Abstract
We investigated the administration of intravenous (i.v.) busulfan (Bu) combined with melphalan (Mel) in patients with acute lymphoblastic leukemia (ALL) undergoing allogeneic hematopoietic stem cell transplantation (SCT). Forty-seven patients with median age 33 years (range 20–61) received a matched sibling (n=27) or matched unrelated donor transplant (n=20) for ALL in first complete remission (n=26), second complete remission (n=13), or with more advanced disease (n=8). Bu was infused daily for 4 days, either at fixed dose 130 mg/m2 (5 patients) or using pharmacokinetic dose adjustment (42 patients), to target an average daily AUC of 5,000 uMol-min, determined by a test dose of i.v. Bu at 32 mg/m2. This was followed by a rest day, then two daily doses of Mel at 70 mg/m2. Stem cells were infused on the following day. The 2-year overall survival (OS), progression-free survival (PFS), and non-relapse mortality (NRM) rates were 35% (95% confidence interval [CI] 23%–51%), 31% (95% CI 21%–48%), and 37% (95% CI 23%–50%), respectively. Acute non-relapse mortality (NRM) at 100 days was favorable at 12% (95%CI 5%–24%); however, the 2-year NRM was significantly higher for patients older than 40 years, 58% vs. 20%, mainly due to graft versus host disease.
Keywords: Acute lymphoblastic leukemia, allogeneic hematopoietic stem cell transplantation, busulfan
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (SCT) is an effective, potentially curative treatment option for adults with acute lymphoblastic leukemia (ALL). Survival rates range broadly from 20–60%, depending primarily on disease status at time of transplant (1). Non-relapse mortality (NRM) remains a major problem, occurring in 20% to 45% of patients receiving a standard, radiation-based, myeloablative preparative regimen (1–3). The results of the MRC/ECOG trial underscored the impact of NRM. This randomized trial was designed to investigate the efficacy of total body irradiation (TBI) and etoposide with allogeneic SCT as compared to continued chemotherapy in adult patients with ALL in first remission. Although allogeneic SCT afforded significantly better protection against disease relapse and better overall survival (OS) for the transplant group (53% vs. 45%, p=.01), the 2-year NRM rate of 36% in high-risk patients obviated any survival benefit for this group. High-risk was defined as an elevated WBC count at diagnosis, age greater than 35 years, or the presence of the Philadelphia (Ph) chromosome (3). In efforts to reduce the toxicities associated with TBI and allogeneic SCT(4–7), we investigated a non-radiation-based, myeloablative preparative regimen for adult patients with ALL.
Alkylating agents form the backbone of most transplant preparative regimens. Several groups have evaluated the combination of busulfan and melphalan. Both of these agents are directly active, and display linear pharmacokinetics in the dose ranges utilized with hematopoietic transplantation (8–10). Both agents penetrate into the central nervous system (CNS) (11). Myelosuppression is the primary toxicity of both drugs and the nonhematopoietic toxicity profiles are generally non-overlapping. This myeloablative combination has been studied in patients with a wide range of advanced hematologic malignancies in both the autologous (12–15), and allogeneic setting (16–20). Small et al. studied 43 patients with advanced leukemia (5 with ALL), including 34 with active disease and reported 3-year OS of 37%(17). Importantly, the NRM at 30 days and 100 days were 0% and 16%, respectively.
Once daily i.v. busulfan administration has been noted to be safe (21). There is linear pharmacokinetics (PK), with highly reproducible intra- and inter-patient systemic exposure (10); this has allowed the identification of an optimized therapeutic interval (22). Based on these considerations, we hypothesized that i.v. busulfan administered once daily for 4 days with PK-guidance, followed by melphalan administered over 2 days, would constitute a safe and effective myeloablative regimen in patients undergoing allogeneic SCT for ALL.
PATIENTS AND METHODS
Patient eligibility
This was a prospective, phase II single arm study conducted between August 2005 to October 2009 investigating the combination of i.v. busulfan and melphalan in patients with ALL. Patients were required to be between 18 and 65 years of age, with an HLA matched related or unrelated donor (defined as an HLA- A, B serologic matched and DRB1 molecular matched donor). Additional eligibility criteria included creatinine clearance of ≥60 ml/min, alanine aminotransferase ≤ 3 times the upper normal limit, a Zubrod performance status of 0 or 1, no evidence of uncontrolled infection, and negative serology for hepatitis B, C and HIV. Patients were required to have adequate cardiac function demonstrated by left ventricular ejection fraction > 40%, and good lung function demonstrated by forced expiratory volume in 1 second, forced vital capacity, and diffusing capacity of lung for CO2 corrected for hemoglobin of more than 50% of predicted. Patients with active CNS disease were excluded.
Preparative regimen
The transplant preparative regimen consisted of i.v. busulfan administered either as a fixed dose of 130 mg/m2 infused over 3 hours once daily for 4 days or to target an average daily AUC of 5,000 μMol-min ± 12%. In a prior study, we demonstrated that a daily Bu dose of 130 mg/m2 produced a median daily AUC of approximately 4,900 μMol-min (23); patients had the option of receiving either fixed dose or PK-directed dosing. The therapeutic dose was determined by the drug clearance rate determined by PK testing using a test dose of i.v. busulfan administered at 32 mg/m2 and infused over 45 minutes approximately 48 hours before the first therapeutic busulfan dose. If necessary, a second busulfan dose adjustment was made following the first therapeutic dose analysis in efforts to keep the total course AUC at 20,000 μMol-min. Collection of blood and methods for PK analyses were performed as previously reported. (10, 14, 24). The busulfan administrations were followed by a rest day to allow for glutathione repletion, and melphalan was administered at a fixed dose of 70 mg/m2 infused over 30 minutes once daily for 2 days. The allogeneic progenitor cells were infused on the following day.
