. Produced by the Gram-negative bacterium Vibrio cholerae, cholera toxin consists of an enzymatically active A subunit and a homopentameric membrane-binding B subunit, CTxB (Figure 1). CTxB targets the toxin to host cells by binding with high avidity up to five GM1 gangliosides, themselves raft components, in the plasma membrane [2]. Importantly, CTxB is non-toxic and commercially available, and the receptor for CTxB is widely expressed in the plasma membrane of many cell types. As a result, CTxB can easily be added exogenously to cells or model membranes containing its lipid receptor, and when appropriately labelled, can be directly visualized by fluorescence microscopy. Because CTxB binds to multiple copies of its lipid receptor, it has the capacity to locally remodel underlying membrane structure. In this chapter, we summarize evidence that CTxB associates with and cross-links lipid rafts, discuss how CTxB binding modulates the architecture and dynamics of membrane domains, and describe the functional consequences of this cross-linking behaviour on toxin entry into cells via endocytosis.
Figure 1. Structure of cholera toxin.
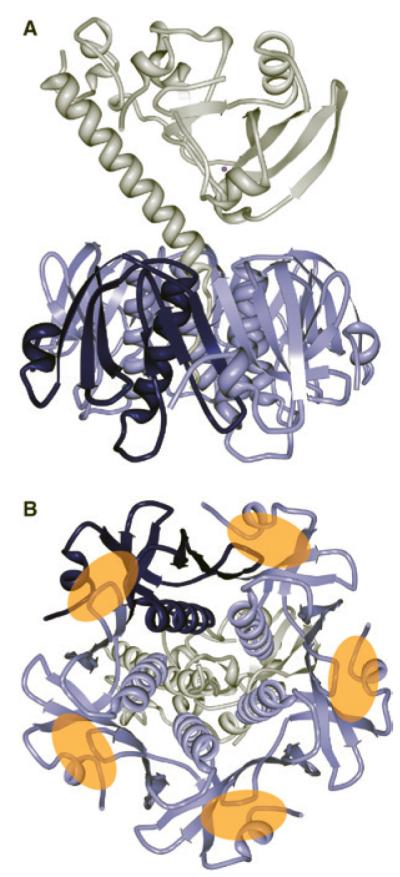
(A) Ribbon diagram of the molecular structure of cholera holotoxin with the A subunit in white. The homopentamic B subunit is labelled in blues, with one of the five proteins that make up the B subunit highlighted in dark blue. (B) Bottom-up view of cholera toxin. The binding sites on the B subunit for the five GM1 receptor lipids (denoted with orange ovals) are between adjacent members of the B subunit. Structure based on the crystal structure (PDB code 1S5E) from [39].
Evidence that CTxB associates with lipid rafts and functions as a raft cross-linker
The observation that CTxB associates with DRMs (detergent-resistant membranes) provided some of the earliest evidence supporting an association between CTxB and lipid rafts [3-5]. In this experiment, cell membranes are fractionated, typically with cold detergent, and the membrane fraction that does not dissolve in the detergent (the DRM) is considered to contain lipid rafts [6,7]. Interestingly, the addition of CTxB to cells causes GM1 to become more detergent resistant [8]. This suggests that CTxB actively changes the properties of the membrane upon binding.
More recent evidence that CTxB alters membrane organization comes from studies using GUVs (giant unilamellar vesicles) produced from purified lipids. GUVs allow for the visualization with fluorescence microscopy of Lo and Ld (liquid disordered) domains, which are the model membrane analogues of raft and non-raft domains in live cells, respectively. CTxB and fluorescent analogues of GM1 have been imaged in phase separated GUVs and in most cases are found enriched in the Lo region [9-11], supporting the DRM-based assignment of CTxB to lipid rafts. Importantly, these studies not only revealed that CTxB may be associated with rafts, but also that it may be modulating rafts. In a key paper, CTxB was added to GUVs composed of a mixture of lipids containing the CTxB receptor ganglioside GM1 and close to a phase boundary. Despite lacking detectable phase separation prior to the addition of CTxB, the GUVs reorganized into distinct, resolvable CTxB-enriched Lo and CTxB-depleted Ld fractions following labelling with CTxB (Figure 2) [9]. This experiment indicated for the first time that CTxB is capable of inducing the formation of rafts. Importantly, this process also led to the reorganization of another protein within the GUVs, suggesting that it also represents a general mechanism for controlling protein localization.