Patients that were Ph+, and were in molecular remission after SCT, were started on maintenance therapy with imatinib mesylate upon normalization of blood counts following SCT to continue for one year; patients in continued molecular remission at one year following SCT stopped TKI, but those with persistent minimal residual disease (MRD) stayed on treatment.
Donors
All donors were human leukocyte antigen (HLA)-A, -B, and -DR compatible with the patients. HLA typing for class I antigens was performed using standard serologic or low-resolution molecular techniques, followed by high-resolution molecular typing using polymerase chain reaction for class I and II antigens for confirmatory typing of sibling donors; high-resolution molecular typing of class I and II antigens was performed for all unrelated donors.
Peripheral blood stem cells were obtained from donors using standard mobilization protocols and apheresis techniques, with a target progenitor cell dose of 4 × 106 CD34+ cells/kg and minimal acceptable dose of 2 × 106 CD34+ cells/kg; bone marrow was used if peripheral blood could not be used. Stem cells from all related donors were collected at M. D. Anderson Cancer Center. Peripheral blood progenitor cells or bone marrow harvests from unrelated donors, was obtained through the National Marrow Donor Program.
Supportive care
Phenytoin 600 mg orally was used during and one day after completion of i.v. busulfan therapy, starting the evening before the first dose (25). Graft versus host disease (GVHD) prophylaxis consisted of a combination of tacrolimus and methotrexate. Methotrexate (5 mg/m2) was given intravenously on days +1, +3, +6, and +11. Tacrolimus was administered at a dose to maintain levels between 5 and 15 ng/ml, and tapered at the discretion of the treating physician. Patients who received unrelated donor products additionally received rabbit anti-thymocyte globulin for a total 4 mg/kg infused over three days beginning three days prior to SCT. Patients who experienced grade II or higher acute GVHD received intravenous methylprednisolone at a dosage of at least 0.5 mg/kg every 6 hours and, if possible, were enrolled in treatment protocols for GVHD.
Institutional transplant guidelines for antimicrobial, antifungal, and antiviral prophylaxis were followed as previously reported. (26)
Definitions and clinical outcome variables
Criteria for complete response included normal cytogenetics, the absence of circulating blasts, less than 5% marrow blasts, and a platelet count of 100 × 109/L or higher. Standard morphologic criteria, conventional cytogenetic analysis by G-banding, or both were used to diagnose recurrent disease. The disease stage at transplantation was defined using established criteria. Response was documented as best response occurring after day 30 following SCT. Molecular response measured by quantitative polymerase chain reaction (PCR) analysis for BCR-ABL rearrangement was obtained when possible. Hematologic recovery was defined on the date that the patient had an absolute neutrophil count of 0.5 × 109/L or higher for 3 consecutive days. Platelet recovery was defined as occurring on the first of 7 consecutive days with a platelet count of 20 × 109/L or higher without transfusion support. Failure to engraft by day +30 was considered primary engraftment failure. Hematopoietic chimerism was evaluated in bone marrow (using unsorted cells) or peripheral blood (with myeloid and T-lineage sorting) by restriction fragment length polymorphisms using PCR methods to determine donor engraftment. Mixed chimerism was defined as the presence of any detectable (>1%) recipient DNA in addition to donor-derived DNA in myeloid or T-lineage cells.
Overall survival was estimated from the time of SCT until death from any cause, and patients still alive at last follow-up were considered censored. Progression-free survival was estimated from SCT until the date of progression or death from any cause. Patients alive and progression free at last follow-up were considered censored. Non-relapse mortality (NRM) was defined as death from any cause other than disease progression or relapse. The diagnosis of GVHD was confirmed by biopsy when feasible but was ultimately determined by clinical presentation. Acute GVHD (aGVHD) was clinically graded as 0 to IV based on standard criteria (27); chronic GVHD (cGVHD) was classified as none, limited, or extensive (28). Acute GVHD, which persisted or progressed after day 100, was also scored as cGVHD in this study.
Statistical methods
The primary outcomes for this single-arm trial were safety and overall survival. Bayesian early stopping rules based on the observed rates of these 2 outcomes, as compared to historical data, were implemented (29). The Kaplan-Meier estimator (30) was used to assess OS and PFS probabilities in months. OS or PFS distributions were compared between subgroups using the log rank test (31). Descriptive statistics were used to summarize patient demographics.
RESULTS
Patient and treatment characteristics
Patient demographics and baseline disease characteristics are listed in Table 1. Forty-seven patients with median age 33 years (range 20–61) were evaluated on this study. The median number of prior treatment regimens was 2 (range 1–4), with median 12 months from time of diagnosis to transplant. The majority of patients had high risk features at diagnosis, with 30% (n=14) having an elevated WBC count at diagnosis, 45% (n=21) having the Philadelphia chromosome, and 49% (n=23) taking greater than 4 weeks to achieve first remission. At the time of transplant, 55% (n=26) were in CR1, 28% (n=13) were in CR2, and 18% (n=8) were beyond CR2 or had active disease. Seven patients had extramedullary involvement of disease at time of diagnosis. Eighty-nine percent of patients (n=42) had PK-directed busulfan dosing.
Table 1.
Patient characteristics at diagnosis, among 47 total
| Patient Characteristic | No. (%) |
|---|---|
| Median age (range) | 33 (20–61) years |
| Sex, male/female | 28/19 |
| Disease lineage | |
| B-lineage | 41 (87) |
| T-lineage | 6 (13) |
| WBC count at diagnosis | |
| <30×109/L | 24 (51) |
| 30–100×109/L | 8 (17) |
| >100×109/L | 6 (13) |
| Unknown | 9 (19) |
| Cytogenetics at diagnosis | |
| Diploid | 5 (11) |
| Other | 12 (26) |
| Ph+ | 21 (45) |
| Hyperdiploid | 3 (6) |
| Unknown | 6 (12) |
| Time to achieve CR | |
| Within 4 weeks | 19 (40) |
| >4 weeks | 23 (49) |
| Unknown | 5 (11) |
| Disease status at transplant | |
| CR1 | 26 (55) |
| MRD present | 6 |
| CR2 | 13 (28) |
| MRD present | 5 |
| CR3 or greater remission1 | 5 (11) |
| MRD present | 1 |
| Not in remission | 3 (6) |
| Median lines of chemotherapy pre-SCT (range) | 2 (1–4) |
| Median months to SCT (range) | 12 (3–131) |
One patient received prior allogeneic SCT
Eleven patients with Ph+ ALL were started on median dose 200 mg imatinib (range 100mg–400mg) maintenance therapy at a median of 2 months (range 1–5) following SCT. Three patients remain on imatinib due to fluctuating levels of MRD, 4 patients stopped imatinib after completing one year of maintenance, 1 patient stopped after 1 month due to thrombocytopenia, and 3 patients stopped due to disease progression. The remaining 10 patients with Ph+ ALL were not able to receive intended maintenance due to low counts in the setting of active GVHD or infection. Of note, all of the Ph+ patients received imatinib as part of their therapy prior to SCT.
Graft content and engraftment
Stem cell graft characteristics and hematopoietic recovery data are summarized in Table 2. Approximately half of the patients received a matched related donor transplant (57%) and the remaining matched unrelated donor SCT (43%). The source of stem cells was peripheral blood for the majority of patients. The median TNC count and CD34+ cell doses were 7 × 108/kg (range 1–23) and 5 × 106/kg (range 1–14), respectively. The median time to neutrophil and platelet recovery were 11 (range 10–22) and 13 days (range 8–61), respectively.
Table 2.
Graft characteristics at transplant, and hematopoietic recovery
| Characteristic | No. (%) |
|---|---|
| Donor type | |
| Matched related | 27 (57) |
| Matched unrelated | 20 (43) |
| Stem cell source | |
| Bone marrow | 7 (15) |
| Peripheral blood | 40 (85) |
| Graft composition, median (range) | |
| Total nucleated cells | 7 (1–23) ×108/kg |
| CD34+ | 5 (1–14) ×106/kg |
| CD3+ | 197 (6–407) ×106/kg |
| Days to ANC, median (range) | 11 (10–22) |
| Days to platelet, median (range)1 | 13 (8–61) |
Six patients did not have platelet recovery.
Overall survival
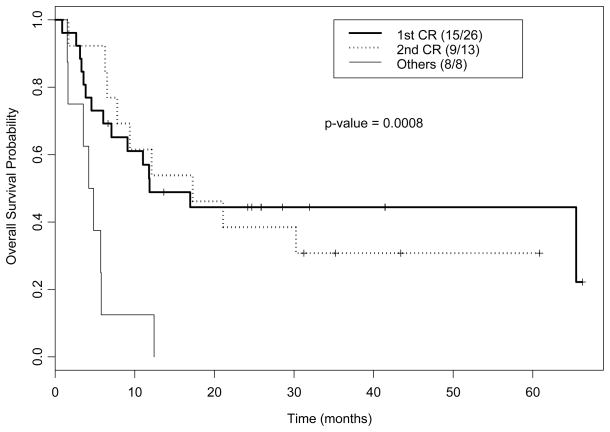
Fourteen patients were alive at a median follow-up of 38 months (range 7–77) among survivors, with 1- and 2-year OS rates of 46% (95% confidence interval [CI] 34%–63%) and 35% (95%CI 23%–51%), respectively. As expected, patients in CR1 or CR2 had significantly better outcomes than patients with more advanced disease at time of transplant, with none of the 8 patients transplanted beyond CR2 remaining alive (Figure 1).
Figure 1.
Kaplan-Meier plot for overall survival by disease status.