Figure 2. CTxB binding results in the stabilization of large raft-like Lo domains in giant unilamellar vesicles.
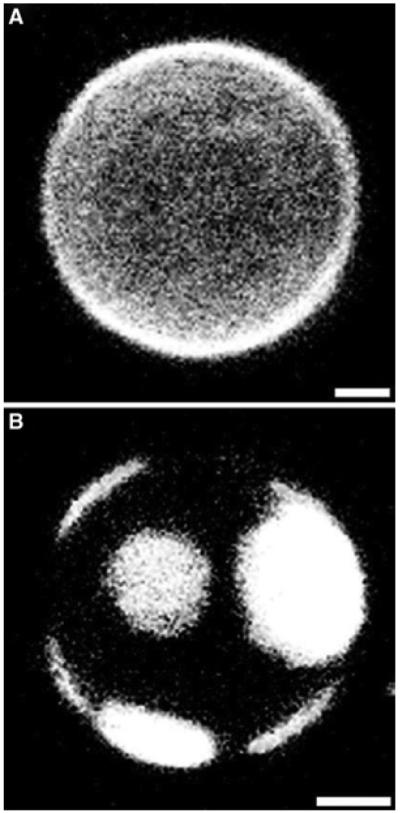
GUVs containing rhodamine-labelled LAT peptides were examined before (A) and after (B) the addition of Alexa Fluor® 488-conjugated CTxB. Whereas LAT peptides appear uniformly dispersed in the absence of toxin, following the addition of CTxB, LAT becomes enriched in large Ld domains. Scale bars, 5 µm. LAT, linker for activation of T-cells. From A.T. Hammond, F.A. Heberle, T. Baumgart, D. Holowka, B. Baird and G.W. Feigenson (2005). Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc Natl Acad Sci USA 102(18), 6320–6325. Copyright 2005, The National Academy of Sciences, USA.
A potential limitation of these types of studies is that the simplicity of GUVs may not accurately represent what occurs in the much more complex cell membrane. More recent studies have thus turned to cell-derived plasma membrane vesicles as a model to further evaluate the properties of membrane domains [10-12]. Unlike in intact cells, plasma membrane vesicles lack an underlying cytoskeleton, allowing for domains to coalesce and be directly visualized by fluorescence microscopy. An advantage of this model system is that the cell-derived vesicles retain the high level of complexity of protein and lipid composition and high protein-to-lipid ratio that exists in native cell plasma membranes. Similar to the case of GUVs, CTxB induced large scale separation of raft and non-raft associating fluorescent probes in cytoskeleton-free plasma membrane vesicles, which were still attached to cells [10]. Thus, the ability of CTxB to reorganize membranes to form large stabilized rafts appears to be conserved in both simple lipid-based systems and in more complex membrane environments more closely reflecting that of the plasma membrane.
Biophysical studies of the dynamics of CTxB-cross-linked domains in cell membranes
Although biochemical analysis and model membrane studies indicate that CTxB associates with and is capable of cross-linking lipid rafts, the exact properties of CTxB-stabilized rafts in live cells have been difficult to elucidate. In many live cell fluorescence microscopy studies, CTxB appears to be uniformly distributed at the cell surface [13,14]. However, rafts may still be present but cannot be resolved with conventional fluorescence techniques. One approach for probing rafts below the resolution limit is to look for their impact on the lateral diffusion of putative raft components within cellular membranes.
In 1975, Saffman and Delbrück [15] described the dependence of diffusion within a lipid bilayer on the hydrodynamic radius (analogous to the area at the surface of the membrane) of the diffusing particle, the viscosity of the membrane and the thickness of the membrane. Working from this equation, a variety of theories have been put forward about how the association of putative raft-associated proteins such as CTxB with lipid rafts may affect their diffusion within the membrane [14,16-18]. For example, by diffusing as a group, the hydrodynamic radius of a raft-associated protein may effectively match that of its raft complex. Or, the local viscosity in the raft may be increased as the result of its ordered nature, such that diffusion is slower within the raft than outside. Interestingly, biophysical studies of cholera toxin diffusion in live cells using techniques such as FCS (fluorescence correlation spectroscopy) and FRAP (fluorescence recovery after photobleaching) have revealed that its diffusion is slower than many other membrane anchored proteins [14,19]. For example, CTxB diffusion in live cells is of the order of 0.1–0.2 µm/s2, while transmembrane proteins often diffuse at between 0.15 and 0.6 µm/s2. In contrast, lipids (including fluorescent GM1 analogues) and lipid anchored proteins diffuse at 1.3–2.5 µm/s2 and 0.4–1.2 µm/s2, respectively [13,14,16,20]. One plausible explanation for this slow diffusion is that CTxB may be associated with cross-linked rafts.