Response, relapse, and progression-free survival
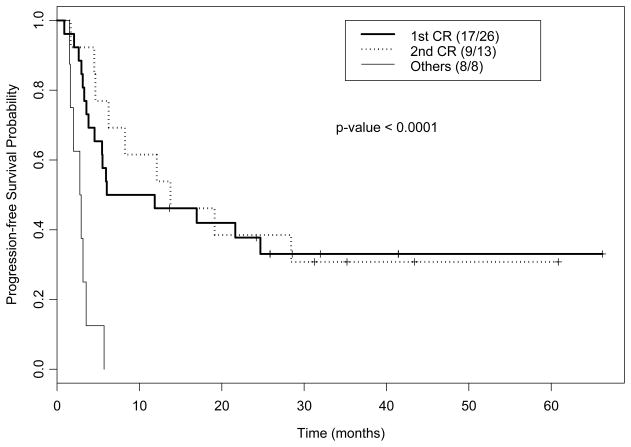
One patient had an early death at 20 days following transplant due to pulmonary hemorrhage, thus 46 patients were evaluable for response. All patients maintained or achieved complete remission. All except for one patient achieved 100% donor engraftment at a median of 32 days (range 25–47) following SCT; one patient remained mixed chimera with 97% donor cells by unsorted chimerism analysis in bone marrow, and 99% donor cells in myeloid lineage and 30% donor in T-lineage cells by cell sorted chimerism analysis in peripheral blood, and eventually relapsed 5 months following transplant. Thirty-five patients relapsed or died at a median of 6 months (95% CI: 5–22) following SCT with 1- and 2-year PFS rates of 43% (95% CI 31%–59%) and 31% (95% CI 21%–48%), respectively. Again, PFS for patients transplanted in CR1 or CR2 were significantly better than for patients transplanted with advanced disease (Figure 2). The incidence of progression for the entire group at 1- and 2-years was 26% (95% CI 14%–39%) and 32% (95% CI 19%–46%), respectively. The incidence of progression in CR1 patients was lower at 19%(95% CI 7%–36%) and 23%(95% CI 9%–41%) at 1- and 2-years, respectively. Six patients had an extramedullary site of disease at time of relapse; 4 had bone marrow relapse concurrent with extramedullary relapse and 2 patients had an isolated relapse (stomach and cheek). Among these 6 patients, 4 had a history of extramedullary disease prior to transplant.
Figure 2.
Kaplan-Meier plot for progression-free survival by disease status.
Toxicity, NRM, and GVHD
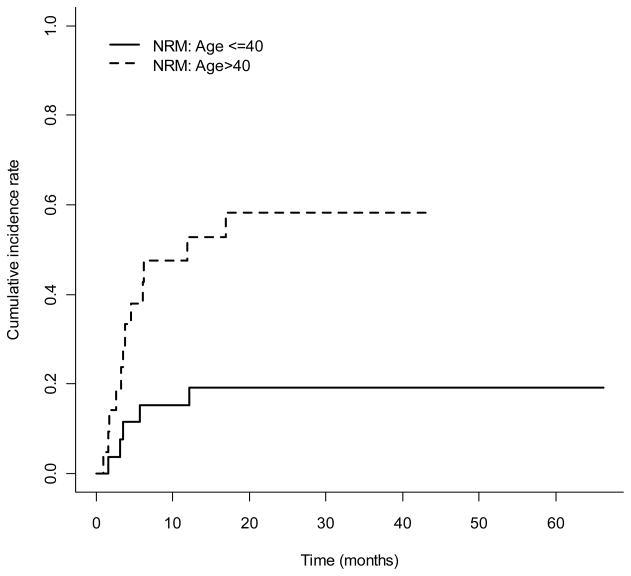
Regimen-related toxicities are detailed in Table 3. The most commonly observed toxicities involved the GI tract, and were grades I or II nausea and vomiting (94%), mucositis (70%), and diarrhea (57%). Nineteen percent of patients developed reversible transaminitis or hyperbilirubinemia. One patient, with multiple lines of prior therapy, developed grade 4 bilirubin elevation beginning at day 4 of the transplant conditioning regimen, peaking at 40 mg/dL at time of his death at day 46 following SCT from candidemia lusitaniae (identified post mortem) hepatic infiltration, sepsis and multi-organ failure. Two patients developed reversible VOD, ascertained using Jones’ criteria (32), at 24 days and 33 days following SCT. There were 2 cases of grade 2 diffuse alveolar hemorrhage (DAH), without isolation of definite infectious causes, which responded to steroid therapy. One fatal case of DAH occurred within 30 days of the transplant conditioning regimen in a 52 year-old patient who had received a matched unrelated donor transplant. Significant CNS toxicity was not observed, but one patient who was unable to take his prophylactic phenytoin because of nausea and vomiting suffered a seizure. The cumulative incidences of NRM at 100 days, 1-year, and 2-years were 13% (95% CI, 5%–24%), 32% (95% CI, 19%–46%), and 37% (95% CI, 23%–50%), respectively. Non-relapse mortality was significantly higher in patients greater than 40 years-old (Figure 3). There were 33 deaths, with primary causes infection (n=2), multi-organ failure (n=1), DAH (n=1), recurrent breast cancer (n=1), acute GVHD (n=8), chronic GVHD (n=4), or relapse (n=16).
Table 3.
Regimen related toxicity in 47 patients
| Toxicity | Grade I | Grade II | Grade III | Grade IV | Grade V |
|---|---|---|---|---|---|
| Liver | |||||
| Bilirubin elevation | 1 (2) | 1 (2) | 0 (0) | 1 (2) | |
| VOD* | 0 (0) | 0 (0) | 2 (4) | 0 (0) | |
| Gastrointestinal tract | |||||
| Diarrhea | 18 (38) | 9 (19) | 4 (9) | 0(0) | |
| Nausea | 22 (47) | 22 (47) | 1 (2) | 0 (0) | |
| Mucositis | 7 (15) | 26 (55) | 12 (26) | 0 (0) | |
| Urinary tract/kidney | |||||
| Creatinine elevation | 0 (0) | 1 (2) | 0 (0) | 0 (0) | |
| Hemorrhagic cystitis | 1 (2) | 0 (0) | 0 (0) | 0 (0) | |
| Skin | |||||
| Rash | 5 (11) | 4 (9) | 0 (0) | 0 (0) | |
| Neurologic | |||||
| Seizures | 0 (0) | 1 (2) | 0 (0) | 0 (0) | |
| Neuropathy | 1 (2) | 4 (9) | 0 (0) | 0 (0) | |
| Headache | 2 (4) | 1 (2) | 0 (0) | 0 (0) | |
| Pulmonary/pleural | |||||
| Pleural effusion | 1 (2) | 2 (4) | 1 (2) | 0 (0) | |
| Pulm hemorrhage | 2 (4) | 1 (2) | |||
| Cardiovascular | |||||
| Hypertension | 0 (0) | 3 (6) | 0 (0) | 0 (0) | |
| Flu-like/ATG related sx | 9 (19) | 6 (13) | |||
VOD; veno-occlusive disease of the liver.
Figure 3.
The cumulative incidence rate of NRM over time by age group (p-value = 0.008)
The cumulative incidence of grades II to IV and III–IV acute GVHD were 54% and 26%, respectively; there was no statistically significant difference between matched related and unrelated donors. The cumulative incidence of chronic GVHD was 40%, with 26% experiencing extensive GVHD. Again, no difference was noted between allotypes (Table 4).
Table 4.
Incidence of acute and chronic GVHD
| No. (%) | |
|---|---|
| Acute GVHD, 46 evaluable | |
| Grades 2–4 | 25 (54) |
| Grade 3–4 | 12 (26) |
| Chronic, 42 evaluable | |
| limited and/or extensive | 18 (43) |
| extensive | 11 (26) |
DISCUSSION
This is a large, single institution series of adult patients with high-risk ALL treated with a myeloablative, non-radiation based, transplant preparative regimen, with relatively long follow-up. The eradication of MRD in 11 patients, and the clearance of active disease in 3 patients at the time of transplant, attest to the anti-leukemic activity of the busulfan and melphalan combination, which has been demonstrated in children and adults with advanced myeloid and lymphoid malignancies (12, 13, 33, 34). Importantly, despite our high-risk patient population, including 45% Ph+ patients, and a median 12 months from diagnosis to transplant, our 2-year OS rates of approximately 40% for patients in first or second remission were comparable to the survival rates reported in the CIBMTR registry study of patients transplanted in CR1 or CR2 using mainly TBI-based (88%) preparative regimens. (34) One important concern is the control of “sanctuary” sites with a non-TBI regimen. We noted one case of CNS relapse concurrent with marrow relapse in a patient with T-lineage ALL with a prior history of mediastinal mass and lymphadenopathy, and one case of testicular relapse concurrent with marrow relapse in a patient with prior history of testicular involvement and radiation therapy to the testes. These rates appear comparable to TBI-containing regimens, but our study population is too small to fully address this question.
Approximately 50% of our Ph+ patients received post transplant imatinib maintenance therapy. Similar to our previous reported findings, the use of imatinib maintenance did not appear to impact the PFS rate (data not shown) (25).
Our regimen was well tolerated with an early NRM rate of 12% at 100 days. Furthermore, in our 12 patients up to age 35 years and transplanted in CR1, our 2-year NRM rate was only 8% compared with 20% for a much larger patient cohort, but with similar characteristics, reported in the MRC-ECOG trial (3). We postulated that using intravenous, PK-guided busulfan, which allows for more precise dose delivery with a tighter range in systemic drug exposure, would result in decreased regimen-related toxicity. The assumed benefits from PK-directed busulfan dosing were extrapolated from our previous observations of an optimal therapeutic interval for busulfan within the window of 3800 to 6080 μMol-min (22). Thus, we targeted a (median) daily dose AUC of 5000 μMol-min for the present study. We noted prompt engraftment in all patients, no unexpected toxicities, and only two cases of reversible VOD in two heavily pre-treated patients. This favorable toxicity profile is in distinct contrast to toxicities of fatal VOD in earlier studies that used oral busulfan (12, 16, 35).
As expected, there was a higher risk of NRM in older patients. In patients greater than 40 years-old, there was one regimen-related death resulting from pulmonary hemorrhage at 17 days following transplant, and the NRM rate was 55% at 2 years (Figure 3). Increased rates of GVHD accounted for the higher, late NRM noted in this population. The rate of grades 2–4 acute GVHD was 65% in older patients, compared with 46% in patients up to age 40-years. The incidence of GVHD was comparable to that reported by Marks and colleagues for ALL patients receiving myeloablative SCT regimens (36). Of the 21 patients greater than 40 years-old, 9 of the 12 deaths were related to acute or chronic GVHD. The incidence of chronic extensive GVHD was similar in both age groups.