To test if cross-linking of rafts could account for the slow diffusion of CTxB, confocal FRAP was performed on both wild-type toxin and chimaeric toxins containing only one or two functional receptor binding sites. The prediction was that if the toxin cross-links rafts into larger rafts then reducing the number of binding sites should produce smaller rafts that can diffuse faster. Interestingly, the mutant toxin diffused similarly to wild-type CTxB [3]. Also, FRAP measurements were made on the membrane-binding subunit of another toxin with the ability to cross-link rafts, Shiga toxin. STxB (Shiga toxin B-subunit) binds to up to 15 copies of its glycolipid receptor, Gb3, and thus would be predicted to cross-link domains even more strongly than does CTxB, which binds up to five GM1 molecules. Strikingly, instead of diffusing more slowly as one might expect based on its more extensive cross-linking potential, the measured diffusional mobility of STxB was even faster than that of CTxB [13]. As an alternative approach, FRAP was performed on raft and non-raft markers in the absence or presence of CTxB, with the prediction that CTxB cross-linking of rafts would result in slowed diffusion of other markers which became trapped in the enlarged rafts. Again, this approach yielded no evidence for raft coalescence, as the diffusional mobilities of the probes examined were unaltered by the presence of CTxB [13]. However, it is important to note that using particle tracking, a model GPI (glycosylphosphatidylinositol)-anchored protein did diffuse more slowly in the presence of CTxB than in the absence of CTxB, signifying that CTxB binding can induce changes in plasma membrane organization that affect other proteins [21]. Taken together, these findings suggest that raft cross-linking may occur in live cells but that cross-linking alone cannot entirely explain the slow diffusion of CTxB.
Of course, other features besides rafts, such as the cytoskeleton, protein-to-lipid ratio, and membrane topography all impact diffusion as well. A growing body of literature supports a role for cortical actin in slowing the diffusion of specific membrane components, and at least four studies have now identified actin filaments as perturbing CTxB diffusion [10,13,19,22]. How actin near the cytoplasmic face of the plasma membrane could be affecting the diffusion of the CTxB–GM1 complex on the outer leaflet of the plasma membrane is unclear and is an important avenue for future research. One potential mechanism could involve the actin-dependent formation of ordered plasma membrane domains in the outer leaflet of the plasma membrane [23].
Functional consequences of cross-linking for toxin entry
From a functional perspective, CTxB is required to enable cholera toxin to enter cells and be taken into a retrograde trafficking pathway that ultimately allows the A subunit to access the cytoplasm and induce a pathogenic cell response [24]. Thus, the ability of CTxB to cross-link and stabilize lipid rafts might be expected to contribute importantly to its mode of endocytic uptake and eventual intoxification of cells. This idea has been tested in studies utilizing a chimaeric mutant cholera toxin containing zero, one or two functional binding sites for GM1 [3]. The chimaeric toxin displayed reduced endocytosis and toxicity compared with the wild-type toxin, implicating an important role for multivalent binding in CTxB functionality. In a follow up study, the mutant toxin with only one binding site was examined. This mutant could induce toxicity, but at a lowered efficiency than wild type toxin [25].