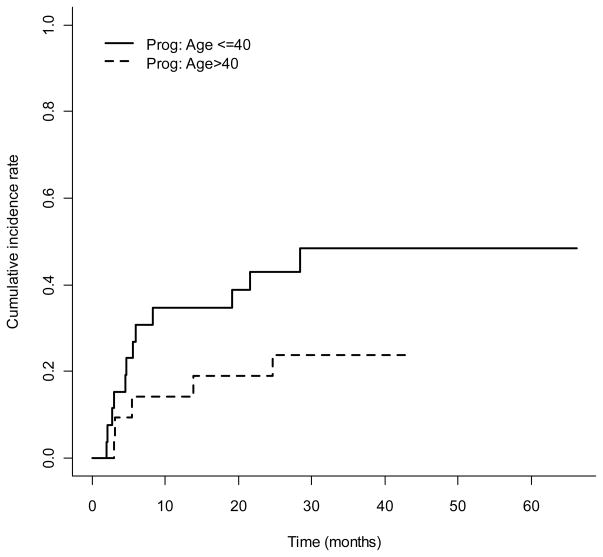
In conclusion, we have demonstrated the efficacy of a chemotherapy-only preparative regimen for allogeneic SCT in patients with high-risk ALL. These findings corroborate recently published results of i.v. busulfan combined with cyclophosphamide with excellent disease control, and low TRM with the replacement of i.v. for oral busulfan historically used in this regimen (37). As we continue to build on these results, we will need to “personalize” treatment approaches for patients modifying treatment intensity based on patient and disease characteristics, to optimize overall outcomes. Leukemia relapse remains the major cause of treatment failure with a high rate of progression in the younger patients (Figure 4), while GVHD remains a major problem in older patients. Thus, in our current transplant protocol for ALL patients, we target a higher daily busulfan AUC of 5500 μMol-min for younger patients and lower AUC of 4000 μMol-min for older patients. Furthermore, based on in vitro (38) and in vivo (21, 23, 39, 40) studies suggesting favorable synergy between nucleoside analogs and busulfan, we have replaced melphalan with clofarabine in efforts to reduce GI and pulmonary toxicity, while possibly improving anti-ALL activity (41, 42). Early observations are encouraging (43). Further modifications will be needed to better control GVHD.
Figure 4.
The cumulative incidence rate of progression over time by age group
Footnotes
The authors declare no conflicts of interest.
References
- 1.Oliansky DM, Larson RA, Weisdorf D, Dillon H, Ratko TA, Wall D, et al. The Role of Cytotoxic Therapy with Hematopoietic Stem Cell Transplantation in the Treatment of Adult Acute Lymphoblastic Leukemia: Update of the 2006 Evidence-Based Review. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2011.09.002. Epub 2011/08/02. [DOI] [PubMed] [Google Scholar]
- 2.Sutton L, Kuentz M, Cordonnier C, Blaise D, Devergie A, Guyotat D, et al. Allogeneic bone marrow transplantation for adult acute lymphoblastic leukemia in first complete remission: factors predictive of transplant-related mortality and influence of total body irradiation modalities. Bone Marrow Transplant. 1993;12(6):583–9. Epub 1993/12/01. [PubMed] [Google Scholar]
- 3.Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111(4):1827–33. doi: 10.1182/blood-2007-10-116582. Epub 2007/12/01. [DOI] [PubMed] [Google Scholar]
- 4.Kelsey CR, Horwitz ME, Chino JP, Craciunescu O, Steffey B, Folz RJ, et al. Severe pulmonary toxicity after myeloablative conditioning using total body irradiation: an assessment of risk factors. Int J Radiat Oncol Biol Phys. 81(3):812–8. doi: 10.1016/j.ijrobp.2010.06.058. Epub 2010/10/12. [DOI] [PubMed] [Google Scholar]
- 5.Cohen A, van Lint MT, Uderzo C, Rovelli A, Lavagetto A, Vitale V, et al. Growth in patients after allogeneic bone marrow transplant for hematological diseases in childhood. Bone Marrow Transplant. 1995;15(3):343–8. Epub 1995/03/01. [PubMed] [Google Scholar]
- 6.Liesner RJ, Leiper AD, Hann IM, Chessells JM. Late effects of intensive treatment for acute myeloid leukemia and myelodysplasia in childhood. J Clin Oncol. 1994;12(5):916–24. doi: 10.1200/JCO.1994.12.5.916. Epub 1994/05/01. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo JD, Curtis RE, Socie G, Sobocinski KA, Gilbert E, Landgren O, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175–83. doi: 10.1182/blood-2008-05-158782. Epub 2008/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan M, Ljungman P, Bolme P, Ringden O, Syruckova Z, Bekassy A, et al. Busulfan bioavailability. Blood. 1994;84(7):2144–50. Epub 1994/10/01. [PubMed] [Google Scholar]
- 9.Jones RB. Clinical pharmacology of melphalan and its implications for clinical resistance to anticancer agents. Cancer Treat Res. 2002;112:305–22. doi: 10.1007/978-1-4615-1173-1_15. Epub 2002/12/17. [DOI] [PubMed] [Google Scholar]
- 10.Madden T, de Lima M, Thapar N, Nguyen J, Roberson S, Couriel D, et al. Pharmacokinetics of once-daily IV busulfan as part of pretransplantation preparative regimens: a comparison with an every 6-hour dosing schedule. Biol Blood Marrow Transplant. 2007;13(1):56–64. doi: 10.1016/j.bbmt.2006.08.037. Epub 2007/01/16. [DOI] [PubMed] [Google Scholar]
- 11.Balis FM, Poplack DG. Central nervous system pharmacology of antileukemic drugs. Am J Pediatr Hematol Oncol. 1989;11(1):74–86. doi: 10.1097/00043426-198921000-00017. Epub 1989/01/01. [DOI] [PubMed] [Google Scholar]