One interesting model for how multivalent binding can impact endocytosis states that by cross-linking rafts the toxin is able to physically induce membrane bending and assist in the formation of endocytic carriers. This model was originally developed using STxB [26]. However, these same mechanisms have been proposed to also facilitate CTxB uptake [27]. This model proposes that the toxins, through raft cross-linking, become highly enriched at specific locations on the cell surface. The toxins then collectively bend the membrane into an invagination. The cell recognizes the invaginations as nascent endocytic structures and responds by cleaving the structures, releasing them from the membrane so that they can be trafficked into the cell [28]. The majority of the data supporting this model were collected in cells under pharmacological perturbations that inhibit scission (including ATP depletion, actin depolymerization and inhibition of dynamin) and thus trap these endocytic structures at the plasma membrane. Under these conditions, the nascent structures elongate into distinct, micrometrelong invaginations allowing them to be readily visualized (Figure 3). Parallel in vitro studies showed that when bound to the outer leaflet of GUVs, both STxB and CTxB induce the formation of inwardly facing membrane tubules, revealing their intrinsic ability to generate membrane curvature [26,27].
Figure 3. CTxB accumulates in long tubular plasma membrane invaginations under conditions where scission is blocked.
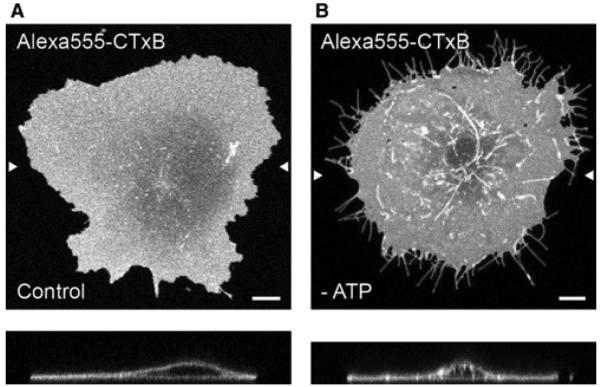
(A) CTxB bound to live COS-7 cells appears uniformly distributed across the plasma membrane. In the xz-section (lower panel), the plasma membrane is largely planar. (B) In cells where scission of nascent endocytic vesicles from the plasma membrane has been blocked by ATP depletion, CTxB accumulates in long tubular plasma membrane invaginations. Arrow heads mark the position of the xz-section. Scale bars, 10 µm. Adapted from Day, C. A. and A. K. Kenworthy (2012). Mechanisms underlying the confined diffusion of cholera toxin B-subunit in intact cell membranes. PLoS ONE 7(4), e34923.
The notion that toxin binding actively contributes to membrane deformation and endocytosis is an attractive one because it implies that the ability of CTxB to cross-link and stabilize rafts in model membranes is physiologically relevant. This model also offers a potential explanation for why the toxicity of cholera toxin is decreased for the one- and two-binding-site mutants. It is important to note however that our understanding of these toxin-induced structures is still in its infancy. Although cleavage of the invaginations is thought to occur by an actin-dependent mechanism [28], many questions remain to be fully addressed, including if and how additional cellular machinery contributes to membrane bending and scission and where these specific endocytic carriers are ultimately delivered within the cell.
A strong case can also be made for an alternative model, i.e. that CTxB endocytosis occurs through native endocytic mechanisms. In fact, it has been shown that CTxB will enter cells through a wide variety of endogenous endocytic pathways, many of which are thought to be raft dependent [20,29-31]. Here also, sorting of CTxB into specific endocytic vesicles could be the result of membrane remodelling by the toxin as opposed to simply passively associating with small clusters of its receptor GM1. This could occur by a mechanism in which CTxB senses and/or contributes to local membrane curvature at nascent sites of endocytosis. Indeed, several in vitro studies suggest CTxB undergoes curvature-sensitive sorting, with a preference for regions of negative curvature [32,33].
Thus, there is conflicting evidence as to whether CTxB enters cells by an endogenous endocytic pathway or by directly curving the membrane to stimulate its own endocytosis.. If CTxB is utilizing endogenous pathways, then the next question is whether receptor cross-linking leads to curvature-dependent sorting of CTxB into specific classes of nascent endocytic pits. Clearly, more work is needed to address these issues.