- 12.Cony-Makhoul P, Marit G, Boiron JM, Puntous M, Reiffers J. Busulphan and melphalan prior to autologous transplantation for myeloid malignancies. Bone Marrow Transplant. 1995;16(1):69–70. Epub 1995/07/01. [PubMed] [Google Scholar]
- 13.Meloni G, Capria S, Vignetti M, Alimena G, de Fabritiis P, Montefusco E, et al. Ten-year follow-up of a single center prospective trial of unmanipulated peripheral blood stem cell autograft and interferon-alpha in early phase chronic myeloyd leukemia. Haematologica. 2001;86(6):596–601. Epub 2001/06/22. [PubMed] [Google Scholar]
- 14.Kebriaei P, Madden T, Kazerooni R, Wang X, Thall PF, Ledesma C, et al. Intravenous busulfan plus melphalan is a highly effective, well-tolerated preparative regimen for autologous stem cell transplantation in patients with advanced lymphoid malignancies. Biol Blood Marrow Transplant. 2011;17(3):412–20. doi: 10.1016/j.bbmt.2010.07.016. Epub 2010/08/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanes M, de la Rubia J, Lahuerta JJ, Gonzalez JD, Ribas P, Solano C, et al. Single daily dose of intravenous busulfan and melphalan as a conditioning regimen for patients with multiple myeloma undergoing autologous stem cell transplantation: a phase II trial. Leuk Lymphoma. 2009;50(2):216–22. doi: 10.1080/10428190802630170. Epub 2009/02/07. [DOI] [PubMed] [Google Scholar]
- 16.Vey N, De Prijck B, Faucher C, Stoppa AM, Sainty D, Lafage M, et al. A pilot study of busulfan and melphalan as preparatory regimen prior to allogeneic bone marrow transplantation in refractory or relapsed hematological malignancies. Bone Marrow Transplant. 1996;18(3):495–9. Epub 1996/09/01. [PubMed] [Google Scholar]
- 17.Small TN, Young JW, Castro-Malaspina H, Prockop S, Wilton A, Heller G, et al. Intravenous busulfan and melphalan, tacrolimus, and short-course methotrexate followed by unmodified HLA-matched related or unrelated hematopoietic stem cell transplantation for the treatment of advanced hematologic malignancies. Biol Blood Marrow Transplant. 2007;13(2):235–44. doi: 10.1016/j.bbmt.2006.10.005. Epub 2007/01/24. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe T, Kajiume T, Abe T, Kawano Y, Iwai A, Iwai T, et al. Allogeneic peripheral blood stem cell transplantation in children with hematologic malignancies from HLA-matched siblings. Med Pediatr Oncol. 2000;34(3):171–6. doi: 10.1002/(sici)1096-911x(200003)34:3<171::aid-mpo2>3.0.co;2-0. Epub 2000/03/01. [DOI] [PubMed] [Google Scholar]
- 19.Matsuyama T, Kojima S, Kato K. Allogeneic bone marrow transplantation for childhood leukemia following a busulfan and melphalan preparative regimen. Bone Marrow Transplant. 1998;22(1):21–6. doi: 10.1038/sj.bmt.1701276. Epub 1998/07/25. [DOI] [PubMed] [Google Scholar]
- 20.Martino R, Badell I, Brunet S, Sureda A, Torras A, Cubells J, et al. High-dose busulfan and melphalan before bone marrow transplantation for acute nonlymphoblastic leukemia. Bone Marrow Transplant. 1995;16(2):209–12. Epub 1995/08/01. [PubMed] [Google Scholar]
- 21.Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8(9):468–76. doi: 10.1053/bbmt.2002.v8.pm12374451. Epub 2002/10/11. [DOI] [PubMed] [Google Scholar]
- 22.Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8(9):477–85. doi: 10.1053/bbmt.2002.v8.pm12374452. Epub 2002/10/11. [DOI] [PubMed] [Google Scholar]
- 23.de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104(3):857–64. doi: 10.1182/blood-2004-02-0414. Epub 2004/04/10. [DOI] [PubMed] [Google Scholar]
- 24.Schumitzky DDAaA. ADAPT II User’s Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Los Angeles, CA: 1997. [Google Scholar]
- 25.Andersson BS, Madden T, Tran HT, Hu WW, Blume KG, Chow DS, et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant. 2000;6(5A):548–54. doi: 10.1016/s1083-8791(00)70064-4. Epub 2000/11/09. [DOI] [PubMed] [Google Scholar]
- 26.Kebriaei P, Saliba R, Rondon G, Chiattone A, Luthra R, Anderlini P, et al. Long-Term Follow-up of Allogeneic Hematopoietic Stem Cell Transplantation for Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: Impact of Tyrosine Kinase Inhibitors on Treatment Outcomes. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.08.011. Epub 2011/08/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8. Epub 1995/06/01. [PubMed] [Google Scholar]