Conclusions and future directions
A growing body of evidence suggests CTxB, one of the most widely studied markers of lipid rafts, is capable of remodelling and organizing otherwise small and transient rafts to form stabilized membrane domains that can be readily detected in simplified membrane models. These stabilized domains share some of the predicted properties of lipid rafts, presumably form as the results of CTxB’s ability to cross-link multiple copies of its lipid receptor, GM1, and appear to be required for cholera toxin’s intracellular trafficking and subsequent intoxification of host cells. Much remains to be learned, however, about the properties of these putative domains in living cells and their function in directing cholera toxin for endocytic uptake and subsequent retrograde trafficking. Methods that combine super-resolution imaging with other techniques sensitive to dynamics and membrane order [18,34] or other emerging high-resolution approaches that allow for direct visualization of protein clustering in intact cells [35] should prove capable of detecting and characterizing CTxB-stabilized domains in intact cells. Indeed, sub-diffraction imaging techniques have now begun to reveal the nanoscale features of CTxB-enriched domains [36]. Our understanding of the properties of CTxB–GM1 complexes should also continue to be greatly increased by in vitro approaches. These include elegant biophysical approaches being used to study CTxB’s curvature sensing and generating abilities [26,27,32,33], follow the early stages of the formation of CTxB-enriched domains [37] and uncover the impact of CTxB binding on underlying membrane structure [38]. The development of new tools such as fluorescent glycolipid analogues and mutants toxins [20,25,27] are also now making it possible to directly probe the contributions of both cholera toxin and its receptor to the assembly, dynamics and function of these domains. Ultimately, the principles that emerge from these studies should increase our understanding of general mechanisms that contribute to the organization and function of protein–lipid assemblies in cells. In addition, they may provide insights into the long-sought connections between the structure and function of lipid rafts.
Summary.
The homopentameric B-subunit of cholera toxin, CTxB, is a widely studied putative raft marker.
CTxB binds to up to five copies of its lipid receptor, ganglioside GM1, on the plasma membrane of host cells.
CTxB is capable of inducing the formation of stabilized rafts in simplified membrane models, such as GUVs and giant plasma membrane-derived vesicles.
Diffusion measurements are an example of a biophysical approach that has been used to probe the properties of CTxB–GM1 complexes in model membranes and intact cells.
Although CTxB undergoes much slower diffusion than free GM1, its slow diffusion is not simply the result of cross-linking rafts.
Multivalent receptor binding is important for the proper trafficking and function of CTxB.
Current experimental evidence supports competing models for the mechanism by which receptor cross-linking regulates the initial uptake of CTxB into cells.
CTxB is capable of sensing and generating membrane curvature and this activity is likely to play a role in sorting of CTxB in the endocytic pathway.
Emerging imaging approaches and biophysical techniques should enable more detailed investigations into the properties and functions of CTxB-cross-linked rafts in the future.
Acknowledgments
We thank Ilya Levental, Wayne Lencer and Ludger Johannes for stimulating discussions and apologize to those whose work could not be cited owing to space limitations. This work was supported by the National Institutes of Health [grant number RO1 GM073846] (to A.K.) and a Mayo Clinic Kidney Disease Research Training Grant [grant number 5 T32 DK007013-35] (to C.D.). The funding sources had no role in the preparation of the report or the decision to submit the paper for publication.
References
- 1.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 2.Heyningen SV. Cholera toxin: interaction of subunits with ganglioside GM1. Science. 1974;183:656–657. doi: 10.1126/science.183.4125.656. [DOI] [PubMed] [Google Scholar]
- 3.Wolf AA, Jobling MG, Saslowsky DE, Kern E, Drake KR, Kenworthy AK, Holmes RK, Lencer WI. Attenuated endocytosis and toxicity of a mutant cholera toxin with decreased ability to cluster ganglioside GM1 molecules. Infect. Immun. 2008;76:1476–1484. doi: 10.1128/IAI.01286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shogomori H, Futerman AH. Cholera toxin is found in detergent-insoluble rafts/ domains at the cell surface of hippocampal neurons but is internalized via a raft-independent mechanism. J. Biol. Chem. 2001;276:9182–9188. doi: 10.1074/jbc.M009414200. [DOI] [PubMed] [Google Scholar]