- 28.Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57(2):267–76. Epub 1981/02/01. [PubMed] [Google Scholar]
- 29.Thall PF, Wathen JK. Covariate-adjusted adaptive randomization in a sarcoma trial with multi-stage treatments. Stat Med. 2005;24(13):1947–64. doi: 10.1002/sim.2077. Epub 2005/04/05. [DOI] [PubMed] [Google Scholar]
- 30.Meier P, Kaplan EL. Non-parametric estimation from incomplete observations. Journal of the American Statistical Association. 1958:53. [Google Scholar]
- 31.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;50:163–70. [PubMed] [Google Scholar]
- 32.Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44(6):778–83. doi: 10.1097/00007890-198712000-00011. Epub 1987/12/01. [DOI] [PubMed] [Google Scholar]
- 33.Lahuerta JJ, Mateos MV, Martinez-Lopez J, Grande C, de la Rubia J, Rosinol L, et al. Busulfan 12 mg/kg plus melphalan 140 mg/m2 versus melphalan 200 mg/m2 as conditioning regimens for autologous transplantation in newly diagnosed multiple myeloma patients included in the PETHEMA/GEM2000 study. Haematologica. 95(11):1913–20. doi: 10.3324/haematol.2010.028027. Epub 2010/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grigg AP, Stone J, Milner AD, Schwarer AP, Wolf M, Prince HM, et al. Phase II study of autologous stem cell transplant using busulfan-melphalan chemotherapy-only conditioning followed by interferon for relapsed poor prognosis follicular non-Hodgkin lymphoma. Leuk Lymphoma. 51(4):641–9. doi: 10.3109/10428191003611428. Epub 2010/03/12. [DOI] [PubMed] [Google Scholar]
- 35.Lahuerta JJ, Martinez-Lopez J, Grande C, Blade J, de la Serna J, Alegre A, et al. Conditioning regimens in autologous stem cell transplantation for multiple myeloma: a comparative study of efficacy and toxicity from the Spanish Registry for Transplantation in Multiple Myeloma. Br J Haematol. 2000;109(1):138–47. doi: 10.1046/j.1365-2141.2000.01979.x. Epub 2000/06/10. [DOI] [PubMed] [Google Scholar]
- 36.Marks DI, Wang T, Perez WS, Antin JH, Copelan E, Gale RP, et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010;116(3):366–74. doi: 10.1182/blood-2010-01-264077. Epub 2010/04/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang W, Wang L, Zhao WL, Chen YB, Shen ZX, Hu J. Intravenous busulfan-cyclophosphamide as a preparative regimen before allogeneic hematopoietic stem cell transplantation for adult patients with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2011;17(10):1555–61. doi: 10.1016/j.bbmt.2011.04.003. Epub 2011/05/10. [DOI] [PubMed] [Google Scholar]
- 38.Valdez BC, Li Y, Murray D, Champlin RE, Andersson BS. The synergistic cytotoxicity of clofarabine, fludarabine and busulfan in AML cells involves ATM pathway activation and chromatin remodeling. Biochemical pharmacology. 2011;81(2):222–32. doi: 10.1016/j.bcp.2010.09.027. Epub 2010/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersson BS, Valdez BC, de Lima M, Wang X, Thall PF, Worth LL, et al. Clofarabine +/− fludarabine with once daily i.v. busulfan as pretransplant conditioning therapy for advanced myeloid leukemia and MDS. Biol Blood Marrow Transplant. 2011;17(6):893–900. doi: 10.1016/j.bbmt.2010.09.022. Epub 2010/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santarone S, Pidala J, Di Nicola M, Field T, Alsina M, Ayala E, et al. Fludarabine and pharmacokinetic-targeted busulfan before allografting for adults with acute lymphoid leukemia. Biol Blood Marrow Transplant. 2011;17(10):1505–11. doi: 10.1016/j.bbmt.2011.02.011. Epub 2011/03/10. [DOI] [PubMed] [Google Scholar]
- 41.O’Connor D, Sibson K, Caswell M, Connor P, Cummins M, Mitchell C, et al. Early UK experience in the use of clofarabine in the treatment of relapsed and refractory paediatric acute lymphoblastic leukaemia. Br J Haematol. 2011;154(4):482–5. doi: 10.1111/j.1365-2141.2011.08752.x. Epub 2011/06/22. [DOI] [PubMed] [Google Scholar]
- 42.Jeha S, Gandhi V, Chan KW, McDonald L, Ramirez I, Madden R, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103(3):784–9. doi: 10.1182/blood-2003-06-2122. Epub 2003/10/11. [DOI] [PubMed] [Google Scholar]
- 43.Kebriaei PCL, Culotta K, Shpall E, Ciurea S, Alousi A, Popat U, de Lima MDT, Champlin RE, Andersson BS, editors. Clofarabine (Clo) and Busulfan as a Novel Reduced Toxicity Conditioning Regimen for Allogeneic Hematopoietic Cell Transplantation (HCT) in Patients (pts) with Acute Lymphoblastic Leukemia (ALL) American Society of Hematology. Orlando, Florida: 2010. [Google Scholar]