- 5.Badizadegan K, Dickinson BL, Wheeler HE, Blumberg RS, Holmes RK, Lencer WI. Heterogeneity of detergent-insoluble membranes from human intestine containing caveolin-1 and ganglioside GM1. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G895–G904. doi: 10.1152/ajpgi.2000.278.6.G895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rietveld A, Simons K. The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim. Biophys. Acta. 1998;1376:467–479. doi: 10.1016/s0304-4157(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 7.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 8.Hagmann J, Fishman PH. Detergent extraction of cholera toxin and gangliosides from cultured cells and isolated membranes. Biochim. Biophys. Acta. 1982;720:181–187. doi: 10.1016/0167-4889(82)90010-6. [DOI] [PubMed] [Google Scholar]
- 9.Hammond AT, Heberle FA, Baumgart T, Holowka D, Baird B, Feigenson GW. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc. Natl. Acad. Sci. U.S.A. 2005;102:6320–6325. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lingwood D, Ries J, Schwille P, Simons K. Plasma membranes are poised for activation of raft phase coalescence at physiological temperature. Proc. Natl. Acad. Sci. U.S.A. 2008;105:10005–10010. doi: 10.1073/pnas.0804374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumgart T, Hammond AT, Sengupta P, Hess ST, Holowka DA, Baird BA, Webb WW. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. U.S.A. 2007;104:3165–3170. doi: 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sezgin E, Kaiser HJ, Baumgart T, Schwille P, Simons K, Levental I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat. Protoc. 2012;7:1042–1051. doi: 10.1038/nprot.2012.059. [DOI] [PubMed] [Google Scholar]
- 13.Day CA, Kenworthy AK. Mechanisms underlying the confined diffusion of cholera toxin B-subunit in intact cell membranes. PLoS ONE. 2012;7:e34923. doi: 10.1371/journal.pone.0034923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenworthy AK, Nichols BJ, Remmert CL, Hendrix GM, Kumar M, Zimmerberg J, Lippincott-Schwartz J. Dynamics of putative raft-associated proteins at the cell surface. J. Cell Biol. 2004;165:735–476. doi: 10.1083/jcb.200312170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saffman PG, Delbruck M. Brownian motion in biological membranes. Proc. Natl. Acad. Sci. U.S.A. 1975;72:3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenne PF, Wawrezinieck L, Conchonaud F, Wurtz O, Boned A, Guo XJ, Rigneault H, He HT, Marguet D. Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. EMBO J. 2006;25:3245–3256. doi: 10.1038/sj.emboj.7601214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meder D, Moreno MJ, Verkade P, Vaz WL, Simons K. Phase coexistence and connectivity in the apical membrane of polarized epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 2006;103:329–334. doi: 10.1073/pnas.0509885103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggeling C, Ringemann C, Medda R, Schwarzmann G, Sandhoff K, Polyakova S, Belov VN, Hein B, von Middendorff C, Schonle A, Hell SW. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature. 2009;457:1159–1162. doi: 10.1038/nature07596. [DOI] [PubMed] [Google Scholar]
- 19.Bacia K, Scherfeld D, Kahya N, Schwille P. Fluorescence correlation spectroscopy relates rafts in model and native membranes. Biophys. J. 2004;87:1034–1043. doi: 10.1529/biophysj.104.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chinnapen DJ, Hsieh WT, te Welscher YM, Saslowsky DE, Kaoutzani L, Brandsma E, D’Auria L, Park H, Wagner JS, Drake KR, et al. Lipid sorting by ceramide structure from plasma membrane to ER for the cholera toxin receptor ganglioside GM1. Dev. Cell. 2012;23:573–586. doi: 10.1016/j.devcel.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinaud F, Michalet X, Iyer G, Margeat E, Moore HP, Weiss S. Dynamic partitioning of a glycosyl-phosphatidylinositol-anchored protein in glycosphingolipid-rich microdomains imaged by single-quantum dot tracking. Traffic. 2009;10:691–712. doi: 10.1111/j.1600-0854.2009.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaqaman K, Kuwata H, Touret N, Collins R, Trimble WS, Danuser G, Grinstein S. Cytoskeletal control of CD36 diffusion promotes its receptor and signaling function. Cell. 2011;146:593–606. doi: 10.1016/j.cell.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinic J, Ashrafzadeh P, Parmryd I. Actin filaments attachment at the plasma membrane in live cells cause the formation of ordered lipid domains. Biochim. Biophys. Acta. 2013;1828:1102–1111. doi: 10.1016/j.bbamem.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Cho JA, Chinnapen DJ, Aamar E, Te Welscher YM, Lencer WI, Massol R. Insights on the trafficking and retro-translocation of glycosphingolipid-binding bacterial toxins. Front. Cell. Infect. Microbiol. 2012;2:51. doi: 10.3389/fcimb.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jobling MG, Yang Z, Kam WR, Lencer WI, Holmes RK. A single native ganglioside GM1-binding site is sufficient for cholera toxin to bind to cells and complete the intoxication pathway. MBio. 2012;3:e00401–12. doi: 10.1128/mBio.00401-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Römer W, Berland L, Chambon V, Gaus K, Windschiegl B, Tenza D, Aly MR, Fraisier V, Florent JC, Perrais D, Lamaze C, Raposo G, Steinem C, Sens P, Bassereau P, Johannes L. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450:670–675. doi: 10.1038/nature05996. [DOI] [PubMed] [Google Scholar]
- 27.Ewers H, Römer W, Smith AE, Bacia K, Dmitrieff S, Chai W, Mancini R, Kartenbeck J, Chambon V, Berland L, et al. GM1 structure determines SV40-induced membrane invagination and infection. Nat. Cell Biol. 2010;12(suppl. 1–12):11–18. doi: 10.1038/ncb1999. [DOI] [PubMed] [Google Scholar]
- 28.Römer W, Pontani LL, Sorre B, Rentero C, Berland L, Chambon V, Lamaze C, Bassereau P, Sykes C, Gaus K, Johannes L. Actin dynamics drive membrane reorganization and scission in clathrin-independent endocytosis. Cell. 2010;140:540–553. doi: 10.1016/j.cell.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Massol RH, Larsen JE, Fujinaga Y, Lencer WI, Kirchhausen T. Cholera toxin toxicity does not require functional Arf6- and dynamin-dependent endocytic pathways. Mol. Biol. Cell. 2004;15:3631–3641. doi: 10.1091/mbc.E04-04-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howes MT, Kirkham M, Riches J, Cortese K, Walser PJ, Simpson F, Hill MM, Jones A, Lundmark R, Lindsay MR, et al. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J. Cell Biol. 2010;190:675–691. doi: 10.1083/jcb.201002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torgersen ML, Skretting G, van Deurs B, Sandvig K. Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 2001;114:3737–3747. doi: 10.1242/jcs.114.20.3737. [DOI] [PubMed] [Google Scholar]
- 32.Tian A, Baumgart T. Sorting of lipids and proteins in membrane curvature gradients. Biophys. J. 2009;96:2676–2688. doi: 10.1016/j.bpj.2008.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh WT, Hsu CJ, Capraro BR, Wu T, Chen CM, Yang S, Baumgart T. Curvature sorting of peripheral proteins on solid-supported wavy membranes. Langmuir. 2012;28:12838–12843. doi: 10.1021/la302205b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owen DM, Williamson DJ, Magenau A, Gaus K. Sub-resolution lipid domains exist in the plasma membrane and regulate protein diffusion and distribution. Nat. Commun. 2012;3:1256. doi: 10.1038/ncomms2273. [DOI] [PubMed] [Google Scholar]
- 35.de Jonge N, Peckys DB, Kremers GJ, Piston DW. Electron microscopy of whole cells in liquid with nanometer resolution. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2159–2164. doi: 10.1073/pnas.0809567106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Zanten TS, Gomez J, Manzo C, Cambi A, Buceta J, Reigada R, Garcia-Parajo MF. Direct mapping of nanoscale compositional connectivity on intact cell membranes. Proc. Natl. Acad. Sci. U.S.A. 2010;107:15437–15442. doi: 10.1073/pnas.1003876107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stefl M, Sachl R, Humpolickova J, Cebecauer M, Machan R, Kolarova M, Johansson LB, Hof M. Dynamics and size of cross-linking-induced lipid nanodomains in model membranes. Biophys. J. 2012;102:2104–2113. doi: 10.1016/j.bpj.2012.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watkins EB, Miller CE, Majewski J, Kuhl TL. Membrane texture induced by specific protein binding and receptor clustering: active roles for lipids in cellular function. Proc. Natl. Acad. Sci. U.S.A. 2011;108:6975–6980. doi: 10.1073/pnas.1014579108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Neal CJ, Amaya EI, Jobling MG, Holmes RK, Hol WG. Crystal structures of an intrinsically active cholera toxin mutant yield insight into the toxin activation mechanism. Biochemistry. 2004;43:3772–3782. doi: 10.1021/bi0360152. [DOI] [PubMed] [Google Scholar]


